|
|
市場調査レポート
商品コード
1452623
アジア太平洋のT細胞療法:2030年までの市場予測 - 地域別分析 - モダリティ別、治療タイプ別、適応症別Asia Pacific T Cell Therapy Market Forecast to 2030 - Regional Analysis - by Modality (Research and Commercialized), Therapy Type [CAR T-cell Therapy and T-cell Receptor (TCR)-based], and Indication (Hematologic Malignancies and Solid Tumors) |
||||||
| アジア太平洋のT細胞療法:2030年までの市場予測 - 地域別分析 - モダリティ別、治療タイプ別、適応症別 |
|
出版日: 2024年01月15日
発行: The Insight Partners
ページ情報: 英文 76 Pages
納期: 即納可能
|
- 全表示
- 概要
- 図表
- 目次
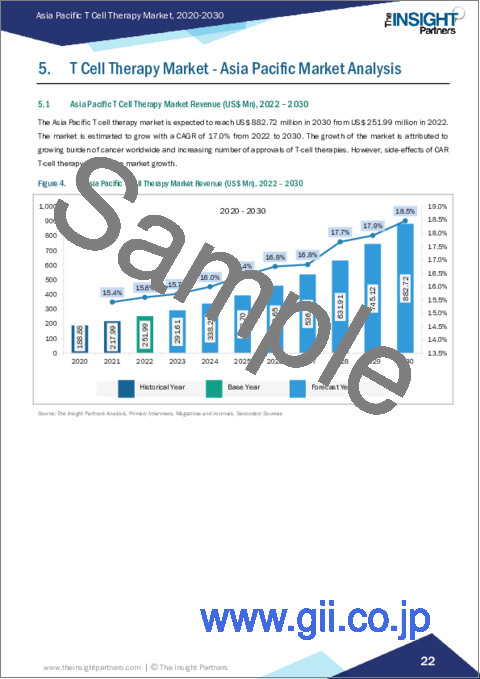
アジア太平洋のT細胞療法市場は、2022年に2億5,199万米ドルと評価され、2030年には8億8,272万米ドルに達すると予測され、2022年から2030年までのCAGRは17.0%で成長すると予測されています。
T細胞療法の承認件数の増加がアジア太平洋のT細胞療法市場を後押し
標的を絞った治療、より迅速で効率的な回復、副作用の軽減などがT細胞療法の利点です。世界的には、細胞療法は様々な承認が得られることから広く採用されています。例えば、2023年にはヤンセンのCAR-T細胞療法CARVYKTI(Ciltacabtagene Autoleucel)は、オーストラリアのTherapeutic Goods Administrationから多発性骨髄腫患者の4次治療として承認されました。
2023年中国国家薬品監督管理局は、イノベント・バイオロジクス社とIASOバイオテクノロジー社(以下、 IASOバイオ社)によるフカソ(イクエカブタジェンオートロイセル)の再発・難治性多発性骨髄腫(RRMM)成人患者に対する使用を承認しました。2023年6月、欧州医薬品庁は本療法の新薬承認申請を承認しました。
2023年初のヒト化CD19標的キメラ抗原受容体T細胞(CAR-T細胞)療法製品であるNexCAR19(Actalycabtagene autoleucel)が、インドで再発/難治性のB細胞リンパ腫および白血病の症例に使用されることが中央医薬品標準管理機構(CDSO)により承認されました。IITボンベイで培養されているImmuno Adoptive Cell Therapy。
2023年佛山凧生物科技有限公司が開発した自己CAR T細胞療法であるYikaida(axicabtagene ciloleucel)が、大細胞型B細胞リンパ腫患者のセカンドライン治療薬として中国国家医薬品監督管理局(NMPA)から承認を取得。
2020年厚生労働省は、公益財団法人神戸医療産業都市推進機構(以下「神戸医療産業都市推進機構」)に対し、日本国内におけるキムリア(一般名:チサゲンレキュセル)の製造販売承認を付与しました。ノバルティスは本日、この発表を行いました。今回の承認により、FBRIはアジアで唯一のCAR-T細胞療法の商業的製造施設となりました。
このように、T細胞療法の承認件数の増加が市場の成長を後押ししています。
アジア太平洋のT細胞療法市場概要
アジア太平洋のT細胞療法市場は、中国、日本、インド、韓国、オーストラリア、その他アジア太平洋に区分されます。アジア太平洋の市場は、CAR T細胞療法ががん治療に効率的であることから、大きな成長が見込まれており、同地域の市場成長を押し上げています。同地域ではCAR T細胞療法に関する多くの臨床試験が進行中であり、アジア太平洋に新たなビジネスチャンスをもたらしています。中国は、病院が実施するCAR T細胞臨床試験において世界最大の国です。中国ではCAR-T産業チェーンが形成されつつあり、このような新しい治療法へのアクセスに有利になるような規制改革が進行中であり、期待されています。中国では2021年9月に、JWセラピューティクスのrelmacabtagene autoleucelという初のCAR T細胞療法が、2ライン以上の全身療法後の再発または難治性の大細胞型B細胞リンパ腫(r/r LBCL)の成人患者の治療薬として承認されました。
アジア太平洋のT細胞療法市場の収益と2030年までの予測(US$Mn)
アジア太平洋のT細胞療法市場のセグメンテーション
アジア太平洋のT細胞療法市場は、モダリティ、治療タイプ、適応症、国別に区分されます。
モダリティ別に見ると、アジア太平洋のT細胞療法市場は研究用と商業用に二分されます。2022年の市場シェアは、商業化セグメントの方が大きいです。
治療タイプに基づき、アジア太平洋のT細胞療法市場はCAR T細胞療法とT細胞受容体(TCR)ベースに分けられます。2022年の市場シェアはCAR T細胞療法が上回った。
適応症に基づくと、アジア太平洋のT細胞療法市場は血液悪性腫瘍と固形腫瘍に二分されます。2022年には血液悪性腫瘍分野が最大の市場シェアを占めました。
国別に見ると、アジア太平洋のT細胞療法市場は中国、日本、オーストラリア、韓国、その他アジア太平洋に区分されます。2022年のアジア太平洋のT細胞療法市場は中国が支配的でした。
Bristol-Myers Squibb Co、Gilead Sciences Inc、Innovent Biologics Inc、Janssen Global Services LLC、JW(Cayman)Therapeutics Co Ltd、Legend Biotech Corp、Novartis AGは、アジア太平洋のT細胞療法市場で事業を展開する大手企業の一部です。
目次
第1章 イントロダクション
第2章 エグゼクティブサマリー
- 主要洞察
- アジア太平洋のT細胞療法市場:国別
第3章 調査手法
- 調査範囲
- 2次調査
- 1次調査
第4章 アジア太平洋のT細胞療法市場:主要産業力学
- 市場促進要因
- 世界のがん罹患率の増加
- T細胞療法の承認数の増加
- 市場抑制要因
- CAR T細胞療法の副作用
- 市場機会
- T細胞療法への投資の増加
- 今後の動向
- 臨床試験中のCAR T細胞療法数の増加
- インパクト分析
第5章 T細胞療法市場:アジア太平洋市場分析
- アジア太平洋のT細胞療法市場売上高、2022年~2030年
第6章 アジア太平洋のT細胞療法市場:収益と2030年までの予測 - モダリティ別
- 市場収益シェア(%)、2022年および2030年
- 研究
- 商業化
第7章 アジア太平洋のT細胞療法市場:収益と2030年までの予測 - 治療タイプ別
- 市場収益シェア(%)、2022年および2030年
- CAR T細胞療法
- T細胞受容体(TCR)ベース
第8章 アジア太平洋のT細胞療法市場:収益と2030年までの予測 - 適応症別
- 市場収益シェア(%)、2022年および2030年
- 血液悪性腫瘍
- 固形がん
第9章 アジア太平洋のT細胞療法市場:国別分析
- アジア太平洋のT細胞療法市場:国別分析
- 中国
- 日本
- オーストラリア
- 韓国
- その他アジア太平洋
第10章 T細胞療法市場-業界情勢
- T細胞療法市場における成長戦略
- 有機的成長戦略
- 無機的成長戦略
第11章 企業プロファイル
- Legend Biotech Corp
- Janssen Global Services LLC
- Gilead Sciences Inc
- Bristol-Myers Squibb Co
- Novartis AG
- JW(Cayman)Therapeutics Co Ltd
- Innovent Biologics Inc
第12章 付録
List Of Tables
- Table 1. Asia Pacific T Cell Therapy Market Segmentation
- Table 2. Asia Pacific T Cell Therapy Market, by Hematological Malignancies - Revenue and Forecast to 2030 (US$ Million)
- Table 3. FDA-Approved Autologous CAR T-Cell Therapies Registered in Asia Pacific
- Table 4. China: Asia Pacific T Cell Therapy Market, by Modality - Revenue and Forecast to 2030 (US$ Million)
- Table 5. China: Asia Pacific T Cell Therapy Market, by Therapy Type - Revenue and Forecast to 2030 (US$ Million)
- Table 6. China: Asia Pacific T Cell Therapy Market, by Indication - Revenue and Forecast to 2030 (US$ Million)
- Table 7. China: Asia Pacific T Cell Therapy Market, For Hematologic Malignancies by Indication - Revenue and Forecast to 2030 (US$ Million)
- Table 8. Japan: Asia Pacific T Cell Therapy Market, by Modality - Revenue and Forecast to 2030 (US$ Million)
- Table 9. Japan: Asia Pacific T Cell Therapy Market, by Therapy Type - Revenue and Forecast to 2030 (US$ Million)
- Table 10. Japan: Asia Pacific T Cell Therapy Market, by Indication - Revenue and Forecast to 2030 (US$ Million)
- Table 11. Japan: Asia Pacific T Cell Therapy Market, For Hematologic Malignancies by Indication - Revenue and Forecast to 2030 (US$ Million)
- Table 12. Australia: Asia Pacific T Cell Therapy Market, by Modality - Revenue and Forecast to 2030 (US$ Million)
- Table 13. Australia: Asia Pacific T Cell Therapy Market, by Therapy Type - Revenue and Forecast to 2030 (US$ Million)
- Table 14. Australia: Asia Pacific T Cell Therapy Market, by Indication - Revenue and Forecast to 2030 (US$ Million)
- Table 15. Australia: Asia Pacific T Cell Therapy Market, For Hematologic Malignancies by Indication - Revenue and Forecast to 2030 (US$ Million)
- Table 16. South Korea: Asia Pacific T Cell Therapy Market, by Modality - Revenue and Forecast to 2030 (US$ Million)
- Table 17. South Korea: Asia Pacific T Cell Therapy Market, by Therapy Type - Revenue and Forecast to 2030 (US$ Million)
- Table 18. South Korea: Asia Pacific T Cell Therapy Market, by Indication - Revenue and Forecast to 2030 (US$ Million)
- Table 19. South Korea: Asia Pacific T Cell Therapy Market, For Hematologic Malignancies by Indication - Revenue and Forecast to 2030 (US$ Million)
- Table 20. Rest of Asia Pacific: Asia Pacific T Cell Therapy Market, by Modality - Revenue and Forecast to 2030 (US$ Million)
- Table 21. Rest of Asia Pacific: Asia Pacific T Cell Therapy Market, by Therapy Type - Revenue and Forecast to 2030 (US$ Million)
- Table 22. Rest of Asia Pacific: Asia Pacific T Cell Therapy Market, by Indication - Revenue and Forecast to 2030 (US$ Million)
- Table 23. Rest of Asia Pacific: Asia Pacific T Cell Therapy Market, For Hematologic Malignancies by Indication - Revenue and Forecast to 2030 (US$ Million)
- Table 24. Recent Organic Growth Strategies in Asia Pacific T Cell Therapy Market
- Table 25. Recent Inorganic Growth Strategies in the Asia Pacific T Cell Therapy Market
- Table 26. Glossary of Terms, Asia Pacific T Cell Therapy Market
List Of Figures
- Figure 1. Asia Pacific T Cell Therapy Market Segmentation, By Country
- Figure 2. Asia Pacific T Cell Therapy Market - Key Industry Dynamics
- Figure 3. Impact Analysis of Drivers and Restraints
- Figure 4. Asia Pacific T Cell Therapy Market Revenue (US$ Mn), 2022 - 2030
- Figure 5. Asia Pacific T Cell Therapy Market Revenue Share, by Modality, 2022 & 2030 (%)
- Figure 6. Research: Asia Pacific T Cell Therapy Market - Revenue and Forecast to 2030 (US$ Million)
- Figure 7. Commercialized: Asia Pacific T Cell Therapy Market - Revenue and Forecast to 2030 (US$ Million)
- Figure 8. Asia Pacific T Cell Therapy Market Revenue Share, by Therapy Type 2022 & 2030 (%)
- Figure 9. CAR T-cell Therapy: Asia Pacific T Cell Therapy Market - Revenue and Forecast to 2030 (US$ Million)
- Figure 10. T-cell Receptor (TCR)-based: Asia Pacific T Cell Therapy Market - Revenue and Forecast to 2030 (US$ Million)
- Figure 11. Asia Pacific T Cell Therapy Market Revenue Share, by Indication, 2022 & 2030 (%)
- Figure 12. Hematologic Malignancies: Asia Pacific T Cell Therapy Market - Revenue and Forecast to 2030 (US$ Million)
- Figure 13. Solid Tumor: Asia Pacific T Cell Therapy Market - Revenue and Forecast to 2030 (US$ Million)
- Figure 14. Asia Pacific T Cell Therapy Market, by Key Country - Revenue, 2022 (US$ Million)
- Figure 15. Asia Pacific T Cell Therapy Market, By Key Countries, 2022 and 2030 (%)
- Figure 16. China: Asia Pacific T Cell Therapy Market Revenue and Forecast to 2030 (US$ Mn)
- Figure 17. Japan: Asia Pacific T Cell Therapy Market Revenue and Forecast to 2030 (US$ Mn)
- Figure 18. Australia: Asia Pacific T Cell Therapy Market Revenue and Forecast to 2030 (US$ Mn)
- Figure 19. South Korea: Asia Pacific T Cell Therapy Market Revenue and Forecast to 2030 (US$ Mn)
- Figure 20. Rest of Asia Pacific: Asia Pacific T Cell Therapy Market Revenue and Forecast to 2030 (US$ Mn)
- Figure 21. Growth Strategies in T Cell Therapy Market
The Asia Pacific T cell therapy market was valued at US$ 251.99 million in 2022 and is expected to reach US$ 882.72 million by 2030; it is estimated to grow at a CAGR of 17.0% from 2022 to 2030.
Increasing Number of T-Cell Therapy Approvals Fuels the Asia Pacific T Cell Therapy Market
Targeted treatment, faster and more efficient recovery, and reduced side effects are among the advantages of t cell therapy. Globally, cell therapies are widely adopted owing to the availability of various approvals. For instance, in 2023: Janssen's CAR-T cell therapy CARVYKTI (Ciltacabtagene Autoleucel) has been approved as a fourth-line treatment for multiple myeloma patients by the Therapeutic Goods Administration, Australia.
In 2023: The Chinese National Medical Products Administration has approved the use of Fucaso (equecabtagene autoleucel) by Innovent Biologics and IASO Biotechnology (IASO Bio) for the treatment of adult patients with relapsed or refractory multiple myeloma (RRMM). In June 2023, the agency approved the therapy's new drug application (NDA).
In 2023: The first humanized CD19-targeted Chimeric Antigen Receptor T cell (CAR-T cell) therapy product, NexCAR19 (Actalycabtagene autoleucel), has been approved by the Central Drugs Standard Control Organization (CDSO) for use in cases of relapsed/refractory B-cell lymphomas and leukemia in India. Immuno Adoptive Cell Therapy is incubated at IIT Bombay.
In 2023: Yikaida (axicabtagene ciloleucel), an autologous CAR T-cell therapy developed by Fosun Kite Biotechnology, was granted approval by China's National Medical Products Administration (NMPA) for use as a second-line treatment for patients with large B-cell lymphoma.
In 2020: The Ministry of Health, Labor and Welfare (MHLW) of Japan has granted the Foundation for Biomedical Research and Innovation at Kobe ("FBRI") a marketing license to produce and distribute Kymriah (tisagenlecleucel) for patients in Japan. Novartis made this announcement today. With this clearance, FBRI is now the only authorized commercial manufacturing facility in Asia for CAR-T cell therapy.
Thus, the increasing number of approvals for T-cell therapy is fueling the market growth.
Asia Pacific T Cell Therapy Market Overview
The Asia Pacific T-cell therapy market is segmented into China, Japan, India, South Korea, Australia, and the Rest of Asia Pacific. The market in Asia Pacific is expected to witness significant growth as CAR T-cell therapy is efficient in treating cancer, elevating the market growth in the region. Many clinical trials for CAR T-cell therapy are ongoing in the region, offering new opportunities in APAC. China is the largest country in the world for CAR T-cell clinical trials, which hospitals conduct. The CAR-T industry chain is being formed in China, including ongoing and expected regulatory reforms to benefit access to such novel therapies. China approved its first CAR T-cell therapy, relmacabtagene autoleucel of JW Therapeutics, in September 2021 for the treatment of adult patients with relapsed or refractory large B-cell lymphoma (r/r LBCL) after two or more lines of systemic therapy.
Asia Pacific T Cell Therapy Market Revenue and Forecast to 2030 (US$ Mn)
Asia Pacific T Cell Therapy Market Segmentation
The Asia Pacific T cell therapy market is segmented into modality , therapy type, indication, and country.
Based on modality, the Asia Pacific T cell therapy market is bifurcated into research and commercialized. The commercialized segment held a larger market share in 2022.
Based on therapy type, the Asia Pacific T cell therapy market is divided into CAR T-cell therapy and T-cell Receptor (TCR)-based. The CAR T-cell therapy segment held a larger market share in 2022.
Based on indication, the Asia Pacific T cell therapy market is bifurcated into hematologic malignancies and solid tumors. The hematologic malignancies segment held the largest market share in 2022.
Based on country, the Asia Pacific T cell therapy market is segmented into China, Japan, Australia, South Korea, and the Rest of Asia Pacific. China dominated the Asia Pacific T cell therapy market in 2022.
Bristol-Myers Squibb Co, Gilead Sciences Inc, Innovent Biologics Inc, Janssen Global Services LLC, JW (Cayman) Therapeutics Co Ltd, Legend Biotech Corp, and Novartis AG are some of the leading companies operating in the Asia Pacific T cell therapy market.
Table Of Contents
1. Introduction
- 1.1 The Insight Partners Research Report Guidance
- 1.2 Market Segmentation
2. Executive Summary
- 2.1 Key Insights
- 2.2 Asia Pacific T Cell Therapy Market, by Country (US$ Million)
3. Research Methodology
- 3.1 Coverage
- 3.2 Secondary Research
- 3.3 Primary Research
4. Asia Pacific T Cell Therapy Market - Key Industry Dynamics
- 4.1 Market Drivers:
- 4.1.1 Growing Burden of Cancer Worldwide
- 4.1.2 Increasing Number of T-Cell Therapy Approvals
- 4.2 Market Restraints
- 4.2.1 Side-effects of CAR T-Cell Therapy
- 4.3 Market Opportunities
- 4.3.1 Growing Investment in T-Cell Therapy
- 4.4 Future Trends
- 4.4.1 Rising Number of CAR T-Cell Therapies in Clinical Trials
- 4.5 Impact Analysis:
5. T Cell Therapy Market - Asia Pacific Market Analysis
- 5.1 Asia Pacific T Cell Therapy Market Revenue (US$ Mn), 2022 - 2030
6. Asia Pacific T Cell Therapy Market - Revenue and Forecast to 2030 - by Modality.
- 6.1 Overview
- 6.2 Asia Pacific T Cell Therapy Market Revenue Share, by Modality, 2022 & 2030 (%)
- 6.3 Research
- 6.3.1 Overview
- 6.3.2 Research: Asia Pacific T Cell Therapy Market - Revenue and Forecast to 2030 (US$ Million)
- 6.4 Commercialized
- 6.4.1 Overview
- 6.4.2 Commercialized: Asia Pacific T Cell Therapy Market - Revenue and Forecast to 2030 (US$ Million)
7. Asia Pacific T Cell Therapy Market - Revenue and Forecast to 2030 - by Therapy Type
- 7.1 Overview
- 7.2 Asia Pacific T Cell Therapy Market Revenue Share, by Therapy Type 2022 & 2030 (%)
- 7.3 CAR T-cell Therapy
- 7.3.1 Overview
- 7.3.2 CAR T-cell Therapy: Asia Pacific T Cell Therapy Market - Revenue and Forecast to 2030 (US$ Million)
- 7.4 T-cell Receptor (TCR)-based.
- 7.4.1 Overview
- 7.4.2 T-cell Receptor (TCR)-based: Asia Pacific T Cell Therapy Market - Revenue and Forecast to 2030 (US$ Million)
8. Asia Pacific T Cell Therapy Market - Revenue and Forecast to 2030 - by Indication.
- 8.1 Overview
- 8.2 Asia Pacific T Cell Therapy Market Revenue Share, by Indication, 2022 & 2030 (%)
- 8.3 Hematologic Malignancies
- 8.3.1 Overview
- 8.3.2 Hematologic Malignancies: Asia Pacific T Cell Therapy Market - Revenue and Forecast to 2030 (US$ Million)
- 8.3.3 Asia Pacific T Cell Therapy Market, by Haematological Malignancies, 2020-2030 (US$ Million)
- 8.4 Solid Tumor
- 8.4.1 Overview
- 8.4.2 Solid Tumor: Asia Pacific T Cell Therapy Market - Revenue and Forecast to 2030 (US$ Million)
9. Asia Pacific T Cell Therapy Market - Country Analysis
- 9.1 Overview
- 9.1.1.1 Asia Pacific T Cell Therapy Market, by Country
- 9.1.1.2 China
- 9.1.1.2.1 China: Asia Pacific T Cell Therapy Market Revenue and Forecast to 2030 (US$ Mn)
- 9.1.1.2.2 China: Asia Pacific T Cell Therapy Market, by Modality, 2020-2030 (US$ Million)
- 9.1.1.2.3 China: Asia Pacific T Cell Therapy Market, by Therapy Type, 2020-2030 (US$ Million)
- 9.1.1.2.4 China: Asia Pacific T Cell Therapy Market, by Indication, 2020-2030 (US$ Million)
- 9.1.1.2.4.1 China: Asia Pacific T Cell Therapy Market, For Hematologic Malignancies by Indication, 2020-2030 (US$ Million)
- 9.1.1.3 Japan
- 9.1.1.3.1 Japan: Asia Pacific T Cell Therapy Market Revenue and Forecast to 2030 (US$ Mn)
- 9.1.1.3.2 Japan: Asia Pacific T Cell Therapy Market, by Modality, 2020-2030 (US$ Million)
- 9.1.1.3.3 Japan: Asia Pacific T Cell Therapy Market, by Therapy Type, 2020-2030 (US$ Million)
- 9.1.1.3.4 Japan: Asia Pacific T Cell Therapy Market, by Indication, 2020-2030 (US$ Million)
- 9.1.1.3.4.1 Japan: Asia Pacific T Cell Therapy Market, For Hematologic Malignancies by Indication, 2020-2030 (US$ Million)
- 9.1.1.4 Australia
- 9.1.1.4.1 Australia: Asia Pacific T Cell Therapy Market Revenue and Forecast to 2030 (US$ Mn)
- 9.1.1.4.2 Australia: Asia Pacific T Cell Therapy Market, by Modality, 2020-2030 (US$ Million)
- 9.1.1.4.3 Australia: Asia Pacific T Cell Therapy Market, by Therapy Type, 2020-2030 (US$ Million)
- 9.1.1.4.4 Australia: Asia Pacific T Cell Therapy Market, by Indication, 2020-2030 (US$ Million)
- 9.1.1.4.4.1 Australia: Asia Pacific T Cell Therapy Market, For Hematologic Malignancies by Indication, 2020-2030 (US$ Million)
- 9.1.1.5 South Korea
- 9.1.1.5.1 South Korea: Asia Pacific T Cell Therapy Market Revenue and Forecast to 2030 (US$ Mn)
- 9.1.1.5.2 South Korea: Asia Pacific T Cell Therapy Market, by Modality, 2020-2030 (US$ Million)
- 9.1.1.5.3 South Korea: Asia Pacific T Cell Therapy Market, by Therapy Type, 2020-2030 (US$ Million)
- 9.1.1.5.4 South Korea: Asia Pacific T Cell Therapy Market, by Indication, 2020-2030 (US$ Million)
- 9.1.1.5.4.1 South Korea: Asia Pacific T Cell Therapy Market, For Hematologic Malignancies by Indication, 2020-2030 (US$ Million)
- 9.1.1.6 Rest of Asia Pacific
- 9.1.1.6.1 Rest of Asia Pacific: Asia Pacific T Cell Therapy Market Revenue and Forecast to 2030 (US$ Mn)
- 9.1.1.6.2 Rest of Asia Pacific: Asia Pacific T Cell Therapy Market, by Modality, 2020-2030 (US$ Million)
- 9.1.1.6.3 Rest of Asia Pacific: Asia Pacific T Cell Therapy Market, by Therapy Type, 2020-2030 (US$ Million)
- 9.1.1.6.4 Rest of Asia Pacific: Asia Pacific T Cell Therapy Market, by Indication, 2020-2030 (US$ Million)
- 9.1.1.6.4.1 Rest of Asia Pacific: Asia Pacific T Cell Therapy Market, For Hematologic Malignancies by Indication, 2020-2030 (US$ Million)
10. T Cell Therapy Market-Industry Landscape
- 10.1 Overview
- 10.2 Growth Strategies in T Cell Therapy Market
- 10.3 Organic Growth Strategies
- 10.3.1 Overview
- 10.4 Inorganic Growth Strategies
- 10.4.1 Overview
11. Company Profiles
- 11.1 Legend Biotech Corp
- 11.1.1 Key Facts
- 11.1.2 Business Description
- 11.1.3 Products and Services
- 11.1.4 Financial Overview
- 11.1.5 SWOT Analysis
- 11.1.6 Key Developments
- 11.2 Janssen Global Services LLC
- 11.2.1 Key Facts
- 11.2.2 Business Description
- 11.2.3 Products and Services
- 11.2.4 Financial Overview
- 11.2.5 SWOT Analysis
- 11.2.6 Key Developments
- 11.3 Gilead Sciences Inc
- 11.3.1 Key Facts
- 11.3.2 Business Description
- 11.3.3 Products and Services
- 11.3.4 Financial Overview
- 11.3.5 SWOT Analysis
- 11.3.6 Key Developments
- 11.4 Bristol-Myers Squibb Co
- 11.4.1 Key Facts
- 11.4.2 Business Description
- 11.4.3 Products and Services
- 11.4.4 Financial Overview
- 11.4.5 SWOT Analysis
- 11.4.6 Key Developments
- 11.5 Novartis AG
- 11.5.1 Key Facts
- 11.5.2 Business Description
- 11.5.3 Products and Services
- 11.5.4 Financial Overview
- 11.5.5 SWOT Analysis
- 11.5.6 Key Developments
- 11.6 JW (Cayman) Therapeutics Co Ltd
- 11.6.1 Key Facts
- 11.6.2 Business Description
- 11.6.3 Products and Services
- 11.6.4 Financial Overview
- 11.6.5 SWOT Analysis
- 11.6.6 Key Developments
- 11.7 Innovent Biologics Inc
- 11.7.1 Key Facts
- 11.7.2 Business Description
- 11.7.3 Products and Services
- 11.7.4 Financial Overview
- 11.7.5 SWOT Analysis
- 11.7.6 Key Developments
12. Appendix
- 12.1 About Us
- 12.2 Glossary of Terms