|
|
市場調査レポート
商品コード
1463593
北米の脊椎固定装置:2030年市場予測-地域別分析-製品タイプ、手術タイプ、適応疾患、エンドユーザー別North America Spinal Fusion Devices Market Forecast to 2030 - Regional Analysis - By Product Type, Surgery Type, Disease Indications, and End User |
||||||
|
|||||||
| 北米の脊椎固定装置:2030年市場予測-地域別分析-製品タイプ、手術タイプ、適応疾患、エンドユーザー別 |
|
出版日: 2024年01月24日
発行: The Insight Partners
ページ情報: 英文 109 Pages
納期: 即納可能
|
- 全表示
- 概要
- 図表
- 目次
北米の脊椎固定装置市場は、2022年に38億6,893万米ドルと評価され、2030年には60億7,564万米ドルに達すると予測され、2022~2030年のCAGRは5.8%で成長すると予測されています。
脊椎機器企業による脊椎技術の新興国市場開拓が北米の脊椎固定機器市場を活性化
脊椎固定装置企業は、技術革新、スピンオフ、M&Aなどの開発・戦略を広く採用しています。これらの戦略により、北米の脊椎固定装置市場は2022年に飛躍的な成長を遂げることが予想されます。以下は、2022年に各社が行った開発の例です。
2022年8月、Kleiner Device Labsは、新しいKG2サージ・フロースルー・インターボディ・システムを使用した初の手術の成功を発表しました。2022年7月19日、米国ニューヨークのブルックリン病院センターで、1レベルの経椎体腰椎椎体間固定術(TLIF)が行われました。Kleiner Device Labsは、新しいKG2サージ・フロースルー・インターボディ・システムは、従来の脊椎手術アプローチに比べ、3倍の骨移植材量を提供できるとしています。同社はまた、KG2サージ・フロースルー・インターボディ・システムは、3Dプリントされたチタン製i-Beam融合インプラントと組み合わされた、患者一人用の骨移植材送達ツールであるとしています。さらに、KG2サージ・フロースルー・インターボディ・システムは、現在の脊椎固定術の多段階、多器具通過を1回の挿入工程に減らします。
2022年3月、Zimmer Biomet Holdings, Incはジンビーの完全分離独立を発表しました。ZimVieは脊椎と歯科市場の主要成長戦略に引き続き注力します。同社は脊椎固定インプラント、手術器具、骨移植片、インプラント、非固定代替品、デジタルケア技術などの製品を開発します。分社化により、ZimVieはナスダックに「ZIMV」のシンボルで登録された独立企業として運営されます。従って、ZimVieは良好な市場シェアを保持することで、市場を大幅に強化することが期待されます。
2022年10月、Spineartは新しい低侵襲脊椎手術(MISS)システム「PERLA TL MIS」を発売しました。同社のMISSシステムは、世界中で利用可能な胸腰椎後方固定システムです。PERLA TL MISの発売は、エンドユーザー(外科医、患者、病院)に利益をもたらす脊椎外科手術の変革に対する同社のコミットメントを示すものと期待されています。このような製品の発売は、今後の市場成長を牽引するものと考えられます。
脊椎業界における発展動向は続いており、北米の脊椎固定装置市場の成長を後押しする戦略的な改善が見られます。
2023年7月、Xtant Medical Holdings, Inc.は、脊椎固定と国内外の生物製剤に関するSurgalign Holdings, Inc.の特定の資産と負債を獲得したと発表しました。Xtant Medical Holdings, Inc.は、複雑な脊椎変形の退行過程における脊椎固定を促進する脊椎インプラントシステムの開発に専念しています。このように、脊椎器具メーカーによる脊椎技術のこうした市場開拓が、市場の成長を促進しています。
北米の脊椎固定装置市場概要
北米の脊椎固定装置市場では、DePuy Synthes、Stryker、Aurora Spine、Alevio Spineなどが米国で事業を展開している主要企業です。これらの企業による製品開拓と市場投入が市場成長を後押ししています。米国では、FDA(食品医薬品局)の認可を受けた最先端の脊椎固定装置が広く採用されています。以下は、最近FDAによって承認された脊椎固定装置一覧です。
2023年5月、CTL Amedica は、生体材料である窒化ケイ素の融合によってのみ製造されるNITRO Interbody Fusion Cage Systemの商品化についてFDA 510(k)認可を取得しました。窒化ケイ素材料はあらゆる画像モダリティと互換性があり、独自の静菌特性を示し、アーチファクトのない画像を記載しています。
2023年1月、Alevio Spine社は、SI-Cure SI Joint Fusion Systemの適応追加の510(K)クリアランスを取得しました。適応拡大には、腰椎または胸腰椎固定術の一部として仙腸関節固定術を受ける骨格的に成熟した患者に対する仙腸関節固定術が含まれます。
2022年6月、米国FDAはAurora SpineのDEXA SOLO-L前方腰椎椎体間固定装置(ALIF)の510K認可を与えました。DEXA技術・プラットフォームに基づいて、3Dプリントされたスタンドアローン装置が前方と側方腰椎椎体間固定術(ALIF &LLIF)用に設計されました。
米国では、高齢者の腰痛(LBP)の蔓延の引き金となる加齢に関連した磨耗が、脊椎固定装置の需要を煽っています。2022年のNational Health Servicesによると、米国における腰痛の生涯発症率は60~90%で、年間発症率は5%と報告されています。また、この情報源によると、毎年新規患者の14.3%がLBPが原因で医師を訪れており、慢性LBPが原因で医師を訪れている人は1,300万人にのぼるといいます。
北米の脊椎固定装置市場の収益と2030年までの予測(金額)
北米の脊椎固定装置市場のセグメンテーション
北米の脊椎固定装置市場は、製品タイプ、手術タイプ、適応疾患、エンドユーザー、国によって区分されます。
製品タイプ別に見ると、北米の脊椎固定装置市場は、胸腰椎固定装置、頸椎固定装置、胴体間固定装置に区分されます。2022年には胸腰椎デバイスセグメントが最大のシェアを占めています。
手術タイプ別では、北米の脊椎固定装置市場は開腹脊椎手術と低侵襲脊椎手術に二分されます。2022年には、開放脊椎手術セグメントが最大のシェアを占めています。
疾患適応症別では、北米の脊椎固定装置市場は変性椎間板、外傷・骨折、複雑な変形、その他に区分されます。変性椎間板セグメントは2022年に最大のシェアを占めました。
エンドユーザー別では、北米の脊椎固定装置市場は病院、専門クリニック、その他に分類されます。病院セグメントが2022年に最大のシェアを占めました。
国別では、北米の脊椎固定装置市場は米国、カナダ、メキシコに区分されます。2022年の北米の脊椎固定装置市場は米国が支配的でした。
ATEC Spine Inc、B. Braun SE、Centinel Spine LLC、DePuy Synthes Inc、Globus Medical Inc、Medtronic Plc、NuVasive Inc、Orthofix Medical Inc、Stryker Corp、ZimVie Incなどが北米の脊椎固定装置市場で事業を展開する大手企業です。
目次
第1章 イントロダクション
第2章 エグゼクティブサマリー
- 主要な洞察
第3章 調査手法
- 調査範囲
- 2次調査
- 1次調査
第4章 北米の脊椎固定装置市場情勢
- 概観
- 北米PEST分析
- エコシステム分析
- バリューチェーンのベンダー一覧
第5章 脊椎固定装置の北米市場:主要産業力学
- 市場促進要因
- 脊椎デバイス企業による脊椎技術の開発の増加
- 脊椎固定術の急増
- 市場抑制要因
- 脊椎固定装置に対する厳しい規制
- 市場機会
- 医療ツーリズムによる低コストの手術
- 市場動向
- 脊椎固定用製品の3Dプリントによる脊椎手術への用途
- 影響分析
第6章 脊椎固定装置市場:北米市場分析
- 北米の脊椎固定装置市場の売上実績、2022~2030年
第7章 北米の脊椎固定装置市場:2030年までの収益と予測:製品タイプ別
- イントロダクション
- 北米の脊椎固定装置市場:2022年と2030年の製品タイプ別売上高シェア(%)
- 胸腰椎デバイス
- 頸椎固定装置
- 椎体間固定装置
第8章 北米の脊椎固定装置市場:2030年までの収益と予測:手術タイプ別
- イントロダクション
- 北米の脊椎固定装置市場:2022年と2030年の手術タイプ別売上高シェア(%)
- 開腹脊椎手術
- 低侵襲脊椎手術
第9章 北米の脊椎固定装置市場:2030年までの収益と予測:適応疾患別
- イントロダクション
- 北米の脊椎固定装置市場:2022年と2030年の適応疾患別売上シェア(%)
- 変性椎間板
- 外傷と骨折
- 複雑な変形
- その他
第10章 北米の脊椎固定装置市場:2030年までの収益と予測:エンドユーザー別
- イントロダクション
- 北米の脊椎固定装置市場:2022年と2030年のエンドユーザー別売上高シェア(%)
- 病院
- 専門クリニック
- その他
第11章 北米の脊椎固定装置市場:国別分析
- 北米
- 米国
- カナダ
- メキシコ
第12章 北米の脊椎固定装置市場の業界情勢
- イントロダクション
- 北米の脊椎固定装置市場における成長戦略
- 有機的成長戦略
- 概要
- 無機的成長戦略
- 概要
第13章 企業プロファイル
- DePuy Synthes Inc
- Stryker Corp
- B. Braun SE
- ATEC Spine Inc
- Globus Medical Inc
- NuVasive Inc
- ZimVie Inc
- Medtronic Plc
- Centinel Spine LLC
- Orthofix Medical Inc
第14章 付録
List Of Tables
- Table 1. North America Spinal Fusion Devices Market Segmentation
- Table 2. List of Vendors
- Table 3. US Spinal Fusion Devices Market Revenue and Forecast to 2030 (US$ Mn) - Product Type
- Table 4. US Spinal Fusion Devices Market Revenue and Forecast to 2030 (US$ Mn) - Thoracolumbar Devices
- Table 5. US Spinal Fusion Devices Market Revenue and Forecast to 2030 (US$ Mn) - Cervical Fixation Devices
- Table 6. US Spinal Fusion Devices Market Revenue and Forecast to 2030 (US$ Mn) - Disease Indication
- Table 7. US Spinal Fusion Devices Market Revenue and Forecast to 2030 (US$ Mn) - Surgery Type
- Table 8. US Spinal Fusion Devices Market Revenue and Forecast to 2030 (US$ Mn) - End User
- Table 9. Canada Spinal Fusion Devices Market Revenue and Forecast to 2030 (US$ Mn) - Product Type
- Table 10. Canada Spinal Fusion Devices Market Revenue and Forecast to 2030 (US$ Mn) - Thoracolumbar Devices
- Table 11. Canada Spinal Fusion Devices Market Revenue and Forecast to 2030 (US$ Mn) - Cervical Fixation Devices
- Table 12. Canada Spinal Fusion Devices Market Revenue and Forecast to 2030 (US$ Mn) - Disease Indication
- Table 13. Canada Spinal Fusion Devices Market Revenue and Forecast to 2030 (US$ Mn) - Surgery Type
- Table 14. Canada Spinal Fusion Devices Market Revenue and Forecast to 2030 (US$ Mn) - End User
- Table 15. Mexico Spinal Fusion Devices Market Revenue and Forecast to 2030 (US$ Mn) - Product Type
- Table 16. Mexico Spinal Fusion Devices Market Revenue and Forecast to 2030 (US$ Mn) - Thoracolumbar Devices
- Table 17. Mexico Spinal Fusion Devices Market Revenue and Forecast to 2030 (US$ Mn) - Cervical Fixation Devices
- Table 18. Mexico Spinal Fusion Devices Market Revenue and Forecast to 2030 (US$ Mn) - Disease Indication
- Table 19. Mexico Spinal Fusion Devices Market Revenue and Forecast to 2030 (US$ Mn) - Surgery Type
- Table 20. Mexico Spinal Fusion Devices Market Revenue and Forecast to 2030 (US$ Mn) - End User
- Table 21. Recent Organic Growth Strategies in North America Spinal Fusion Devices Market
- Table 22. Recent Inorganic Growth Strategies in the North America Spinal Fusion Devices Market
- Table 23. Glossary of Terms, spinal fusion devices market
List Of Figures
- Figure 1. North America Spinal Fusion Devices Market Segmentation, By Country
- Figure 2. North America - PEST Analysis
- Figure 3. North America Spinal Fusion Devices Market - Key Industry Dynamics
- Figure 4. Impact Analysis of Drivers and Restraints
- Figure 5. North America Spinal Fusion Devices Market Revenue (US$ Mn), 2022 - 2030
- Figure 6. North America Spinal Fusion Devices Market Revenue Share, by Product Type, 2022 & 2030 (%)
- Figure 7. Thoracolumbar Devices: North America Spinal Fusion Devices Market - Revenue and Forecast to 2030 (US$ Million)
- Figure 8. Anterior Lumbar Plates: North America Spinal Fusion Devices Market - Revenue and Forecast to 2030 (US$ Million)
- Figure 9. Pedicle Screw and Rods: North America Spinal Fusion Devices Market - Revenue and Forecast to 2030 (US$ Million)
- Figure 10. Others: North America Spinal Fusion Devices Market - Revenue and Forecast to 2030 (US$ Million)
- Figure 11. Cervical Fixation Devices: North America Spinal Fusion Devices Market - Revenue and Forecast to 2030 (US$ Million)
- Figure 12. Anterior Cervical Plates: North America Spinal Fusion Devices Market - Revenue and Forecast to 2030 (US$ Million)
- Figure 13. Hook Fixation Systems: North America Spinal Fusion Devices Market - Revenue and Forecast to 2030 (US$ Million)
- Figure 14. Others: North America Spinal Fusion Devices Market - Revenue and Forecast to 2030 (US$ Million)
- Figure 15. Interbody Fusion Devices: North America Spinal Fusion Devices Market - Revenue and Forecast to 2030 (US$ Million)
- Figure 16. North America Spinal Fusion Devices Market Revenue Share, by Surgery Type, 2022 & 2030 (%)
- Figure 17. Open Spine Surgery: North America Spinal Fusion Devices Market - Revenue and Forecast to 2030 (US$ Million)
- Figure 18. Minimally Invasive Spine Surgery: North America Spinal Fusion Devices Market - Revenue and Forecast to 2030 (US$ Million)
- Figure 19. North America Spinal Fusion Devices Market Revenue Share, by Disease Indications 2022 & 2030 (%)
- Figure 20. Degenerative Disc: North America Spinal Fusion Devices Market - Revenue and Forecast to 2030 (US$ Million)
- Figure 21. Trauma and Fractures: North America Spinal Fusion Devices Market - Revenue and Forecast to 2030 (US$ Million)
- Figure 22. Complex Deformity: North America Spinal Fusion Devices Market - Revenue and Forecast to 2030 (US$ Million)
- Figure 23. Others: North America Spinal Fusion Devices Market - Revenue and Forecast to 2030 (US$ Million)
- Figure 24. North America Spinal Fusion Devices Market Revenue Share, by End User 2022 & 2030 (%)
- Figure 25. Hospitals: North America Spinal Fusion Devices Market- Revenue and Forecast to 2030 (US$ Million)
- Figure 26. Specialty Clinics: North America Spinal Fusion Devices Market - Revenue and Forecast to 2030 (US$ Million)
- Figure 27. Others: North America Spinal Fusion Devices Market - Revenue and Forecast to 2030 (US$ Million)
- Figure 28. North America Spinal Fusion Devices Market by Key Country - Revenue (2022) (US$ Million))
- Figure 29. North America North America Spinal Fusion Devices Market, By Key Countries, 2022 And 2030 (%)
- Figure 30. US Spinal Fusion Devices Market Revenue and Forecast to 2030 (US$ Mn)
- Figure 31. Canada Spinal Fusion Devices Market Revenue and Forecast to 2030 (US$ Mn)
- Figure 32. Mexico Spinal Fusion Devices Market Revenue and Forecast to 2030 (US$ Mn)
- Figure 33. Growth Strategies in North America Spinal Fusion Devices Market
The North America spinal fusion devices market was valued at US$ 3,868.93 million in 2022 and is expected to reach US$ 6,075.64 million by 2030; it is estimated to grow at a CAGR of 5.8% from 2022 to 20 30.
Increasing Developments in Spinal Technology by Spine Device Companies Fuels the North America Spinal Fusion Devices Market
The spine device companies widely adopted innovation, spinoffs, and merger and acquisition developments or strategies. The strategies have helped mark an exponential growth of the North America spinal fusion devices market in 2022. Below are a few instances of developments made by the companies in 2022.
In August 2022, Kleiner Device Labs announced the completion of its first successful surgery using its new KG2 Surge flow-thru interbody system. On July 19, 2022, the single level transforaminal lumbar interbody fusion (TLIF) surgery was performed at the Brooklyn Hospital Center, New York, US. Kleiner Device Labs claims that the new KG2 Surge flow-thru interbody system provided the amount of bone-grafting material three times more than the traditional spinal surgery approaches. The company also claims that their KG2 Surge flow-thru interbody system is a single-patient-use bone graft delivery tool combined with a 3D-printed titanium I-Beam fusion implant. Further, the KG2 Surge flow-thru interbody system will reduce multi-step, multi-instrument pass practice to a single insertion process of current spinal fusion procedures.
In March 2022, Zimmer Biomet Holdings, Inc. announced a complete spinoff of ZimVie. ZimVie will continue to focus on key growth strategies for the spine and dental markets. The company will develop products, including spinal fusion implants, surgical tools, bone grafts, implants, non-fusion alternatives, and digital care technologies. Due to the spinoff, ZimVie will operate as an independent entity registered on the Nasdaq with the symbol 'ZIMV.' Thus, it is expected that ZimVie will significantly enhance the market by holding a good market share.
In October 2022, Spineart launched its new minimally invasive spine surgery (MISS) system, PERLA TL MIS. The company's MISS system is a thoracolumbar posterior fixation system available worldwide. The launch of PERLA TL MIS is anticipated to demonstrate the company's commitment to transforming spine surgery to benefit its end users: surgeons, patients, and hospitals. Such product launches are likely to drive market growth in the coming future.
The trend of developments in the spine industry has continued and witnessed strategic improvements that have fueled the growth of the North America spinal fusion devices market.
In July 2023, Xtant Medical Holdings, Inc. announced that it won certain assets and liabilities of Surgalign Holdings, Inc. related to spinal fixation and domestic and international biologics. Xtant Medical Holdings, Inc. is dedicated to developing spinal implant systems that facilitate spinal fusion in complex spinal deformity degenerative processes. Thus, such developments in spinal technology by spine device companies are catalyzing the market growth.
North America Spinal Fusion Devices Market Overview
DePuy Synthes, Stryker, Aurora Spine, and Alevio Spine are among the major players operating in the North America spinal fusion devices market in the US. Product developments and launches driven by these players favor the market growth. Technologically advanced spinal fusion devices approved by the Food and Drug Administration (FDA) are widely adopted in the US. Following is the list of spinal fusion devices recently approved by the FDA:
In May 2023, CTL Amedica received FDA 510(k) clearance for the commercialization of the NITRO Interbody Fusion Cage System, which is exclusively made by the fusion of biomaterial silicon nitride. Silicon nitride material is compatible with all imaging modalities; it exhibits unique bacteriostatic properties and provides artifact-free imaging.
In January 2023, Alevio Spine received 510 (K) clearance of additional indications for the SI-Cure SI Joint Fusion System. The expanded indication includes sacroiliac fusion for skeletally mature patients undergoing sacropelvic fixation as part of a lumbar or thoracolumbar fusion.
In June 2022, the US FDA granted 510K clearance for Aurora Spine's DEXA SOLO-L anterior lumbar interbody fusion device (ALIF). Based on DEXA Technology Platform, a 3D printed standalone device was designed for anterior and lateral lumbar interbody fusion (ALIF & LLIF) procedures.
Age-related wear-and-tear triggers the prevalence of lower back pain (LBP) among the geriatric population in the US, in turn, fuels the demand for spinal fusion devices. According to National Health Services in 2022, lifetime incidence of LBP in the US is reported to be 60-90%, with annual incidence of 5%. The source also states that 14.3% of new patients visit physicians each year because of LBP, and ~13 million people visit physician due to chronic LBP.
North America Spinal Fusion Devices Market Revenue and Forecast to 2030 (US$ Million)
North America Spinal Fusion Devices Market Segmentation
The North America spinal fusion devices market is segmented based on product type, surgery type, disease indications, end user, and country.
Based on product type, the North America spinal fusion devices market is segmented into thoracolumbar devices, cervical fixation devices, and interbody fusion devices. The thoracolumbar devices segment held the largest share in 2022.
By surgery type, the North America spinal fusion devices market is bifurcated into open spine surgery and minimally invasive spine surgery. The open spine surgery segment held the largest share in 2022.
By disease indications, the North America spinal fusion devices market is segmented into degenerative disc, trauma and fractures, complex deformity, and others. The degenerative disc segment held the largest share in 2022.
In terms of end users, the North America spinal fusion devices market is categorized into hospitals, specialty clinics, and others. The hospitals segment held the largest share in 2022.
Based on country, the North America spinal fusion devices market is segmented into the US, Canada, and Mexico. The US dominated the North America spinal fusion devices market in 2022.
ATEC Spine Inc, B. Braun SE, Centinel Spine LLC, DePuy Synthes Inc, Globus Medical Inc, Medtronic Plc, NuVasive Inc, Orthofix Medical Inc, Stryker Corp, and ZimVie Inc are some of the leading companies operating in the North America spinal fusion devices market.
Table Of Contents
1. Introduction
- 1.1 The Insight Partners Research Report Guidance
- 1.2 Market Segmentation
2. Executive Summary
- 2.1 Key Insights
3. Research Methodology
- 3.1 Coverage
- 3.2 Secondary Research
- 3.3 Primary Research
4. North America Spinal Fusion Devices Market Landscape
- 4.1 Overview
- 4.2 North America PEST Analysis
- 4.3 Ecosystem Analysis
- 4.3.1 List of Vendors in the Value Chain
5. North America Spinal Fusion Devices Market - Key Industry Dynamics
- 5.1 Market Drivers
- 5.1.1 Increasing Developments in Spinal Technology by Spine Device Companies
- 5.1.2 Surging Number of Spinal Fusion Procedures
- 5.2 Market Restraints
- 5.2.1 Stringent Regulations for Spinal Fusion Devices
- 5.3 Market Opportunities
- 5.3.1 Low-Cost Surgery Under Medical Tourism
- 5.4 Market Trends
- 5.4.1 3D Printing for Spinal Fusion Products for Spinal Surgery
- 5.5 Impact Analysis:
6. Spinal Fusion Devices Market - North America Market Analysis
- 6.1 North America Spinal Fusion Devices Market Revenue (US$ Mn), 2022 - 2030
7. North America Spinal Fusion Devices Market - Revenue and Forecast to 2030 - by Product Type
- 7.1 Overview
- 7.2 North America Spinal Fusion Devices Market Revenue Share, by Product Type, 2022 & 2030 (%)
- 7.3 Thoracolumbar Devices
- 7.3.1 Overview
- 7.3.2 Thoracolumbar Devices: North America Spinal Fusion Devices Market - Revenue and Forecast to 2030 (US$ Million)
- 7.3.3 Anterior Lumbar Plates
- 7.3.3.1 Overview
- 7.3.3.2 Anterior Lumbar Plates: North America Spinal Fusion Devices Market - Revenue and Forecast to 2030 (US$ Million)
- 7.3.4 Pedicle Screw and Rods
- 7.3.4.1 Overview
- 7.3.4.2 Pedicle Screw and Rods: North America Spinal Fusion Devices Market - Revenue and Forecast to 2030 (US$ Million)
- 7.3.5 Others
- 7.3.5.1 Overview
- 7.3.5.2 Others: North America Spinal Fusion Devices Market - Revenue and Forecast to 2030 (US$ Million)
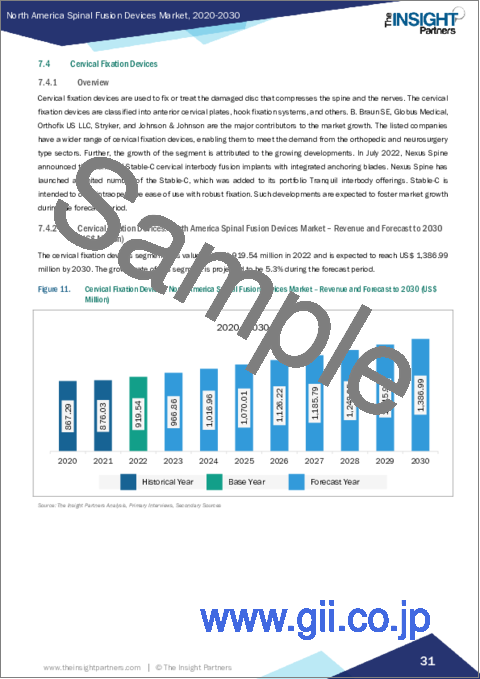
- 7.4 Cervical Fixation Devices
- 7.4.1 Overview
- 7.4.2 Cervical Fixation Devices: North America Spinal Fusion Devices Market - Revenue and Forecast to 2030 (US$ Million)
- 7.4.3 Anterior Cervical Plates
- 7.4.3.1 Overview
- 7.4.3.2 Anterior Cervical Plates: North America Spinal Fusion Devices Market - Revenue and Forecast to 2030 (US$ Million)
- 7.4.4 Hook Fixation Systems
- 7.4.4.1 Overview
- 7.4.4.2 Hook Fixation Systems: North America Spinal Fusion Devices Market - Revenue and Forecast to 2030 (US$ Million)
- 7.4.5 Others
- 7.4.5.1 Overview
- 7.4.5.2 Others: North America Spinal Fusion Devices Market - Revenue and Forecast to 2030 (US$ Million)
- 7.5 Interbody Fusion Devices
- 7.5.1 Overview
- 7.5.2 Interbody Fusion Devices: North America Spinal Fusion Devices Market - Revenue and Forecast to 2030 (US$ Million)
8. North America Spinal Fusion Devices Market - Revenue and Forecast to 2030 - by Surgery Type
- 8.1 Overview
- 8.2 North America Spinal Fusion Devices Market Revenue Share, by Surgery Type, 2022 & 2030 (%)
- 8.3 Open Spine Surgery
- 8.3.1 Overview
- 8.3.2 Open Spine Surgery: North America Spinal Fusion Devices Market - Revenue and Forecast to 2030 (US$ Million)
- 8.4 Minimally Invasive Spine Surgery
- 8.4.1 Overview
- 8.4.2 Minimally Invasive Spine Surgery: North America Spinal Fusion Devices Market - Revenue and Forecast to 2030 (US$ Million)
9. North America Spinal Fusion Devices Market - Revenue and Forecast to 2030 - by Disease Indications
- 9.1 Overview
- 9.2 North America Spinal Fusion Devices Market Revenue Share, by Disease Indications 2022 & 2030 (%)
- 9.3 Degenerative Disc
- 9.3.1 Overview
- 9.3.2 Degenerative Disc: North America Spinal Fusion Devices Market - Revenue and Forecast to 2030 (US$ Million)
- 9.4 Trauma and Fractures
- 9.4.1 Overview
- 9.4.2 Trauma and Fractures: North America Spinal Fusion Devices Market - Revenue and Forecast to 2030 (US$ Million)
- 9.5 Complex Deformity
- 9.5.1 Overview
- 9.5.2 Complex Deformity: North America Spinal Fusion Devices Market - Revenue and Forecast to 2030 (US$ Million)
- 9.6 Others
- 9.6.1 Overview
- 9.6.2 Others: North America Spinal Fusion Devices Market - Revenue and Forecast to 2030 (US$ Million)
10. North America Spinal Fusion Devices Market - Revenue and Forecast to 2030 - by End User
- 10.1 Overview
- 10.2 North America Spinal Fusion Devices Market Revenue Share, by End User 2022 & 2030 (%)
- 10.3 Hospitals
- 10.3.1 Overview
- 10.3.2 Hospitals: North America Spinal Fusion Devices Market- Revenue and Forecast to 2030 (US$ Million)
- 10.4 Specialty Clinics
- 10.4.1 Overview
- 10.4.2 Specialty Clinics: North America Spinal Fusion Devices Market - Revenue and Forecast to 2030 (US$ Million)
- 10.5 Others
- 10.5.1 Overview
- 10.5.2 Others: North America Spinal Fusion Devices Market - Revenue and Forecast to 2030 (US$ Million)
11. North America Spinal Fusion Devices Market - Country Analysis
- 11.1 Overview
- 11.1.1 North America North America Spinal Fusion Devices Market, by Country
- 11.1.1.1 US
- 11.1.1.1.1 Overview
- 11.1.1.1.2 US Spinal Fusion Devices Market Revenue and Forecast to 2030 (US$ Mn)
- 11.1.1.1.3 US Spinal Fusion Devices Market, by Product Type
- 11.1.1.1.3.1 US Spinal Fusion Devices Market, by Thoracolumbar Devices
- 11.1.1.1.3.2 US Spinal Fusion Devices Market, by Cervical Fixation Devices
- 11.1.1.1.4 US Spinal Fusion Devices Market, by Disease Indication
- 11.1.1.1.5 US Spinal Fusion Devices Market, by Surgery Type
- 11.1.1.1.6 US Spinal Fusion Devices Market, by End User
- 11.1.1.2 Canada
- 11.1.1.2.1 Overview
- 11.1.1.2.2 Canada Spinal Fusion Devices Market Revenue and Forecast to 2030 (US$ Mn)
- 11.1.1.2.3 Canada Spinal Fusion Devices Market, by Product Type
- 11.1.1.2.3.1 Canada Spinal Fusion Devices Market, by Thoracolumbar Devices
- 11.1.1.2.3.2 Canada Spinal Fusion Devices Market, by Cervical Fixation Devices
- 11.1.1.2.4 Canada Spinal Fusion Devices Market, by Disease Indication
- 11.1.1.2.5 Canada Spinal Fusion Devices Market, by Surgery Type
- 11.1.1.2.6 Canada Spinal Fusion Devices Market, by End User
- 11.1.1.3 Mexico
- 11.1.1.3.1 Overview
- 11.1.1.3.2 Mexico Spinal Fusion Devices Market Revenue and Forecast to 2030 (US$ Mn)
- 11.1.1.3.3 Mexico Spinal Fusion Devices Market, by Product Type
- 11.1.1.3.3.1 Mexico Spinal Fusion Devices Market, by Thoracolumbar Devices
- 11.1.1.3.3.2 Mexico Spinal Fusion Devices Market, by Cervical Fixation Devices
- 11.1.1.3.4 Mexico Spinal Fusion Devices Market, by Disease Indication
- 11.1.1.3.5 Mexico Spinal Fusion Devices Market, by Surgery Type
- 11.1.1.3.6 Mexico Spinal Fusion Devices Market, by End User
- 11.1.1.1 US
- 11.1.1 North America North America Spinal Fusion Devices Market, by Country
12. North America Spinal Fusion Devices Market Industry Landscape
- 12.1 Overview
- 12.2 Growth Strategies in North America Spinal Fusion Devices Market
- 12.3 Organic Growth Strategies
- 12.3.1 Overview
- 12.4 Inorganic Growth Strategies
- 12.4.1 Overview
13. Company Profiles
- 13.1 DePuy Synthes Inc
- 13.1.1 Key Facts
- 13.1.2 Business Description
- 13.1.3 Products and Services
- 13.1.4 Financial Overview
- 13.1.5 SWOT Analysis
- 13.1.6 Key Developments
- 13.2 Stryker Corp
- 13.2.1 Key Facts
- 13.2.2 Business Description
- 13.2.3 Products and Services
- 13.2.4 Financial Overview
- 13.2.5 SWOT Analysis
- 13.2.6 Key Developments
- 13.3 B. Braun SE
- 13.3.1 Key Facts
- 13.3.2 Business Description
- 13.3.3 Products and Services
- 13.3.4 Financial Overview
- 13.3.5 SWOT Analysis
- 13.3.6 Key Developments
- 13.4 ATEC Spine Inc
- 13.4.1 Key Facts
- 13.4.2 Business Description
- 13.4.3 Products and Services
- 13.4.4 Financial Overview
- 13.4.5 SWOT Analysis
- 13.4.6 Key Developments
- 13.5 Globus Medical Inc
- 13.5.1 Key Facts
- 13.5.2 Business Description
- 13.5.3 Products and Services
- 13.5.4 Financial Overview
- 13.5.5 SWOT Analysis
- 13.5.6 Key Developments
- 13.6 NuVasive Inc
- 13.6.1 Key Facts
- 13.6.2 Business Description
- 13.6.3 Products and Services
- 13.6.4 Financial Overview
- 13.6.5 SWOT Analysis
- 13.6.6 Key Developments
- 13.7 ZimVie Inc
- 13.7.1 Key Facts
- 13.7.2 Business Description
- 13.7.3 Products and Services
- 13.7.4 Financial Overview
- 13.7.5 SWOT Analysis
- 13.7.6 Key Developments
- 13.8 Medtronic Plc
- 13.8.1 Key Facts
- 13.8.2 Business Description
- 13.8.3 Products and Services
- 13.8.4 Financial Overview
- 13.8.5 SWOT Analysis
- 13.8.6 Key Developments
- 13.9 Centinel Spine LLC
- 13.9.1 Key Facts
- 13.9.2 Business Description
- 13.9.3 Products and Services
- 13.9.4 Financial Overview
- 13.9.5 SWOT Analysis
- 13.9.6 Key Developments
- 13.10 Orthofix Medical Inc
- 13.10.1 Key Facts
- 13.10.2 Business Description
- 13.10.3 Products and Services
- 13.10.4 Financial Overview
- 13.10.5 SWOT Analysis
- 13.10.6 Key Developments
14. Appendix
- 14.1 About Us
- 14.2 Glossary of Terms