|
|
市場調査レポート
商品コード
1452617
北米の細胞・遺伝子治療向け製造サービス:2030年までの市場予測 - 地域別分析 - タイプ別、適応症別、用途別、エンドユーザー別North America Cell and Gene Therapy Manufacturing Services Market Forecast to 2030 - Regional Analysis - by Type, Indication (Cancer, Orthopedics, and Others), Application (Clinical Manufacturing and Commercial Manufacturing), and End User |
||||||
|
|||||||
| 北米の細胞・遺伝子治療向け製造サービス:2030年までの市場予測 - 地域別分析 - タイプ別、適応症別、用途別、エンドユーザー別 |
|
出版日: 2024年01月15日
発行: The Insight Partners
ページ情報: 英文 110 Pages
納期: 即納可能
|
全表示
- 概要
- 図表
- 目次
北米の細胞・遺伝子治療向け製造サービス市場は、2022年の41億5,327万米ドルから2030年には147億1,172万米ドルに成長すると予測されています。2022年から2030年までのCAGRは17.1%と推定されます。
細胞・遺伝子治療向け製造のアウトソーシング人気の高まりが北米の細胞・遺伝子治療向け製造サービス市場を牽引
細胞・遺伝子治療向け製造は複雑なプロセスであるため、業務の適切な実行と監督が極めて重要です。細胞・遺伝子治療メーカーは、生物学やプロセス工学に精通した有資格者の数が限られています。さらに、経験豊富なチームにとって、手作業でオープンな製造方法を用いて最初の臨床試験に到達する試みを管理し、その後、より商業的に適したプロセスを構築することは厄介なことです。そのため、これらの企業は、臨床試験と商業化プロセスを加速するために、開発・製造受託機関(CDMO)との協力を選択します。CDMOは、製品開発、製造、臨床試験サポート、商業化サービスを細胞・遺伝子治療企業に契約ベースで提供します。CDMOと提携することで、細胞・遺伝子治療メーカーにとって、スケーラビリティ、市場投入までのスピード、オーバーヘッドコストのない技術的専門知識へのアクセス、コスト効率が可能になります。2022年4月、サーモジェネシスは米国カリフォルニア州にCDMO施設を設立し、T細胞受容体(TCR)、キメラ抗原受容体-T細胞(CAR-T細胞)、腫瘍浸潤白血球(TIL)、iPSC、ナチュラルキラー細胞(NK)、間葉系幹細胞(MSC)の製造に関する専門知識を活用して、細胞・遺伝子治療メーカーにCDMOサービスを提供しています。細胞治療や遺伝子治療の製造をCDMOに委託することは、製造業者にとって費用対効果が高いことが証明されています。このように、CDMOへの細胞・遺伝子治療向け製造のアウトソーシング志向の高まりが、北米の細胞・遺伝子治療向け製造サービス市場の成長を後押ししています。
北米の細胞・遺伝子治療向け製造サービス市場概要
細胞・遺伝子治療(CGT)は、治療ニーズが未解決の重篤な希少疾患に苦しむ患者を治療します。CGTの製造は非常に複雑なプロセスであり、インフラや専門知識の不足が大きな制約要因となっています。中間体や最終製品に関連する物流の課題も、企業のCGT製造能力を制限しています。CGTの製造プロセスには、「アフェレーシス」による自己細胞の抽出、専門ラボへの派遣、患者への投与のためのクリニックへの送り返しが含まれ、これらすべてが厳格な品質管理のもとに行われなければならないです。米国食品医薬品局(USFDA)が承認したCGT医薬品はわずか7種類に過ぎず、新製品のパイプラインは実験的治療法で~1,200種類に達します。その半数は第2相臨床試験中であり、年間売上成長率は、Chemical &Engineering Newsレポート2022年版の予測によれば、細胞治療で15%、遺伝子治療で~30%です。2022年3月31日、CELL Technologies Inc.は、痛みや関節炎を対象とした幹細胞プログラムの臨床データをカナダ保健省に提出したと発表しました。このように、上記の要因が予測期間中の細胞・遺伝子治療向け製造サービス市場の成長を促進すると予想されます。このように、上記の要因が北米の細胞・遺伝子治療向け製造サービス市場の成長の要因となっています。
北米の細胞・遺伝子治療向け製造サービス市場の収益と2030年までの予測(金額)
北米の細胞・遺伝子治療向け製造サービス市場セグメンテーション
北米の細胞・遺伝子治療向け製造サービス市場は、タイプ、適応症、用途、エンドユーザー、国に区分されます。
タイプ別では、北米の細胞・遺伝子治療向け製造サービス市場は細胞治療と遺伝子治療に二分されます。2022年、北米の細胞・遺伝子治療向け製造サービス市場では、細胞治療分野が大きなシェアを記録しました。細胞療法分野はさらに自己由来と同種異系に区分されます。遺伝子治療分野はさらにウイルスベクターと非ウイルスベクターに区分されます。
適応症に基づき、北米の細胞・遺伝子治療向け製造サービス市場はがん、整形外科、その他に区分されます。2022年、北米の細胞・遺伝子治療向け製造サービス市場では、がん分野が最大のシェアを記録しました。
用途別では、北米の細胞・遺伝子治療向け製造サービス市場は臨床製造と商業製造に区分されます。2022年には、商業用製造セグメントが北米の細胞・遺伝子治療向け製造サービス市場で最大のシェアを記録しました。
エンドユーザーに基づき、北米の細胞・遺伝子治療向け製造サービス市場は製薬・バイオテクノロジー企業と契約研究機関(CRO)に二分されます。2022年、北米の細胞・遺伝子治療向け製造サービス市場では、製薬・バイオテクノロジー企業セグメントがより大きなシェアを記録しました。
国別に見ると、北米の細胞・遺伝子治療向け製造サービス市場は米国、カナダ、メキシコに区分されます。2022年、米国は北米の細胞・遺伝子治療向け製造サービス市場で最大のシェアを記録しました。
Catalent Inc、Charles River Laboratories International Inc、FUJIFILM Holdings Corp、Lonza Group AG、Merck KgaA、National Resilience Inc、Nikon Corp、Oxford BioMedica Plc、Takara Bio Inc、Thermo Fisher Scientific Inc、WuXi AppTec Co Ltdは、北米の細胞・遺伝子治療向け製造サービス市場で事業を展開している大手企業です。
目次
第1章 イントロダクション
第2章 エグゼクティブサマリー
- 主要な洞察
第3章 調査手法
- 調査範囲
- 2次調査
- 1次調査
第4章 北米の細胞・遺伝子治療向け製造サービス市場- 主要産業力学
- 主な市場促進要因
- 細胞・遺伝子治療の承認件数の増加
- 細胞・遺伝子治療向け製造アウトソーシングの人気上昇
- 市場抑制要因
- 細胞・遺伝子治療向け製造の高コスト
- 市場機会
- 企業による戦略的取り組み
- 今後の動向
- 細胞・遺伝子治療向け製造サービスの自動化
- 影響分析
第5章 細胞・遺伝子治療向け製造サービス市場:北米市場分析
- 北米の細胞・遺伝子治療向け製造サービス市場収益、2022年~2030年
第6章 北米の細胞・遺伝子治療向け製造サービス市場:収益と2030年までの予測 - タイプ別
- 細胞療法
- 遺伝子治療
第7章 北米の細胞・遺伝子治療向け製造サービス市場:分析と2030年までの予測 - 適応症別
- がん
- 整形外科
- その他
第8章 北米の細胞・遺伝子治療向け製造サービス市場:収益と2030年までの予測 - 用途別
- 臨床製造
- 商業用製造
第9章 北米の細胞・遺伝子治療向け製造サービス市場:収益と2030年までの予測 - エンドユーザー別
- 製薬会社およびバイオテクノロジー企業
- CRO(医薬品開発業務受託機関)
第10章 北米の細胞・遺伝子治療向け製造サービス市場:収益と2030年までの予測 - 国別分析
- 北米
- 米国
- カナダ
- メキシコ
第11章 北米の細胞・遺伝子治療向け製造サービス市場:業界情勢
- 細胞・遺伝子治療向け製造サービス市場における成長戦略
- 有機的成長戦略
- 無機的成長戦略
第12章 企業プロファイル
- Thermo Fisher Scientific Inc
- Merck KGaA
- Charles River Laboratories International Inc
- Lonza Group AG
- WuXi AppTec Co Ltd
- Catalent Inc
- Takara Bio Inc
- Nikon Corp
- FUJIFILM Holdings Corp
- National Resilience Inc
- Oxford BioMedica Plc
List Of Tables
- Table 1. North America Cell and Gene Therapy Manufacturing Services Market Segmentation
- Table 2. US: Cell and Gene Therapy Manufacturing Services Market, by Type, 2020-2030 (US$ Million)
- Table 3. US: Cell and Gene Therapy Manufacturing Services Market, by Cell Therapy, 2020-2030 (US$ Million)
- Table 4. US: Cell and Gene Therapy Manufacturing Services Market, by Gene Therapy, 2020-2030 (US$ Million)
- Table 5. US: Cell and Gene Therapy Manufacturing Services Market, by Indication, 2020-2030 (US$ Million)
- Table 6. US: Cell and Gene Therapy Manufacturing Services Market, by Application, 2020-2030 (US$ Million)
- Table 7. US: Cell and Gene Therapy Manufacturing Services Market, by End User, 2020-2030 (US$ Million)
- Table 8. Canada: Cell and Gene Therapy Manufacturing Services Market, by Type, 2020-2030 (US$ Million)
- Table 9. Canada: Cell and Gene Therapy Manufacturing Services Market, by Cell Therapy, 2020-2030 (US$ Million)
- Table 10. Canada: Cell and Gene Therapy Manufacturing Services Market, by Gene Therapy, 2020-2030 (US$ Million)
- Table 11. Canada: Cell and Gene Therapy Manufacturing Services Market, by Gene Therapy, 2020-2030 (US$ Million)
- Table 12. Canada: Cell and Gene Therapy Manufacturing Services Market, by Application, 2020-2030 (US$ Million)
- Table 13. Canada: Cell and Gene Therapy Manufacturing Services Market, by End User, 2020-2030 (US$ Million)
- Table 14. Mexico: Cell and Gene Therapy Manufacturing Services Market, by Type, 2020-2030 (US$ Million)
- Table 15. Mexico: Cell and Gene Therapy Manufacturing Services Market, by Cell Therapy, 2020-2030 (US$ Million)
- Table 16. Mexico: Cell and Gene Therapy Manufacturing Services Market, by Gene Therapy, 2020-2030 (US$ Million)
- Table 17. Mexico: Cell and Gene Therapy Manufacturing Services Market, by Indication, 2020-2030 (US$ Million)
- Table 18. Mexico: Cell and Gene Therapy Manufacturing Services Market, by Application, 2020-2030 (US$ Million)
- Table 19. Mexico: Cell and Gene Therapy Manufacturing Services Market, by End User, 2020-2030 (US$ Million)
- Table 20. Recent Organic Growth Strategies in Cell and Gene Therapy Manufacturing Services Market
- Table 21. Recent Inorganic Growth Strategies in the Cell and Gene Therapy Manufacturing Services Market
List Of Figures
- Figure 1. North America Cell and Gene Therapy Manufacturing Services Market Segmentation, By Country
- Figure 2. North America Cell and Gene Therapy Manufacturing Services Market - Key Industry Dynamics
- Figure 3. Impact Analysis of Drivers and Restraints
- Figure 4. North America Cell and Gene Therapy Manufacturing Services Market Revenue (US$ Mn), 2022 - 2030
- Figure 5. North America Cell and Gene Therapy Manufacturing Services Market Revenue Share, by Type 2022 & 2030 (%)
- Figure 6. Cell Therapy: North America Cell and Gene Therapy Manufacturing Services Market - Revenue and Forecast to 2030 (US$ Million)
- Figure 7. Autologous: North America Cell and Gene Therapy Manufacturing Services Market - Revenue and Forecast to 2030 (US$ Million)
- Figure 8. Allogenic: North America Cell and Gene Therapy Manufacturing Services Market - Revenue and Forecast to 2030 (US$ Million)
- Figure 9. Gene Therapy: North America Cell and Gene Therapy Manufacturing Services Market - Revenue and Forecast to 2030 (US$ Million)
- Figure 10. Viral Vector: North America Cell and Gene Therapy Manufacturing Services Market - Revenue and Forecast to 2030 (US$ Million)
- Figure 11. Non-Viral Vector: North America Cell and Gene Therapy Manufacturing Services Market - Revenue and Forecast to 2030 (US$ Million)
- Figure 12. North America Cell and Gene Therapy Manufacturing Services Market, by Indication 2022 & 2030 (%)
- Figure 13. Cancer: North America Cell and Gene Therapy Manufacturing Services Market - Revenue and Forecast to 2030 (US$ Million)
- Figure 14. Orthopedics: North America Cell and Gene Therapy Manufacturing Services Market - Revenue and Forecast to 2030 (US$ Million)
- Figure 15. Others: North America Cell and Gene Therapy Manufacturing Services Market - Revenue and Forecast to 2030 (US$ Million)
- Figure 16. North America Cell and Gene Therapy Manufacturing Services Market Revenue Share, by Application 2022 & 2030 (%)
- Figure 17. Clinical Manufacturing: North America Cell and Gene Therapy Manufacturing Services Market - Revenue and Forecast to 2030 (US$ Million)
- Figure 18. Commercial Manufacturing: North America Cell and Gene Therapy Manufacturing Services Market - Revenue and Forecast to 2030 (US$ Million)
- Figure 19. North America Cell and Gene Therapy Manufacturing Services Market Revenue Share, by End User 2022 & 2030 (%)
- Figure 20. Pharmaceutical & Biotechnology Companies: North America Cell and Gene Therapy Manufacturing Services Market - Revenue and Forecast to 2030 (US$ Million)
- Figure 21. Contract Research Organizations (CROs): North America Cell and Gene Therapy Manufacturing Services Market - Revenue and Forecast to 2030 (US$ Million)
- Figure 22. North America: Cell and Gene Therapy Manufacturing Services Market, by Key Region - Revenue (2022) (US$ Million)
- Figure 23. North America: Cell and Gene Therapy Manufacturing Services Market, by Region, 2022 & 2030 (%)
- Figure 24. US: Cell and Gene Therapy Manufacturing Services Market - Revenue and Forecast to 2030 (US$ Million)
- Figure 25. Canada: Cell and Gene Therapy Manufacturing Services Market - Revenue and Forecast to 2030 (US$ Million)
- Figure 26. Mexico: Cell and Gene Therapy Manufacturing Services Market - Revenue and Forecast to 2030 (US$ Million)
- Figure 27. Growth Strategies in Cell and Gene Therapy Manufacturing Services Market
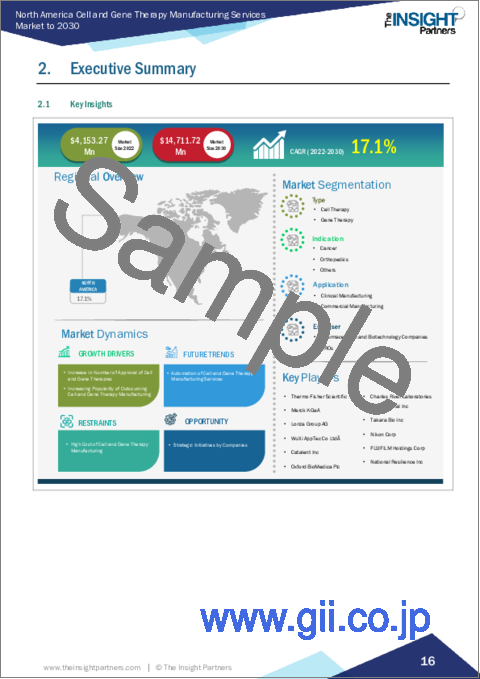
The North America cell and gene therapy manufacturing services market is expected to grow from US$ 4,153.27 million in 2022 to US$ 14,711.72 million by 2030. It is estimated to grow at a CAGR of 17.1% from 2022 to 2030.
Increasing Popularity of Outsourcing Cell and Gene Therapy Manufacturing Drives North America Cell and Gene Therapy Manufacturing Services Market
Cell and gene therapy manufacturing is a complex process, which makes the proper execution and overseeing of the operation crucial. Cell and gene therapy manufacturers have a limited number of qualified personnel who know biological and process engineering. Moreover, for experienced teams, managing the attempts to reach the first clinical trial using a manual, and open manufacturing method and then building a more commercially suitable process can be tricky. Therefore, these enterprises choose to work with contract development and manufacturing organizations (CDMOs) to accelerate their clinical studies and commercialization process. CDMOs provide product development, manufacturing, clinical trial support, and commercialization services to cell and gene therapy companies on a contract basis. Partnering with a CDMO enables scalability, speed to market, access to technical expertise without overhead costs, and cost efficiencies for cell and gene therapy manufacturers. In April 2022, ThermoGenesis established a CDMO facility in California, US to provide CDMO services to cell and gene therapy manufacturers, using its expertise in T-cell receptor (TCR), chimeric antigen receptor-T cell (CAR-T cell), tumor-infiltrating leukocyte (TIL), iPSC, natural killer cell (NK), and mesenchymal stem cell (MSC) manufacturing. Outsourcing cell and gene therapy manufacturing to CDMOs proves cost-effective for manufacturers. Thus, the increasing preference for outsourcing growing cell and gene therapy manufacturing to CDMOs fuels the North America cell and gene therapy manufacturing services market growth.
North America Cell and Gene Therapy Manufacturing Services Market Overview
Cell and gene therapies (CGTs) treat patients suffering from serious and rare diseases with unaddressed therapeutic needs. Manufacturing CGTs is a highly complex process, with the insufficiency of infrastructure and expertise being a major limiting factor. Logistical challenges associated with intermediates and the final product also limit the CGT manufacturing capacity of companies. The CGT manufacturing process involves the extraction of autologous cells through "apheresis," dispatching them to specialized labs, and sending them back to clinics for administration into patients, all of which must be performed with strict quality control. The US Food and Drug Administration (USFDA) has approved only 7 CGT drugs, with the pipeline of new products reaching ~1,200 experimental therapies. Half of these are in Phase 2 clinical trials, with estimates of annual sales growth accounting for 15% for cell therapies and ~30% for gene therapies, as per the estimates of the Chemical & Engineering News report 2022. On March 31, 2022, CELL Technologies Inc. announced the submission of the clinical data of its stem cell program in pain and arthritis for approval by Health Canada to help patients access evidence-based and regulatory-approved stem cell procedures across Canada. Thus, the abovementioned factors are expected to promote the cell and gene therapy manufacturing services market growth during the forecast period. Thus, the abovementioned factors are responsible for the growth of the North America cell and gene therapy manufacturing services market.
North America Cell and Gene Therapy Manufacturing Services Market Revenue and Forecast to 2030 (US$ Million)
North America Cell and Gene Therapy Manufacturing Services Market Segmentation
The North America cell and gene therapy manufacturing services market is segmented into type, indication, application, end user, and country.
Based on type, the North America cell and gene therapy manufacturing services market is bifurcated into cell therapy and gene therapy. In 2022, the cell therapy segment registered a larger share in the North America cell and gene therapy manufacturing services market. The cell therapy segment is further segmented into autologous and allogenic. The gene therapy segment is further segmented into viral and non-viral vector.
Based on indication, the North America cell and gene therapy manufacturing services market is segmented into cancer, orthopedics, and others. In 2022, the cancer segment registered the largest share in the North America cell and gene therapy manufacturing services market.
Based on application, the North America cell and gene therapy manufacturing services market is segmented into clinical manufacturing and commercial manufacturing. In 2022, the commercial manufacturing segment registered the largest share in the North America cell and gene therapy manufacturing services market.
Based on end user, the North America cell and gene therapy manufacturing services market is bifurcated into pharmaceutical and biotechnology companies and contract research organization (CROs). In 2022, the pharmaceutical and biotechnology companies segment registered a larger share in the North America cell and gene therapy manufacturing services market.
Based on country, the North America cell and gene therapy manufacturing services market is segmented into the US, Canada, Mexico. In 2022, the US registered the largest share in the North America cell and gene therapy manufacturing services market.
Catalent Inc, Charles River Laboratories International Inc, FUJIFILM Holdings Corp, Lonza Group AG, Merck KgaA, National Resilience Inc, Nikon Corp, Oxford BioMedica Plc, Takara Bio Inc, Thermo Fisher Scientific Inc, and WuXi AppTec Co Ltd are some of the leading companies operating in the North America cell and gene therapy manufacturing services market.
Table Of Contents
1. Introduction
- 1.1 The Insight Partners Research Report Guidance
- 1.2 Market Segmentation
2. Executive Summary
- 2.1 Key Insights
3. Research Methodology
- 3.1 Coverage
- 3.2 Secondary Research
- 3.3 Primary Research
4. North America Cell and Gene Therapy Manufacturing Services Market - Key Industry Dynamics
- 4.1 Key Market Drivers:
- 4.1.1 Increase in Number of Approval of Cell and Gene Therapies
- 4.1.2 Increasing Popularity of Outsourcing Cell and Gene Therapy Manufacturing
- 4.2 Market Restraints
- 4.2.1 High Cost of Cell and Gene Therapy Manufacturing
- 4.3 Market Opportunities
- 4.3.1 Strategic Initiatives by Companies
- 4.4 Future Trends
- 4.4.1 Automation of Cell and Gene Therapy Manufacturing Services
- 4.5 Impact Analysis:
5. Cell and Gene Therapy Manufacturing Services Market - North America Market Analysis
- 5.1 North America Cell and Gene Therapy Manufacturing Services Market Revenue (US$ Mn), 2022 - 2030
6. North America Cell and Gene Therapy Manufacturing Services Market - Revenue and Forecast to 2030 - by Type
- 6.1 Overview
- 6.2 North America Cell and Gene Therapy Manufacturing Services Market Revenue Share, by Type 2022 & 2030 (%)
- 6.3 Cell Therapy
- 6.3.1 Overview
- 6.3.2 Cell Therapy: North America Cell and Gene Therapy Manufacturing Services Market - Revenue and Forecast to 2030 (US$ Million)
- 6.3.2.1 Autologous
- 6.3.2.1.1 Overview
- 6.3.2.1.2 Autologous: North America Cell and Gene Therapy Manufacturing Services Market - Revenue and Forecast to 2030 (US$ Million)
- 6.3.2.2 Allogenic
- 6.3.2.2.1 Overview
- 6.3.2.2.2 Allogenic: North America Cell and Gene Therapy Manufacturing Services Market - Revenue and Forecast to 2030 (US$ Million)
- 6.3.2.1 Autologous
- 6.4 Gene Therapy
- 6.4.1 Overview
- 6.4.2 Gene Therapy: North America Cell and Gene Therapy Manufacturing Services Market - Revenue and Forecast to 2030 (US$ Million)
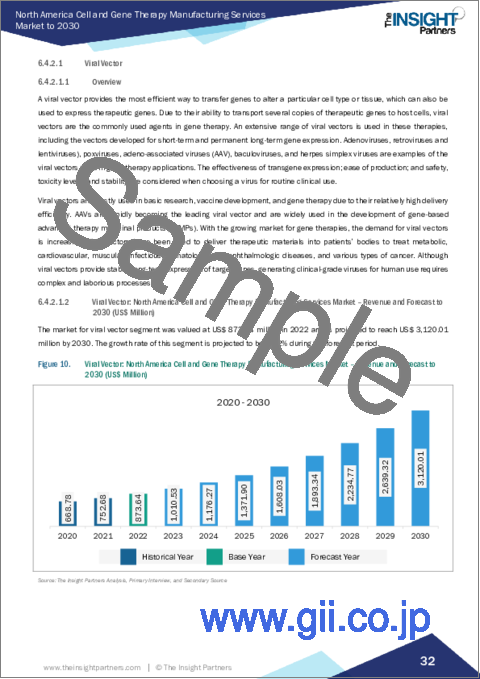
- 6.4.2.1 Viral Vector
- 6.4.2.1.1 Overview
- 6.4.2.1.2 Viral Vector: North America Cell and Gene Therapy Manufacturing Services Market - Revenue and Forecast to 2030 (US$ Million)
- 6.4.2.2 Non-Viral Vector
- 6.4.2.2.1 Overview
- 6.4.2.2.2 Non-Viral Vector: North America Cell and Gene Therapy Manufacturing Services Market - Revenue and Forecast to 2030 (US$ Million)
- 6.4.2.1 Viral Vector
7. North America Cell and Gene Therapy Manufacturing Services Market Analysis and Forecasts to 2030 - by Indication
- 7.1 Overview
- 7.2 North America Cell and Gene Therapy Manufacturing Services Market, by Indication 2022 & 2030 (%)
- 7.3 Cancer
- 7.3.1 Overview
- 7.3.2 Cancer: North America Cell and Gene Therapy Manufacturing Services Market - Revenue and Forecast to 2030 (US$ Million)
- 7.4 Orthopedics
- 7.4.1 Overview
- 7.4.2 Orthopedics: North America Cell and Gene Therapy Manufacturing Services Market - Revenue and Forecast to 2030 (US$ Million)
- 7.5 Others
- 7.5.1 Overview
- 7.5.2 Others: North America Cell and Gene Therapy Manufacturing Services Market - Revenue and Forecast to 2030 (US$ Million)
8. North America Cell and Gene Therapy Manufacturing Services Market - Revenue and Forecast to 2030 - by Application
- 8.1 Overview
- 8.2 North America Cell and Gene Therapy Manufacturing Services Market Revenue Share, by Application 2022 & 2030 (%)
- 8.4 Clinical Manufacturing
- 8.4.1 Overview
- 8.4.2 Clinical Manufacturing: North America Cell and Gene Therapy Manufacturing Services Market - Revenue and Forecast to 2030 (US$ Million)
- 8.5 Commercial Manufacturing
- 8.5.1 Overview
- 8.5.2 Commercial Manufacturing: North America Cell and Gene Therapy Manufacturing Services Market - Revenue and Forecast to 2030 (US$ Million)
9. North America Cell and Gene Therapy Manufacturing Services Market - Revenue and Forecast to 2030 - by End User
- 9.1 Overview
- 9.2 North America Cell and Gene Therapy Manufacturing Services Market Revenue Share, by End User 2022 & 2030 (%)
- 9.4 Pharmaceutical and Biotechnology Companies
- 9.4.1 Overview
- 9.4.2 Pharmaceutical & Biotechnology Companies: North America Cell and Gene Therapy Manufacturing Services Market - Revenue and Forecast to 2030 (US$ Million)
- 9.5 Contract Research Organizations (CROs)
- 9.5.1 Overview
- 9.5.2 Contract Research Organizations (CROs): North America Cell and Gene Therapy Manufacturing Services Market - Revenue and Forecast to 2030 (US$ Million)
10. North America Cell and Gene Therapy Manufacturing Services Market - Revenue and Forecast to 2030 - Country Analysis
- 10.1 Overview
- 10.1.1 North America: Cell and Gene Therapy Manufacturing Services Market, by Region, 2022 & 2030 (%)
- 10.1.1.1 US: Cell and Gene Therapy Manufacturing Services Market - Revenue and Forecast to 2030 (US$ Million)
- 10.1.1.1.1 US: Cell and Gene Therapy Manufacturing Services Market, by Type, 2020-2030 (US$ Million)
- 10.1.1.1.1.1 US: Cell and Gene Therapy Manufacturing Services Market, by Cell Therapy, 2020-2030 (US$ Million)
- 10.1.1.1.1.2 US: Cell and Gene Therapy Manufacturing Services Market, by Gene Therapy, 2020-2030 (US$ Million)
- 10.1.1.1.2 US: Cell and Gene Therapy Manufacturing Services Market, by Indication, 2020-2030 (US$ Million)
- 10.1.1.1.3 US: Cell and Gene Therapy Manufacturing Services Market, by Application, 2020-2030 (US$ Million)
- 10.1.1.1.4 US: Cell and Gene Therapy Manufacturing Services Market, by End User, 2020-2030 (US$ Million)
- 10.1.1.2 Canada: Cell and Gene Therapy Manufacturing Services Market - Revenue and Forecast to 2030 (US$ Million)
- 10.1.1.2.1 Canada: Cell and Gene Therapy Manufacturing Services Market, by Type, 2020-2030 (US$ Million)
- 10.1.1.2.1.1 Canada: Cell and Gene Therapy Manufacturing Services Market, by Cell Therapy, 2020-2030 (US$ Million)
- 10.1.1.2.1.2 Canada: Cell and Gene Therapy Manufacturing Services Market, by Gene Therapy, 2020-2030 (US$ Million)
- 10.1.1.2.2 Canada: Cell and Gene Therapy Manufacturing Services Market, by Gene Therapy, 2020-2030 (US$ Million)
- 10.1.1.2.3 Canada: Cell and Gene Therapy Manufacturing Services Market, by Application, 2020-2030 (US$ Million)
- 10.1.1.2.4 Canada: Cell and Gene Therapy Manufacturing Services Market, by End User, 2020-2030 (US$ Million)
- 10.1.1.3 Mexico: Cell and Gene Therapy Manufacturing Services Market - Revenue and Forecast to 2030 (US$ Million)
- 10.1.1.3.1 Mexico: Cell and Gene Therapy Manufacturing Services Market, by Type, 2020-2030 (US$ Million)
- 10.1.1.3.1.1 Mexico: Cell and Gene Therapy Manufacturing Services Market, by Cell Therapy, 2020-2030 (US$ Million)
- 10.1.1.3.1.2 Mexico: Cell and Gene Therapy Manufacturing Services Market, by Gene Therapy, 2020-2030 (US$ Million)
- 10.1.1.3.2 Mexico: Cell and Gene Therapy Manufacturing Services Market, by Indication, 2020-2030 (US$ Million)
- 10.1.1.3.3 Mexico: Cell and Gene Therapy Manufacturing Services Market, by Application, 2020-2030 (US$ Million)
- 10.1.1.3.4 Mexico: Cell and Gene Therapy Manufacturing Services Market, by End User, 2020-2030 (US$ Million)
- 10.1.1.1 US: Cell and Gene Therapy Manufacturing Services Market - Revenue and Forecast to 2030 (US$ Million)
- 10.1.1 North America: Cell and Gene Therapy Manufacturing Services Market, by Region, 2022 & 2030 (%)
11. North America Cell and Gene Therapy Manufacturing Services Market - Industry Landscape
- 11.1 Overview
- 11.2 Growth Strategies in Cell and Gene Therapy Manufacturing Services Market
- 11.3 Organic Growth Strategies
- 11.3.1 Overview
- 11.4 Inorganic Growth Strategies
- 11.4.1 Overview
12. Company Profiles
- 12.1 Thermo Fisher Scientific Inc
- 12.1.1 Key Facts
- 12.1.2 Business Description
- 12.1.3 Products and Services
- 12.1.4 Financial Overview
- 12.1.5 SWOT Analysis
- 12.1.6 Key Developments
- 12.2 Merck KGaA
- 12.2.1 Key Facts
- 12.2.2 Business Description
- 12.2.3 Products and Services
- 12.2.4 Financial Overview
- 12.2.5 SWOT Analysis
- 12.2.6 Key Developments
- 12.3 Charles River Laboratories International Inc
- 12.3.1 Key Facts
- 12.3.2 Business Description
- 12.3.3 Products and Services
- 12.3.4 Financial Overview
- 12.3.5 SWOT Analysis
- 12.3.6 Key Developments
- 12.4 Lonza Group AG
- 12.4.1 Key Facts
- 12.4.2 Business Description
- 12.4.3 Products and Services
- 12.4.4 Financial Overview
- 12.4.5 SWOT Analysis
- 12.4.6 Key Developments
- 12.5 WuXi AppTec Co Ltd
- 12.5.1 Key Facts
- 12.5.2 Business Description
- 12.5.3 Products and Services
- 12.5.4 Financial Overview
- 12.5.5 SWOT Analysis
- 12.5.6 Key Developments
- 12.6 Catalent Inc
- 12.6.1 Key Facts
- 12.6.2 Business Description
- 12.6.3 Products and Services
- 12.6.4 Financial Overview
- 12.6.5 SWOT Analysis
- 12.6.6 Key Developments
- 12.7 Takara Bio Inc
- 12.7.1 Key Facts
- 12.7.2 Business Description
- 12.7.3 Products and Services
- 12.7.4 Financial Overview
- 12.7.5 SWOT Analysis
- 12.7.6 Key Developments
- 12.8 Nikon Corp
- 12.8.1 Key Facts
- 12.8.2 Business Description
- 12.8.3 Products and Services
- 12.8.4 Financial Overview
- 12.8.5 SWOT Analysis
- 12.8.6 Key Developments
- 12.9 FUJIFILM Holdings Corp
- 12.9.1 Key Facts
- 12.9.2 Business Description
- 12.9.3 Products and Services
- 12.9.4 Financial Overview
- 12.9.5 SWOT Analysis
- 12.9.6 Key Developments
- 12.10 National Resilience Inc
- 12.10.1 Key Facts
- 12.10.2 Business Description
- 12.10.3 Products and Services
- 12.10.4 Financial Overview
- 12.10.5 SWOT Analysis
- 12.10.6 Key Developments
- 12.11 Oxford BioMedica Plc
- 12.11.1 Key Facts
- 12.11.2 Business Description
- 12.11.3 Products and Services
- 12.11.4 Financial Overview
- 12.11.5 SWOT Analysis
- 12.11.6 Key Developments