
|
市場調査レポート
商品コード
1762528
ベクター精製市場:業界動向と世界の予測 - ウイルスベクタータイプ別、精製技術タイプ別、治療タイプ別、治療領域別、事業規模別、主要地域別Vector Purification Market: Industry Trends and Global Forecasts - Distribution by Type Of Viral Vector, Type of Purification Technique, Type Of Therapy, Therapeutic Area, Scale of Operation and Key Geographical Regions |
||||||
カスタマイズ可能
|
|||||||
| ベクター精製市場:業界動向と世界の予測 - ウイルスベクタータイプ別、精製技術タイプ別、治療タイプ別、治療領域別、事業規模別、主要地域別 |
|
出版日: 2025年07月04日
発行: Roots Analysis
ページ情報: 英文 275 Pages
納期: 即日から翌営業日
|
全表示
- 概要
- 図表
- 目次
ベクター精製市場:概要
世界のベクター精製の市場規模は、今年1億5,500万米ドルとなりました。同市場は、予測期間中に21%の有利なCAGRで成長すると予測されています。
市場セグメンテーションと機会分析は、以下のパラメータでセグメント化されています:
ウイルスベクタータイプ
- AAV
- アデノウイルス
- レンチウイルス
- レトロウイルス
- その他
精製技術タイプ
- クロマトグラフィ
- 遠心分離
- ろ過
治療タイプ
- 遺伝子治療
- 細胞療法
- ウイルスワクチン
治療領域
- 腫瘍学的疾患
- 心血管疾患
- 眼科疾患
- 代謝疾患
- 炎症・免疫疾患
- その他
事業規模
- 前臨床/臨床
- 商業
主要地域
- 北米
- 欧州
- アジア太平洋
- その他の地域
ベクター精製市場:成長と動向
ウイルスベクターは、遺伝物質を標的細胞に輸送するために使用される生物学的ツールです。複雑で資源集約的なプロセスであるにもかかわらず、細胞・遺伝子治療の開発・製造は大きな勢いを見せており、後期臨床試験や市場承認に向けて移行する臨床プログラムも増えています。現在、市販されている細胞・遺伝子治療製品は30種類を超え、これらの革新的な治療法の臨床試験は数百件が進行中です。さらに、COVID-19の大流行により、現在臨床試験に介入中の治療法の数が顕著に増加しています。しかし、現在のウイルスベクターの精製方法には多くの工程があり、製品の損失が大きく、収率が低いことが知られています。
次第に、ウイルスベクターに対する需要の高まりと、下流の精製に関する拡張性の欠如やその他の懸念が相まって、この分野の利害関係者は、ウイルス精製のための新規で効果的な解決策を開発するために様々な取り組みを行うようになっています。最近、利害関係者は、アフィニティークロマトグラフィをベースとしたウイルス精製レジメンへの依存度を高め始めています。さらに、フィルタープレート、プレパックドクロマトグラフィカラムおよび樹脂、連結キットなど、ベクター精製のための多様な革新的ソリューションを提供すると主張する企業もいくつかあります。
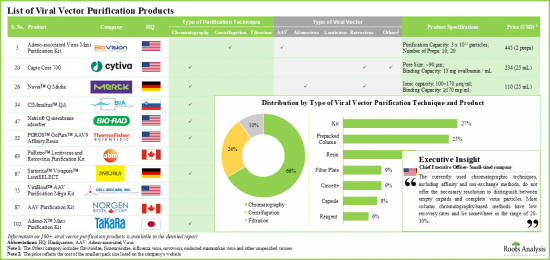
ベクター精製市場:主要インサイト
本レポートでは、世界のベクター精製市場の現状を掘り下げ、業界内の潜在的な成長機会を特定しています。本レポートの主な調査結果は以下の通りです。
- ウイルスの回収率を向上させ、汚染物質や不純物を効果的に除去するために、様々な下流処理技術を用いた100種類以上のウイルスベクター精製製品が開発されています。
- 利用可能なウイルス精製製品のほとんどは、小規模/臨床規模での使用を目的に設計されており、ベクターの種類に応じた特定の要件に対応しています。
- 現在の危機的状況において、ウイルスベクターベースのワクチンの研究開発が急増していることから、大規模ウイルス精製ソリューションの需要は増加傾向にあると予想されます。
- アデノウイルスベクターは細胞治療や遺伝子治療で広く使用されているため、複数の企業がこのようなウイルス用のキット、樹脂、カラムなどの製品を提供しています。
- Bio-Rad Laboratories、Cytiva、Thermo Fisher Scientificなどは北米を拠点とする大手企業です。
- 長年にわたり、様々な疾患にわたる様々なウイルスベクターベースの治療法やワクチンを評価する1,000以上の臨床試験が登録され、世界各地で実施されています。
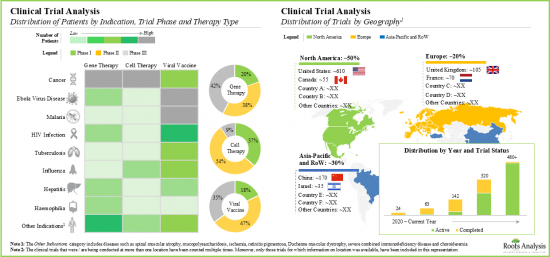
- 既存のウイルス精製プロセスを増強し、さらに最適化するために、いくつかのウイルスベクター医薬品開発・製造企業は、精製製品開発企業と提携する可能性が高いです。
- 世界中の35以上の企業が、自社内あるいは受託製造の一環として、商業規模でさまざまな種類のウイルスベクターを製造する能力を有しています。
- ウイルス細胞治療や遺伝子治療に焦点を当てた研究開発イニシアチブの高まりにより、様々な治療適応症において、様々なタイプのウイルスベクターに対する臨床的・商業的需要が増加すると予想されます。
- 承認された遺伝子組換え療法はわずかであるため、現在の需要は、ウイルスベクターベースの療法に関する様々な臨床試験に登録された患者別牽引されています。
- 現在、商業的需要の40%以上がアデノウイルスに起因していますが、これは市販されている治療法に広く使用されているためです。さらに、レンチウイルスは臨床需要の約35%に寄与しています。
- また、レンチウイルスは臨床需要の約35%に寄与しています。ウイルスベクターの大部分は、腫瘍性疾患を患う患者のために開発されているが、今後数年間は、神経疾患や筋疾患が大きな需要を生み出すと思われます。
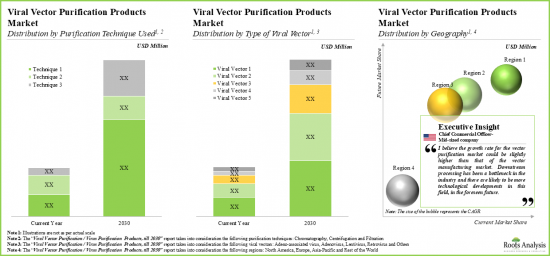
- 市場は予測期間中にCAGR 21%以上で成長すると予想され、その機会はさまざまなタイプの精製技術、ウイルスベクター、主要な地域にわたって分布すると思われます。
ベクター精製市場の参入企業例
- Agilent Technologies
- BIA Separations
- Bio-Rad Laboratories
- BioVision
- Cytiva(formerly GE Lifesciences)
- Merck
- Sartorius
- Takara Bio
- Thermo Fisher Scientific
目次
第1章 序文
第2章 エグゼクティブサマリー
第3章 イントロダクション
- 章の概要
- ウイルス性および非ウイルス性遺伝子導入法
- 遺伝子組み換え治療用ウイルスベクター
- ウイルスベクタータイプ
- ウイルスベクターの応用
- ベクター開発・製造の最新動向
- ベクター製造
- ベクター精製の未来
第4章 市場情勢
- 章の概要
- ウイルスベクター精製製品:市場情勢
- ウイルスベクター精製製品開発者
第5章 企業プロファイル
- 章の概要
- Agilent Technologies
- BIA Separations
- Bio-Rad Laboratories
- BioVision
- Cytiva(formerly GE Lifesciences)
- Merck
- Sartorius
- Takara Bio
- Thermo Fisher Scientific
第6章 戦略的パートナー分析
- 章の概要
- 調査手法と主要なパラメータ
- 潜在的な戦略的パートナー:ウイルスベクターベースの治療法開発者
- 潜在的な戦略的パートナー:ウイルスベクター製造業者
第7章 臨床試験の分析
- 章の概要
- 範囲と調査手法
- ウイルスベクターを用いた治療法:臨床試験分析
- AAVベクターベースを用いた治療法
- アデノウイルスベクターを用いた治療法
- レンチウイルスベクターを用いた治療法
- レトロウイルスベクターを用いた治療法
- その他のウイルスベクターを用いた治療法
第8章 需要分析
- 章の概要
- 前提と調査手法
- ウイルスベクターに対する世界の臨床需要
- ウイルスベクターに対する世界の商業需要
第9章 ケーススタディ:タンジェンシャルフローろ過(TFF)
第10章 ケーススタディ:ウイルスベクター製造業者
第11章 市場規模の評価と機会分析
- 章の概要
- 予測調査手法と主要な前提条件
- ウイルスベクター精製製品市場全体(2035年まで)
- AAVベクター向けウイルスベクター精製製品市場(2035年まで)
- アデノウイルスベクター向けウイルスベクター精製製品市場(2035年まで)
- レンチウイルスベクター向けウイルスベクター精製製品市場(2035年まで)
- レトロウイルスベクター向けウイルスベクター精製製品市場(2035年まで)
- その他のウイルスベクター向けウイルスベクター精製製品市場(2035年まで)
第12章 結論
第13章 エグゼクティブ洞察
第14章 付録1:表形式データ
第15章 付録2:企業・団体一覧
List of Tables
- Table 3.1 Key Comparison of Different Types of Viral Vectors
- Table 4.1 List of Viral Vector Purification Products
- Table 4.2 Viral Vector Purification Products for Chromatography: Additional Details
- Table 4.3 Viral Vector Purification Products for Centrifugation: Additional Details
- Table 4.4 Viral Vector Purification Products for Filtration: Additional Details
- Table 4.5 List of Viral Vector Purification Products Developers
- Table 5.1 Agilent Technologies: Company Snapshot
- Table 5.2 Agilent Technologies: Recent Developments and Future Outlook
- Table 5.3 BIA Separations: Company Snapshot
- Table 5.4 BIA Separations: Recent Developments and Future Outlook
- Table 5.5 Bio-Rad Laboratories: Company Snapshot
- Table 5.6 Bio-Rad Laboratories: Recent Developments and Future Outlook
- Table 5.7 BioVision: Company Snapshot
- Table 5.8 BioVision: Recent Developments and Future Outlook
- Table 5.9 Cytiva (formerly GE Lifesciences): Company Snapshot
- Table 5.10 Cytiva (formerly GE Lifesciences): Recent Developments and Future Outlook
- Table 5.11 Merck: Company Snapshot
- Table 5.12 Merck: Recent Developments and Future Outlook
- Table 5.13 Sartorius: Company Snapshot
- Table 5.14 Sartorius: Recent Developments and Future Outlook
- Table 5.15 Takara Bio: Company Snapshot
- Table 5.16 Takara Bio: Recent Developments and Future Outlook
- Table 5.17 Thermo Fisher Scientific: Company Snapshot
- Table 5.18 Thermo Fisher Scientific: Recent Developments and Future Outlook
- Table 6.1 AAV Vector-based Therapy Developers: Most Likely Partners
- Table 6.2 AAV Vector-based Therapy Developers: Likely Partners
- Table 6.3 AAV Vector-based Therapy Developers: Less Likely Partners
- Table 6.4 AAV Vector-based Therapy Developers: Least Likely Partners
- Table 6.5 Adenoviral Vector-based Therapy Developers: Most Likely Partners
- Table 6.6 Adenoviral Vector-based Therapy Developers: Likely Partners
- Table 6.7 Adenoviral Vector-based Therapy Developers: Less Likely Partners
- Table 6.8 Adenoviral Vector-based Therapy Developers: Least Likely Partners
- Table 6.9 Lentiviral Vector-based Therapy Developers: Most Likely Partners
- Table 6.10 Lentiviral Vector-based Therapy Developers: Likely Partners
- Table 6.11 Lentiviral Vector-based Therapy Developers: Less Likely Partners
- Table 6.12 Lentiviral Vector-based Therapy Developers: Least Likely Partners
- Table 6.13 Retroviral Vector-based Therapy Developers: Most Likely Partners
- Table 6.14 Retroviral Vector-based Therapy Developers: Likely Partners
- Table 6.15 Retroviral Vector-based Therapy Developers: Less Likely Partners
- Table 6.16 Retroviral Vector-based Therapy Developers: Least Likely Partners
- Table 6.17 Other Viral Vector-based Therapy Developers: Most Likely Partners
- Table 6.18 Other Viral Vector-based Therapy Developers: Likely Partners
- Table 6.19 Other Viral Vector-based Therapy Developers: Less Likely Partners
- Table 6.20 Other Viral Vector-based Therapy Developers: Least Likely Partners
- Table 6.21 AAV Vector Manufacturers: Most Likely Partners
- Table 6.22 AAV Vector Manufacturers: Likely Partners
- Table 6.23 AAV Vector Manufacturers: Less Likely Partners
- Table 6.24 AAV Vector Manufacturers: Least Likely Partners
- Table 6.25 Adenoviral Vector Manufacturers: Most Likely Partners
- Table 6.26 Adenoviral Vector Manufacturers: Likely Partners
- Table 6.27 Adenoviral Vector Manufacturers: Less Likely Partners
- Table 6.28 Adenoviral Vector Manufacturers: Least Likely Partners
- Table 6.29 Lentiviral Vector Manufacturers: Most Likely Partners
- Table 6.30 Lentiviral Vector Manufacturers: Likely Partners
- Table 6.31 Lentiviral Vector Manufacturers: Less Likely Partners
- Table 6.32 Lentiviral Vector Manufacturers: Least Likely Partners
- Table 6.33 Retroviral Vector Manufacturers: Most Likely Partners
- Table 6.34 Retroviral Vector Manufacturers: Likely Partners
- Table 6.35 Retroviral Vector Manufacturers: Less Likely Partners
- Table 6.36 Retroviral Vector Manufacturers: Least Likely Partners
- Table 6.37 Other Viral Vector Manufacturers: Most Likely Partners
- Table 6.38 Other Viral Vector Manufacturers: Likely Partners
- Table 6.39 Other Viral Vector Manufacturers: Less Likely Partners
- Table 6.40 Other Viral Vector Manufacturers: Least Likely Partners
- Table 9.1 List of TFF Products
- Table 10.1 List of Commercial Scale Vector Manufacturers
- Table 12.1 Concluding Remarks
- Table 13.1 Virovek: Company Snapshot
- Table 13.2 Vigene Biosciences: Company Snapshot
- Table 13.3 Vibalogics: Company Snapshot
- Table 14.1 Viral Vector Purification Products: Distribution by Type of Product
- Table 14.2 Viral Vector Purification Products: Distribution by Type of Purification Technique
- Table 14.3 Viral Vector Purification Products: Distribution by Scale of Operation
- Table 14.4 Viral Vector Purification Products: Distribution by Type of Viral Vector
- Table 14.5 Viral Vector Purification Products: Distribution by Type of Chromatographic Technique
- Table 14.6 Viral Vector Purification Product Developers: Distribution by Year of Establishment
- Table 14.7 Viral Vector Purification Product Developers: Distribution by Company Size
- Table 14.8 Viral Vector Purification Product Developers: Distribution by Geographical Location
- Table 14.9 Viral Vector based Therapies: Distribution by Trial Registration Year
- Table 14.10 Viral Vector based Therapies: Distribution by Trial Phase
- Table 14.11 Viral Vector based Therapies: Distribution by Trial Status
- Table 14.12 Viral Vector based Therapies: Distribution by Type of Therapy
- Table 14.13 Viral Vector based Therapies: Distribution by Therapeutic Area
- Table 14.14 Viral Vector based Therapies: Distribution by Type of Sponsor / Collaborator
- Table 14.15 Viral Vector based Therapies: Distribution by Geographical Location and Trial Status
- Table 14.16 Viral Vector based Therapies: Most Active Players by Number of Registered Trials
- Table 14.17 Viral Vector based Therapies: Distribution by Patients Enrolled and Trial Phase
- Table 14.18 Viral Vector based Therapies: Distribution by Patients Enrolled and Type of Therapy
- Table 14.19 Viral Vector based Therapies: Distribution by Patients Enrolled and Therapeutic Area
- Table 14.20 Viral Vector based Therapies: Distribution by Patients Enrolled, Trial Status and Geographical Location
- Table 14.21 AAV Vector based Therapies: Distribution by Trial Registration Year
- Table 14.22 AAV Vector based Therapies: Distribution by Trial Phase
- Table 14.23 AAV Vector based Therapies: Distribution by Trial Status
- Table 14.24 AAV Vector based Therapies: Distribution by Type of Therapy
- Table 14.25 AAV Vector based Therapies: Distribution by Therapeutic Area
- Table 14.26 AAV Vector based Therapies: Distribution by Type of Sponsor / Collaborator
- Table 14.27 AAV Vector based Therapies: Distribution by Geographical Location and Trial Status
- Table 14.28 AAV Vector based Therapies: Most Active Players by Number of Registered Trials
- Table 14.29 AAV Vector based Therapies: Distribution by Patients Enrolled and Trial Phase
- Table 14.30 AAV Vector based Therapies: Distribution by Patients Enrolled and Type of Therapy
- Table 14.31 AAV Vector based Therapies: Distribution by Patients Enrolled and Therapeutic Area
- Table 14.32 AAV Vector based Therapies: Distribution by Patients Enrolled, Trial Status and Geographical Location
- Table 14.33 Adenoviral Vector based Therapies: Distribution by Trial Registration Year
- Table 14.34 Adenoviral Vector based Therapies: Distribution by Trial Phase
- Table 14.35 Adenoviral Vector based Therapies: Distribution by Trial Status
- Table 14.36 Adenoviral Vector based Therapies: Distribution by Type of Therapy
- Table 14.37 Adenoviral Vector based Therapies: Distribution by Therapeutic Area
- Table 14.38 Adenoviral Vector based Therapies: Distribution by Type of Sponsor / Collaborator
- Table 14.39 Adenoviral Vector based Therapies: Distribution by Geographical Location and Trial Status
- Table 14.40 Adenoviral Vector based Therapies: Most Active Players by Number of Registered Trials
- Table 14.41 Adenoviral Vector based Therapies: Distribution by Patients Enrolled and Trial Phase
- Table 14.42 Adenoviral Vector based Therapies: Distribution by Patients Enrolled and Type of Therapy
- Table 14.43 Adenoviral Vector based Therapies: Distribution by Patients Enrolled and Therapeutic Area
- Table 14.44 Adenoviral Vector based Therapies: Distribution by Patients Enrolled, Trial Status and Geographical Location
- Table 14.45 Lentiviral Vector based Therapies: Distribution by Trial Registration Year
- Table 14.46 Lentiviral Vector based Therapies: Distribution by Trial Phase
- Table 14.47 Lentiviral Vector based Therapies: Distribution by Trial Status
- Table 14.48 Lentiviral Vector based Therapies: Distribution by Type of Therapy
- Table 14.49 Lentiviral Vector based Therapies: Distribution by Therapeutic Area
- Table 14.50 Lentiviral Vector based Therapies: Distribution by Type of Sponsor / Collaborator
- Table 14.51 Lentiviral Vector based Therapies: Distribution by Geographical Location
- Table 14.52 Lentiviral Vector based Therapies: Most Active Players by Number of Registered Trials
- Table 14.53 Lentiviral Vector based Therapies: Distribution by Patients Enrolled and Trial Phase
- Table 14.54 Lentiviral Vector based Therapies: Distribution by Patients Enrolled and Type of Therapy
- Table 14.55 Lentiviral Vector based Therapies: Distribution by Patients Enrolled and Therapeutic Area
- Table 14.56 Lentiviral Vector based Therapies: Distribution by Patients Enrolled, Trial Status and Geographical Location
- Table 14.57 Retroviral Vector based Therapies: Distribution by Trial Registration Year
- Table 14.58 Retroviral Vector based Therapies: Distribution by Trial Phase
- Table 14.59 Retroviral Vector based Therapies: Distribution by Trial Status
- Table 14.60 Retroviral Vector based Therapies: Distribution by Type of Therapy
- Table 14.61 Retroviral Vector based Therapies: Distribution by Therapeutic Area
- Table 14.62 Retroviral Vector based Therapies: Distribution by Type of Sponsor / Collaborator
- Table 14.63 Retroviral Vector based Therapies: Distribution by Geographical Location
- Table 14.64 Retroviral Vector based Therapies: Most Active Players by Number of Registered Trials
- Table 14.65 Retroviral Vector based Therapies: Distribution by Patients Enrolled and Trial Phase
- Table 14.66 Retroviral Vector based Therapies: Distribution by Patients Enrolled and Type of Therapy
- Table 14.67 Retroviral Vector based Therapies: Distribution by Patients Enrolled and Therapeutic Area
- Table 14.68 Retroviral Vector based Therapies: Distribution by Patients Enrolled, Trial Status and Geographical Location
- Table 14.69 Other Viral Vector based Therapies: Distribution by Trial Registration Year
- Table 14.70 Other Viral Vector based Therapies: Distribution by Trial Phase
- Table 14.71 Other Viral Vector based Therapies: Distribution by Trial Status
- Table 14.72 Other Viral Vector based Therapies: Distribution by Type of Therapy
- Table 14.73 Other Viral Vector based Therapies: Distribution by Therapeutic Area
- Table 14.74 Other Viral Vector based Therapies: Distribution by Type of Sponsor / Collaborator
- Table 14.75 Other Viral Vector based Therapies: Distribution by Geographical Location
- Table 14.76 Other Viral Vector based Therapies: Most Active Players by Number of Registered Trials
- Table 14.77 Other Viral Vector based Therapies: Distribution by Patients Enrolled and Trial Phase
- Table 14.78 Other Viral Vector based Therapies: Distribution by Patients Enrolled and Type of Therapy
- Table 14.79 Other Viral Vector based Therapies: Distribution by Patients Enrolled and Therapeutic Area
- Table 14.80 Other Viral Vector based Therapies: Distribution by Patients Enrolled, Trial Status and Geographical Location
- Table 14.81 Global Clinical Demand for Viral Vectors, Till 2035 (Thousand Patients)
- Table 14.82 Clinical Demand for Viral Vectors: Distribution by Type of Vector (Thousand Patients)
- Table 14.83 Clinical Demand for Viral Vectors: Distribution by Type of Therapy (Thousand Patients)
- Table 14.84 Clinical Demand for Viral Vectors: Distribution by Therapeutic Area (Thousand Patients)
- Table 14.85 Clinical Demand for Viral Vectors: Distribution by Geographical Location (Thousand Patients)
- Table 14.86 Global Commercial Demand for Viral Vectors, Till 2035 (Thousand Patients)
- Table 14.87 Commercial Demand for Viral Vectors: Distribution by Type of Vector (Thousand Patients)
- Table 14.88 Commercial Demand for Viral Vectors: Distribution by Type of Therapy (Thousand Patients)
- Table 14.89 Commercial Demand for Viral Vectors: Distribution by Therapeutic Area (Thousand Patients)
- Table 14.90 Commercial Demand for Viral Vectors: Distribution by Geographical Location (Thousand Patients)
- Table 14.91 Commercial Scale Viral Vector Manufacturers: Distribution by Year of Establishment
- Table 14.92 Commercial Scale Viral Vector Manufacturers: Distribution by Company Size
- Table 14.93 Commercial Scale Viral Vector Manufacturers: Distribution by Type of Viral Vector
- Table 14.94 Commercial Scale Viral Vector Manufacturers: Distribution by Purpose of Production
- Table 14.95 Commercial Scale Viral Vector Manufacturers: Distribution by Geographical Location
- Table 14.96 Overall Viral Vector Purification Products Market, Conservative, Base and Optimistic Scenario, Conservative, Base and Optimistic Scenario, Till 2035 (USD Million)
- Table 14.97 Viral Vector Purification Products Market: Distribution by Type of Viral Vector, Conservative, Base and Optimistic Scenario, Conservative, Base and Optimistic Scenario, Till 2035 (USD Million)
- Table 14.98 Viral Vector Purification Products Market: Distribution by Type of Purification Technique, Conservative, Base and Optimistic Scenario, Conservative, Base and Optimistic Scenario, Till 2035 (USD Million)
- Table 14.99 Viral Vector Purification Products Market: Distribution by Type of Therapy, Conservative, Base and Optimistic Scenario, Conservative, Base and Optimistic Scenario, Till 2035 (USD Million)
- Table 14.100 Viral Vector Purification Products Market: Distribution by Therapeutic Area, Conservative, Base and Optimistic Scenario, Conservative, Base and Optimistic Scenario, Till 2035 (USD Million)
- Table 14.101 Viral Vector Purification Products Market: Distribution by Scale of Operation, Conservative, Base and Optimistic Scenario, Till 2035 (USD Million)
- Table 14.102 Viral Vector Purification Products Market: Distribution by Geographical Location, Conservative, Base and Optimistic Scenario, Till 2035 (USD Million)
- Table 14.103 Viral Vector Purification Products Market for AAV Vectors, Conservative, Base and Optimistic Scenario, Till 2035 (USD Million)
- Table 14.104 AAV Vector Purification Products Market: Distribution by Type of Purification Technique, Conservative, Base and Optimistic Scenario, Till 2035 (USD Million)
- Table 14.105 AAV Vector Purification Products Market: Distribution by Type of Therapy, Conservative, Base and Optimistic Scenario, Till 2035 (USD Million)
- Table 14.106 AAV Vector Purification Products Market: Distribution by Therapeutic Area, Conservative, Base and Optimistic Scenario, Till 2035 (USD Million)
- Table 14.107 AAV Vector Purification Products Market: Distribution by Scale of Operation, Conservative, Base and Optimistic Scenario, Till 2035 (USD Million)
- Table 14.108 AAV Vector Purification Products Market: Distribution by Geographical Location, Conservative, Base and Optimistic Scenario, Till 2035 (USD Million)
- Table 14.109 Viral Vector Purification Products Market for Adenoviral Vectors, Conservative, Base and Optimistic Scenario, Till 2035 (USD Million)
- Table 14.110 Adenoviral Vector Purification Products Market: Distribution by Type of Purification Technique, Conservative, Base and Optimistic Scenario, Till 2035 (USD Million)
- Table 14.111 Adenoviral Vector Purification Products Market: Distribution by Type of Therapy, Conservative, Base and Optimistic Scenario, Till 2035 (USD Million)
- Table 14.112 Adenoviral Vector Purification Products Market: Distribution by Therapeutic Area, Conservative, Base and Optimistic Scenario, Till 2035 (USD Million)
- Table 14.113 Adenoviral Vector Purification Products Market: Distribution by Scale of Operation, Conservative, Base and Optimistic Scenario, Till 2035 (USD Million)
- Table 14.114 Adenoviral Vector Purification Products Market: Distribution by Geographical Location, Conservative, Base and Optimistic Scenario, Till 2035 (USD Million)
- Table 14.115 Viral Vector Purification Products Market for Lentiviral Vectors, Conservative, Base and Optimistic Scenario, Till 2035 (USD Million)
- Table 14.116 Lentiviral Vector Purification Products Market: Distribution by Type of Purification Technique, Conservative, Base and Optimistic Scenario, Till 2035 (USD Million)
- Table 14.117 Lentiviral Vector Purification Products Market: Distribution by Type of Therapy, Conservative, Base and Optimistic Scenario, Till 2035 (USD Million)
- Table 14.118 Lentiviral Vector Purification Products Market: Distribution by Therapeutic Area, Conservative, Base and Optimistic Scenario, Till 2035 (USD Million)
- Table 14.119 Lentiviral Vector Purification Products Market: Distribution by Scale of Operation, Conservative, Base and Optimistic Scenario, Till 2035 (USD Million)
- Table 14.120 Lentiviral Vector Purification Products Market: Distribution by Geographical Location, Conservative, Base and Optimistic Scenario, Till 2035 (USD Million)
- Table 14.121 Viral Vector Purification Products Market for Retroviral Vectors, Conservative, Base and Optimistic Scenario, Till 2035 (USD Million)
- Table 14.122 Retroviral Vector Purification Products Market: Distribution by Type of Purification Technique, Conservative, Base and Optimistic Scenario, Till 2035 (USD Million)
- Table 14.123 Retroviral Vector Purification Products Market: Distribution by Type of Therapy, Conservative, Base and Optimistic Scenario, Till 2035 (USD Million)
- Table 14.124 Retroviral Vector Purification Products Market: Distribution by Therapeutic Area, Conservative, Base and Optimistic Scenario, Till 2035 (USD Million)
- Table 14.125 Retroviral Vector Purification Products Market: Distribution by Scale of Operation, Conservative, Base and Optimistic Scenario, Till 2035 (USD Million)
- Table 14.126 Retroviral Vector Purification Products Market: Distribution by Geographical Location, Conservative, Base and Optimistic Scenario, Till 2035 (USD Million)
- Table 14.127 Viral Vector Purification Products Market for Other Viral Vectors, Conservative, Base and Optimistic Scenario, Till 2035 (USD Million)
- Table 14.128 Other Viral Vector Purification Products Market: Distribution by Type of Purification Technique, Conservative, Base and Optimistic Scenario, Till 2035 (USD Million)
- Table 14.129 Other Viral Vector Purification Products Market: Distribution by Type of Therapy, Conservative, Base and Optimistic Scenario, Till 2035 (USD Million)
- Table 14.130 Other Viral Vector Purification Products Market: Distribution by Therapeutic Area, Conservative, Base and Optimistic Scenario, Till 2035 (USD Million)
- Table 14.131 Other Viral Vector Purification Products Market: Distribution by Scale of Operation, Conservative, Base and Optimistic Scenario, Till 2035 (USD Million)
- Table 14.132 Other Viral Vector Purification Products Market: Distribution by Geographical Location, Conservative, Base and Optimistic Scenario, Till 2035 (USD Million)
List of Figures
- Figure 3.1 Gene Transfer: Viral and Non-Viral Methods
- Figure 3.2 Vector Manufacturing: Different Types of Manufacturers
- Figure 3.3 Vector Manufacturing: Process Steps
- Figure 4.1 Viral Vector Purification Products: Distribution by Type of Product
- Figure 4.2 Viral Vector Purification Products: Distribution by Type of Purification Technique
- Figure 4.3 Viral Vector Purification Products: Distribution by Scale of Operation
- Figure 4.4 Viral Vector Purification Products: Distribution by Type of Viral Vector
- Figure 4.5 Viral Vector Purification Products: Distribution by Type of Chromatographic Technique
- Figure 4.6 Viral Vector Purification Product Developers: Distribution by Year of Establishment
- Figure 4.7 Viral Vector Purification Product Developers: Distribution by Company Size
- Figure 4.8 Viral Vector Purification Product Developers: Distribution by Geographical Location
- Figure 7.1 Viral Vector based Therapies: Distribution by Trial Registration Year
- Figure 7.2 Viral Vector-based Therapies: Distribution by Trial Phase
- Figure 7.3 Viral Vector-based Therapies: Distribution by Trial Status
- Figure 7.4 Viral Vector-based Therapies: Distribution by Type of Therapy
- Figure 7.5 Viral Vector-based Therapies: Distribution by Therapeutic Area
- Figure 7.6 Viral Vector-based Therapies: Distribution by Type of Sponsor / Collaborator
- Figure 7.7 Viral Vector-based Therapies: Distribution by Geographical Location and Trial Status
- Figure 7.8 Viral Vector based Therapies: Most Active Players by Number of Registered Trials
- Figure 7.9 Viral Vector-based Therapies: Distribution by Patients Enrolled and Trial Phase
- Figure 7.10 Viral Vector-based Therapies: Distribution by Patients Enrolled and Type of Therapy
- Figure 7.11 Viral Vector-based Therapies: Distribution by Patients Enrolled and Therapeutic Area
- Figure 7.12 Viral Vector-based Therapies: Distribution by Patients Enrolled, Trial Status and Geographical Location
- Figure 7.13 AAV Vector-based Therapies: Distribution by Trial Registration Year
- Figure 7.14 AAV Vector-based Therapies: Distribution by Trial Phase
- Figure 7.15 AAV Vector-based Therapies: Distribution by Trial Status
- Figure 7.16 AAV Vector-based Therapies: Distribution by Type of Therapy
- Figure 7.17 AAV Vector-based Therapies: Distribution by Therapeutic Area
- Figure 7.18 AAV Vector-based Therapies: Distribution by Type of Sponsor / Collaborator
- Figure 7.19 AAV Vector--based Therapies: Distribution by Geographical Location and Trial Status
- Figure 7.20 AAV Vector-based Therapies: Most Active Players by Number of Registered Trials
- Figure 7.21 AAV Vector-based Therapies: Distribution by Patients Enrolled and Trial Phase
- Figure 7.22 AAV Vector-based Therapies: Distribution by Patients Enrolled and Type of Therapy
- Figure 7.23 AAV Vector-based Therapies: Distribution by Patients Enrolled and Therapeutic Area
- Figure 7.24 AAV Vector-based Therapies: Distribution by Patients Enrolled, Trial Status and Geographical Location
- Figure 7.25 Adenoviral Vector-based Therapies: Distribution by Trial Registration Year
- Figure 7.26 Adenoviral Vector-based Therapies: Distribution by Trial Phase
- Figure 7.27 Adenoviral Vector-based Therapies: Distribution by Trial Status
- Figure 7.28 Adenoviral Vector-based Therapies: Distribution by Type of Therapy
- Figure 7.29 Adenoviral Vector-based Therapies: Distribution by Therapeutic Area
- Figure 7.30 Adenoviral Vector-based Therapies: Distribution by Type of Sponsor / Collaborator
- Figure 7.31 Adenoviral Vector-based Therapies: Distribution by Geographical Location and Trial Status
- Figure 7.32 Adenoviral Vector-based Therapies: Most Active Players by Number of Registered Trials
- Figure 7.33 Adenoviral Vector-based Therapies: Distribution by Patients Enrolled and Trial Phase
- Figure 7.34 Adenoviral Vector-based Therapies: Distribution by Patients Enrolled and Type of Therapy
- Figure 7.35 Adenoviral Vector-based Therapies: Distribution by Patients Enrolled and Therapeutic Area
- Figure 7.36 Adenoviral Vector-based Therapies: Distribution by Patients Enrolled, Trial Status and Geographical Location
- Figure 7.37 Lentiviral Vector-based Therapies: Distribution by Trial Registration Year
- Figure 7.38 Lentiviral Vector-based Therapies: Distribution by Trial Phase
- Figure 7.39 Lentiviral Vector-based Therapies: Distribution by Trial Status
- Figure 7.40 Lentiviral Vector-based Therapies: Distribution by Type of Therapy
- Figure 7.41 Lentiviral Vector-based Therapies: Distribution by Therapeutic Area
- Figure 7.42 Lentiviral Vector-based Therapies: Distribution by Type of Sponsor / Collaborator
- Figure 7.43 Lentiviral Vector-based Therapies: Distribution by Geographical Location
- Figure 7.44 Lentiviral Vector-based Therapies: Most Active Players by Number of Registered Trials
- Figure 7.45 Lentiviral Vector-based Therapies: Distribution by Patients Enrolled and Trial Phase
- Figure 7.46 Lentiviral Vector-based Therapies: Distribution by Patients Enrolled and Type of Therapy
- Figure 7.47 Lentiviral Vector-based Therapies: Distribution by Patients Enrolled and Therapeutic Area
- Figure 7.48 Lentiviral Vector-based Therapies: Distribution by Patients Enrolled, Trial Status and Geographical Location
- Figure 7.49 Retroviral Vector-based Therapies: Distribution by Trial Registration Year
- Figure 7.50 Retroviral Vector-based Therapies: Distribution by Trial Phase
- Figure 7.51 Retroviral Vector-based Therapies: Distribution by Trial Status
- Figure 7.52 Retroviral Vector-based Therapies: Distribution by Type of Therapy
- Figure 7.53 Retroviral Vector-based Therapies: Distribution by Therapeutic Area
- Figure 7.54 Retroviral Vector-based Therapies: Distribution by Type of Sponsor / Collaborator
- Figure 7.55 Retroviral Vector-based Therapies: Distribution by Geographical Location
- Figure 7.56 Retroviral Vector-based Therapies: Most Active Players by Number of Registered Trials
- Figure 7.57 Retroviral Vector-based Therapies: Distribution by Patients Enrolled and Trial Phase
- Figure 7.58 Retroviral Vector-based Therapies: Distribution by Patients Enrolled and Type of Therapy
- Figure 7.59 Retroviral Vector-based Therapies: Distribution by Patients Enrolled and Therapeutic Area
- Figure 7.60 Retroviral Vector-based Therapies: Distribution by Patients Enrolled, Trial Status and Geographical Location
- Figure 7.61 Other Viral Vector-based Therapies: Distribution by Trial Registration Year
- Figure 7.62 Other Viral Vector-based Therapies: Distribution by Trial Phase
- Figure 7.63 Other Viral Vector-based Therapies: Distribution by Trial Status
- Figure 7.64 Other Viral Vector-based Therapies: Distribution by Type of Therapy
- Figure 7.65 Other Viral Vector-based Therapies: Distribution by Therapeutic Area
- Figure 7.66 Other Viral Vector-based Therapies: Distribution by Type of Sponsor / Collaborator
- Figure 7.67 Other Viral Vector-based Therapies: Distribution by Geographical Location
- Figure 7.68 Other Viral Vector-based Therapies: Most Active Players by Number of Registered Trials
- Figure 7.69 Other Viral Vector-based Therapies: Distribution by Patients Enrolled and Trial Phase
- Figure 7.70 Other Viral Vector-based Therapies: Distribution by Patients Enrolled and Type of Therapy
- Figure 7.71 Other Viral Vector-based Therapies: Distribution by Patients Enrolled and Therapeutic Area
- Figure 7.72 Other Viral Vector-based Therapies: Distribution by Patients Enrolled, Trial Status and Geographical Location
- Figure 8.1. Global Clinical Demand for Viral Vectors, Till 2035 (Thousand Patients)
- Figure 8.2. Clinical Demand for Viral Vectors: Distribution by Type of Vector (Thousand Patients)
- Figure 8.3. Clinical Demand for Viral Vectors: Distribution by Type of Therapy (Thousand Patients)
- Figure 8.4. Clinical Demand for Viral Vectors: Distribution by Therapeutic Area (Thousand Patients)
- Figure 8.5. Clinical Demand for Viral Vectors: Distribution by Geographical Location (Thousand Patients)
- Figure 8.6. Global Commercial Demand for Viral Vectors, Till 2035 (Thousand Patients)
- Figure 8.7. Commercial Demand for Viral Vectors: Distribution by Type of Vector (Thousand Patients)
- Figure 8.8. Commercial Demand for Viral Vectors: Distribution by Type of Therapy (Thousand Patients)
- Figure 8.9. Commercial Demand for Viral Vectors: Distribution by Therapeutic Area (Thousand Patients)
- Figure 8.10. Commercial Demand for Viral Vectors: Distribution by Geographical Location (Thousand Patients)
- Figure 10.1 Commercial Scale Viral Vector Manufacturers: Distribution by Year of Establishment
- Figure 10.2 Commercial Scale Viral Vector Manufacturers: Distribution by Company Size
- Figure 10.3 Commercial Scale Viral Vector Manufacturers: Distribution by Type of Viral Vector
- Figure 10.4 Commercial Scale Viral Vector Manufacturers: Distribution by Purpose of Production
- Figure 10.5 Commercial Scale Viral Vector Manufacturers: Distribution by Geographical Location
- Figure 11.1 Overall Viral Vector Purification Products Market, Till 2035 (USD Million)
- Figure 11.2 Viral Vector Purification Products Market: Distribution by Type of Viral Vector, Till 2035 (USD Million)
- Figure 11.3 Viral Vector Purification Products Market: Distribution by Type of Purification Technique, Till 2035 (USD Million)
- Figure 11.4 Viral Vector Purification Products Market: Distribution by Type of Therapy, Till 2035 (USD Million)
- Figure 11.5 Viral Vector Purification Products Market: Distribution by Therapeutic Area, Till 2035 (USD Million)
- Figure 11.6 Viral Vector Purification Products Market: Distribution by Scale of Operation, Till 2035 (USD Million)
- Figure 11.7 Viral Vector Purification Products Market: Distribution by Geographical Location, Till 2035 (USD Million)
- Figure 11.8 Viral Vector Purification Products Market for AAV Vectors, Till 2035 (USD Million)
- Figure 11.9 AAV Vector Purification Products Market: Distribution by Type of Purification Technique, Till 2035 (USD Million)
- Figure 11.10 AAV Vector Purification Products Market: Distribution by Type of Therapy, Till 2035 (USD Million)
- Figure 11.11 AAV Vector Purification Products Market: Distribution by Therapeutic Area, Till 2035 (USD Million)
- Figure 11.12 AAV Vector Purification Products Market: Distribution by Scale of Operation, Till 2035 (USD Million)
- Figure 11.13 AAV Vector Purification Products Market: Distribution by Geographical Location, Till 2035 (USD Million)
- Figure 11.14 Viral Vector Purification Products Market for Adenoviral Vectors, Till 2035 (USD Million)
- Figure 11.15 Adenoviral Vector Purification Products Market: Distribution by Type of Purification Technique, Till 2035 (USD Million)
- Figure 11.16 Adenoviral Vector Purification Products Market: Distribution by Type of Therapy, Till 2035 (USD Million)
- Figure 11.17 Adenoviral Vector Purification Products Market: Distribution by Therapeutic Area, Till 2035 (USD Million)
- Figure 11.18 Adenoviral Vector Purification Products Market: Distribution by Scale of Operation, Till 2035 (USD Million)
- Figure 11.19 Adenoviral Vector Purification Products Market: Distribution by Geographical Location, Till 2035 (USD Million)
- Figure 11.20 Viral Vector Purification Products Market for Lentiviral Vectors, Till 2035 (USD Million)
- Figure 11.21 Lentiviral Vector Purification Products Market: Distribution by Type of Purification Technique, Till 2035 (USD Million)
- Figure 11.22 Lentiviral Vector Purification Products Market: Distribution by Type of Therapy, Till 2035 (USD Million)
- Figure 11.23 Lentiviral Vector Purification Products Market: Distribution by Therapeutic Area, Till 2035 (USD Million)
- Figure 11.24 Lentiviral Vector Purification Products Market: Distribution by Scale of Operation, Till 2035 (USD Million)
- Figure 11.25 Lentiviral Vector Purification Products Market: Distribution by Geographical Location, Till 2035 (USD Million)
- Figure 11.26 Viral Vector Purification Products Market for Retroviral Vectors, Till 2035 (USD Million)
- Figure 11.27 Retroviral Vector Purification Products Market: Distribution by Type of Purification Technique, Till 2035 (USD Million)
- Figure 11.28 Retroviral Vector Purification Products Market: Distribution by Type of Therapy, Till 2035 (USD Million)
- Figure 11.29 Retroviral Vector Purification Products Market: Distribution by Therapeutic Area, Till 2035 (USD Million)
- Figure 11.30 Retroviral Vector Purification Products Market: Distribution by Scale of Operation, Till 2035 (USD Million)
- Figure 11.31 Retroviral Vector Purification Products Market: Distribution by Geographical Location, Till 2035 (USD Million)
- Figure 11.32 Viral Vector Purification Products Market for Other Viral Vectors, Till 2035 (USD Million)
- Figure 11.33 Other Viral Vector Purification Products Market: Distribution by Type of Purification Technique, Till 2035 (USD Million)
- Figure 11.34 Other Viral Vector Purification Products Market: Distribution by Type of Therapy, Till 2035 (USD Million)
- Figure 11.35 Other Viral Vector Purification Products Market: Distribution by Therapeutic Area, Till 2035 (USD Million)
- Figure 11.36 Other Viral Vector Purification Products Market: Distribution by Scale of Operation, Till 2035 (USD Million)
- Figure 11.37 Other Viral Vector Purification Products Market: Distribution by Geographical Location, Till 2035 (USD Million)
VECTOR PURIFICATION MARKET: OVERVIEW
As per Roots Analysis, the global vector purification market valued at USD 155 million in the current year is anticipated to grow at a lucrative CAGR of 21% during the forecast period.
The market sizing and opportunity analysis has been segmented across the following parameters:
Type of Viral Vector
- AAV
- Adenovirus
- Lentivirus
- Retrovirus
- Others
Type of Purification Technique
- Chromatography
- Centrifugation
- Filtration
Type of Therapy
- Gene Therapy
- Cell Therapy
- Viral Vaccines
Therapeutic Area
- Oncological Disorders
- Cardiovascular Disorders
- Ophthalmic Disorders
- Metabolic Disorders
- Inflammation & Immunological Diseases
- Others
Scale of Operation
- Preclinical / Clinical
- Commercial
Key Geographical Regions
- North America
- Europe
- Asia- Pacific
- Rest of the World
VECTOR PURIFICATION MARKET: GROWTH AND TRENDS
Viral vectors are the biological tools that are used to transport genetic material to the target cell. Despite being a complex and resource-intensive process, development and manufacturing of cell and gene therapies has gained substantial momentum, with an increasing number of clinical programs moving to later-phase clinical trials and towards market approval. There are currently over 30 commercially available cell and gene therapy products, along with hundreds of ongoing clinical trials for these innovative therapies. Further, the COVID-19 pandemic has led to a notable rise in the number of therapies that are currently under intervention in clinical trials. However, the current purification methods for viral vectors involve a multitude of steps, which are known to be associated with high product losses and lower yields.
Gradually, rising demand for viral vectors coupled with lack of scalability and other concerns related to downstream purification, have led stakeholders in this domain to undertake various initiatives to develop novel and effective solution for virus purification. Recently, stakeholders have begun relying more on affinity chromatography-based virus purification regimens. Further, there are several companies that claim to offer a diverse range of innovative solutions for vector purification, including filter plates, prepacked chromatography columns and resins, and consolidated kits.

VECTOR PURIFICATION MARKET: KEY INSIGHTS
The report delves into the current state of the global vector purification market and identifies potential growth opportunities within industry. Some key findings from the report include:
- Over 100 viral vector purification products, involving the use of a variety of downstream processing techniques, have been developed to improve virus recovery and facilitate effective removal of contaminants / impurities.
- Most of the available virus purification products have been designed for small / clinical scale use, catering to specific requirements of different types of vectors; North America emerged as the hub of development of such solutions.
- Given the surge in R&D on viral vector-based vaccines in the current crisis, the demand for large scale virus purification solutions is anticipated to be on the rise.
- Adenoviral vectors are widely used in cell and gene therapies; hence, several players offer products, such as kits, resins and columns, for such viruses.
- Majority of developers are based in North America; Bio-Rad Laboratories, Cytiva and Thermo Fisher Scientific are some of the large players based in the region.
- Over the years, more than 1,000 clinical trials, evaluating various viral vector-based therapies and vaccines, across a wide range of diseases, have been registered and are being conducted in different global regions.

- In order to augment and further optimize existing virus purification processes, several viral vector drug developers and manufacturers are likely to forge alliances with purification product developers.
- Over 35 players across the globe are capable of manufacturing different types of viral vectors at commercial scale, either for in-house requirements or as part of contract manufacturing engagements.
- Owing to the rise in R&D initiatives focused on viral cell and gene therapies, the clinical and commercial demand for various types of viral vectors is expected to increase, across a variety of therapeutic indications.
- Since only a few genetically modified therapies have been approved, the current demand is being driven by the patients enrolled in various clinical trials for viral vector-based therapies.
- Currently, over 40% of commercial demand is attributed to adenoviruses due to their wide use in marketed therapies; further, lentiviruses contribute to about 35% of the clinical demand.
- Majority of viral vectors are being developed for patients suffering from oncological disorders; in the coming years, neurological disorders and muscular disorders, are likely to generate a significant demand.
- The market is anticipated to grow at a CAGR of over 21% during the forecast period, and the opportunity is likely to be distributed across different types of purification techniques, viral vectors and key geographical regions.

Example Players in the Vector Purification Market
- Agilent Technologies
- BIA Separations
- Bio-Rad Laboratories
- BioVision
- Cytiva (formerly GE Lifesciences)
- Merck
- Sartorius
- Takara Bio
- Thermo Fisher Scientific
VECTOR PURIFICATION MARKET: RESEARCH COVERAGE
- Market Sizing and Opportunity Analysis: The report features an in-depth analysis of the vector purification market, focusing on key market segments, including [A] type of viral vector, [B] type of purification technique, [C] type of therapy, [D] therapeutic area, [E] scale of operation and [F] key geographical regions.
- Market Landscape: A comprehensive evaluation of companies providing products for purification of viruses / viral vectors, based on several relevant parameters, such as [A] type of product, [B] type of purification technique, [C] scale of operation, [D] type of viral vector and [E] details on other physical and operational parameters of the product. Additionally, a comprehensive evaluation of purification product developers, based on parameters, such as [A] the year of establishment, [B] company size and [C] location of headquarters.
- Company Profiles: In-depth profiles of the players engaged in this domain, focusing on [A] overview of the company, [B] product portfolio and [C] recent developments and an informed future outlook.
- Strategic Partner Analysis: A detailed analysis of the potential strategic partners for viral vector purification product developers, based on various parameters, such as [A] type of viral vector, [B] developer strength, [C] operational strength, [D] therapeutic area, [E] strength of clinical pipeline and [F] strength of preclinical pipeline.
- Clinical Trial Analysis: An in-depth analysis of clinical studies of different viral-vector based therapies, examining factors, such as [A] registration year, [B] phase of development, [C] trial status, [D] type of therapy, [E] therapeutic area, [F] type of sponsor / collaborator, [G] geographical location, [H] number of patients enrolled and [I] key players.
- Demand Analysis: A detailed analysis of the current and future demand for viral vectors, based on various parameters, such as [A] target patient population, [B] dosing frequency, [C] dose strength, [E] type of viral vector, [F] type of therapy, [G] therapeutic are and [h] geographical location.
- Case Study 1: A detailed discussion on tangential flow filtration (TFF), representing the role, advantages and disadvantages of the various techniques used for purification of viral vectors; featuring the details of products used for TFF, including [A] product type, [B] scale of operation, [C] membrane material, [D] flow rate and [E] filtration area.
- Case Study 2: Elaborate assessment of viral vector manufacturers providing commercial scale production, focusing on details, such as [A] year of establishment, [B] company size, [C] type of viral vector, [D] purpose of production, [E] location of headquarters and [F] manufacturing facilities.
KEY QUESTIONS ANSWERED IN THIS REPORT
- How many companies are currently engaged in this market?
- Which are the leading companies in this market?
- What factors are likely to influence the evolution of this market?
- What is the current and future market size?
- What is the CAGR of this market?
- How is the current and future market opportunity likely to be distributed across key market segments?
REASONS TO BUY THIS REPORT
- The report provides a comprehensive market analysis, offering detailed revenue projections of the overall market and its specific sub-segments. This information is valuable to both established market leaders and emerging entrants.
- Stakeholders can leverage the report to gain a deeper understanding of the competitive dynamics within the market. By analyzing the competitive landscape, businesses can make informed decisions to optimize their market positioning and develop effective go-to-market strategies.
- The report offers stakeholders a comprehensive overview of the market, including key drivers, barriers, opportunities, and challenges. This information empowers stakeholders to stay abreast of market trends and make data-driven decisions to capitalize on growth prospects.
ADDITIONAL BENEFITS
- Complimentary PPT Insights Packs
- Complimentary Excel Data Packs for all Analytical Modules in the Report
- 15% Free Content Customization
- Detailed Report Walkthrough Session with Research Team
- Free Updated report if the report is 6-12 months old or older
TABLE OF CONTENTS
1. PREFACE
- 1.1. Scope of the Report
- 1.2. Research Methodology
- 1.2.1. Research Assumptions
- 1.2.2. Project Methodology
- 1.2.3. Forecast Methodology
- 1.2.4. Robust Quality Control
- 1.2.5. Key Considerations
- 1.2.5.1. Demographics
- 1.2.5.2. Economic Factors
- 1.2.5.3. Government Regulations
- 1.2.5.4. Supply Chain
- 1.2.5.5. COVID Impact / Related Factors
- 1.2.5.6. Market Access
- 1.2.5.7. Healthcare Policies
- 1.2.5.8. Industry Consolidation
- 1.3 Key Questions Answered
- 1.4. Chapter Outlines
2. EXECUTIVE SUMMARY
3. INTRODUCTION
- 3.1. Chapter Overview
- 3.2. Viral and Non-Viral Methods of Gene Transfer
- 3.3. Viral Vectors for Genetically Modified Therapies
- 3.4. Types of Viral Vectors
- 3.4.1. Adeno-associated Viral Vectors
- 3.4.2. Adenoviral Vectors
- 3.4.3. Lentiviral Vectors
- 3.4.4. Retroviral Vectors
- 3.4.5. Other Viral Vectors
- 3.4.5.1. Alphavirus
- 3.4.5.2. Foamy Virus
- 3.4.5.3. Herpes Simplex Virus
- 3.4.5.4. Sendai Virus
- 3.4.5.5. Simian Virus
- 3.4.5.6. Vaccinia Virus
- 3.6. Applications of Viral Vectors
- 3.6.1. Cell and Gene Therapy
- 3.6.2. Vaccinology
- 3.7. Current Trends in Vector Development / Manufacturing
- 3.7.1. Vector Engineering
- 3.7.2. Cargo Engineering
- 3.8. Vector Manufacturing
- 3.8.1. Types of Vector Manufacturers
- 3.8.2. Viral Vector Manufacturing Process
- 3.8.3. Challenges Related to Vector Manufacturing
- 3.8.3.1. Vector Purification Process
- 3.8.3.2. Techniques Used for Vector Purification
- 3.8.3.2.1. Centrifugation and Ultra-Centrifugation
- 3.8.3.2.2. Filtration
- 3.8.3.2.3. Chromatography
- 3.8.3.3. Challenges Related to Vector Purification
- 3.9. Future of Vector Purification
4. MARKET LANDSCAPE
- 4.1. Chapter Overview
- 4.2. Viral Vector Purification Products: Overall Market Landscape
- 4.2.1. Analysis by Type of Product
- 4.2.2. Analysis by Type of Purification Technique
- 4.2.3. Analysis by Scale of Operation
- 4.2.4. Analysis by Type of Viral Vector
- 4.2.5. Viral Vector Purification Products for Chromatography
- 4.2.5.1. Analysis by Type of Chromatographic Technique
- 4.2.6. Viral Vector Purification Products for Centrifugation
- 4.2.7. Viral Vector Purification Products for Filtration
- 4.3. Viral Vector Purification Product Developers
- 4.3.1. Analysis by Year of Establishment
- 4.3.2. Analysis by Company Size
- 4.3.3. Analysis by Geographical Location
5. COMPANY PROFILES
- 5.1. Chapter Overview
- 5.2. Agilent Technologies
- 5.2.1. Company Overview
- 5.2.2. Product Portfolio
- 5.2.3. Recent Developments and Future Outlook
- 5.3. BIA Separations
- 5.3.1. Company Overview
- 5.3.2. Product Portfolio
- 5.3.3. Recent Developments and Future Outlook
- 5.4. Bio-Rad Laboratories
- 5.4.1. Company Overview
- 5.4.2. Product Portfolio
- 5.4.3. Recent Developments and Future Outlook
- 5.5. BioVision
- 5.5.1. Company Overview
- 5.5.2. Product Portfolio
- 5.5.3. Recent Developments and Future Outlook
- 5.6. Cytiva (formerly GE Lifesciences)
- 5.6.1. Company Overview
- 5.6.2. Product Portfolio
- 5.6.3. Recent Developments and Future Outlook
- 5.7. Merck
- 5.7.1. Company Overview
- 5.7.2. Product Portfolio
- 5.7.3. Recent Developments and Future Outlook
- 5.8. Sartorius
- 5.8.1. Company Overview
- 5.8.2. Product Portfolio
- 5.8.3. Recent Developments and Future Outlook
- 5.9. Takara Bio
- 5.9.1. Company Overview
- 5.9.2. Product Portfolio
- 5.9.3. Recent Developments and Future Outlook
- 5.10. Thermo Fisher Scientific
- 5.10.1. Company Overview
- 5.10.2. Product Portfolio
- 5.10.3. Recent Developments and Future Outlook
6. STRATEGIC PARTNER ANALYSIS
- 6.1. Chapter Overview
- 6.2. Methodology and Key Parameters
- 6.3. Potential Strategic Partners: Viral Vector-based Therapy Developers
- 6.3.1. Strategic Partner Analysis: AAV Vector-based Therapy Developers
- 6.3.1.1. Most Likely Partners
- 6.3.1.2. Likely Partners
- 6.3.1.3. Less Likely Partners
- 6.3.1.4. Least Likely Partners
- 6.3.2. Strategic Partner Analysis: Adenoviral Vector-based Therapy Developers
- 6.3.2.1. Most Likely Partners
- 6.3.2.2. Likely Partners
- 6.3.2.3. Less Likely Partners
- 6.3.2.4. Least Likely Partners
- 6.3.3. Strategic Partner Analysis: Lentiviral Vector-based Therapy Developers
- 6.3.3.1. Most Likely Partners
- 6.3.3.2. Likely Partners
- 6.3.3.3. Less Likely Partners
- 6.3.3.4. Least Likely Partners
- 6.3.4. Strategic Partner Analysis: Retroviral Vector-based Therapy Developers
- 6.3.4.1. Most Likely Partners
- 6.3.4.2. Likely Partners
- 6.3.4.3. Less Likely Partners
- 6.3.4.4. Least Likely Partners
- 6.3.5. Strategic Partner Analysis: Other Viral Vector-based Therapy Developers
- 6.3.5.1. Most Likely Partners
- 6.3.5.2. Likely Partners
- 6.3.5.3. Less Likely Partners
- 6.3.5.4. Least Likely Partners
- 6.3.1. Strategic Partner Analysis: AAV Vector-based Therapy Developers
- 6.4. Potential Strategic Partners: Viral Vector Manufacturers
- 6.4.1. Strategic Partner Analysis: AAV Vector Manufacturers
- 6.4.1.1. Most Likely Partners
- 6.4.1.2. Likely Partners
- 6.4.1.3. Less Likely Partners
- 6.4.1.4. Least Likely Partners
- 6.4.2. Strategic Partner Analysis: Adenoviral Vector Manufacturers
- 6.4.2.1. Most Likely Partners
- 6.4.2.2. Likely Partners
- 6.4.2.3. Less Likely Partners
- 6.4.2.4. Least Likely Partners
- 6.4.3. Strategic Partner Analysis: Lentiviral Vector Manufacturers
- 6.4.3.1. Most Likely Partners
- 6.4.3.2. Likely Partners
- 6.4.3.3. Less Likely Partners
- 6.4.3.4. Least Likely Partners
- 6.4.4. Strategic Partner Analysis: Retroviral Vector Manufacturers
- 6.4.4.1. Most Likely Partners
- 6.4.4.2. Likely Partners
- 6.4.4.3. Less Likely Partners
- 6.4.4.4. Least Likely Partners
- 6.4.5. Strategic Partner Analysis: Other Viral Vector Manufacturers
- 6.4.5.1. Most Likely Partners
- 6.4.5.2. Likely Partners
- 6.4.5.3. Less Likely Partners
- 6.4.5.4. Least Likely Partners
- 6.4.1. Strategic Partner Analysis: AAV Vector Manufacturers
7. CLINICAL TRIAL ANALYSIS
- 7.1. Chapter Overview
- 7.2. Scope and Methodology
- 7.3. Viral Vector based Therapies: Clinical Trial Analysis
- 7.3.1. Analysis by Trial Registration Year
- 7.3.2. Analysis by Trial Phase
- 7.3.3. Analysis by Trial Status
- 7.3.4. Analysis by Type of Therapy
- 7.3.5. Analysis by Therapeutic Area
- 7.3.6. Analysis by Type of Sponsor / Collaborator
- 7.3.7. Analysis by Geographical Location and Trial Status
- 7.3.8. Most Active Players: Analysis by Number of Registered Trials
- 7.3.9. Analysis by Patients Enrolled and Trial Phase
- 7.3.10. Analysis by Patients Enrolled and Type of Therapy
- 7.3.11. Analysis by Patients Enrolled and Therapeutic Area
- 7.3.12. Analysis by Patients Enrolled, Trial Status and Geographical Location
- 7.4. AAV Vector based Therapies
- 7.4.1. Analysis by Trial Registration Year
- 7.4.2. Analysis by Trial Phase
- 7.4.3. Analysis by Trial Status
- 7.4.4. Analysis by Type of Therapy
- 7.4.5. Analysis by Therapeutic Area
- 7.4.6. Analysis by Type of Sponsor / Collaborator
- 7.4.7. Analysis by Geographical Location and Trial Status
- 7.4.8. Most Active Players: Analysis by Number of Registered Trials
- 7.4.9. Analysis by Patients Enrolled and Trial Phase
- 7.4.10. Analysis by Patients Enrolled and Type of Therapy
- 7.4.11. Analysis by Patients Enrolled and Therapeutic Area
- 7.4.12. Analysis by Patients Enrolled, Trial Status and Geographical Location
- 7.5. Adenoviral Vector based Therapies
- 7.5.1. Analysis by Trial Registration Year
- 7.5.2. Analysis by Trial Phase
- 7.5.3. Analysis by Trial Status
- 7.5.4. Analysis by Type of Therapy
- 7.5.5. Analysis by Therapeutic Area
- 7.5.6. Analysis by Type of Sponsor / Collaborator
- 7.5.7. Analysis by Geographical Location and Trial Status
- 7.5.8. Most Active Players: Analysis by Number of Registered Trials
- 7.5.9. Analysis by Patients Enrolled and Trial Phase
- 7.5.10. Analysis by Patients Enrolled and Type of Therapy
- 7.5.11. Analysis by Patients Enrolled and Therapeutic Area
- 7.5.12. Analysis by Patients Enrolled, Trial Status and Geographical Location
- 7.6. Lentiviral Vector based Therapies
- 7.6.1. Analysis by Trial Registration Year
- 7.6.2. Analysis by Trial Phase
- 7.6.3. Analysis by Trial Status
- 7.6.4. Analysis by Type of Therapy
- 7.6.5. Analysis by Therapeutic Area
- 7.6.6. Analysis by Type of Sponsor / Collaborator
- 7.6.7. Analysis by Geographical Location and Trial Status
- 7.6.8. Most Active Players: Analysis by Number of Registered Trials
- 7.6.9. Analysis by Patients Enrolled and Trial Phase
- 7.6.10. Analysis by Patients Enrolled and Type of Therapy
- 7.6.11. Analysis by Patients Enrolled and Therapeutic Area
- 7.6.12. Analysis by Patients Enrolled, Trial Status and Geographical Location
- 7.7. Retroviral Vector based Therapies
- 7.7.1. Analysis by Trial Registration Year
- 7.7.2. Analysis by Trial Phase
- 7.7.3. Analysis by Trial Status
- 7.7.4. Analysis by Type of Therapy
- 7.7.5. Analysis by Therapeutic Area
- 7.7.6. Analysis by Type of Sponsor / Collaborator
- 7.7.7. Analysis by Geographical Location and Trial Status
- 7.7.8. Most Active Players: Analysis by Number of Registered Trials
- 7.7.9. Analysis by Patients Enrolled and Trial Phase
- 7.7.10. Analysis by Patients Enrolled and Type of Therapy
- 7.7.11. Analysis by Patients Enrolled and Therapeutic Area
- 7.7.12. Analysis by Patients Enrolled, Trial Status and Geographical Location
- 7.8. Other Viral Vector based Therapies
- 7.8.1. Analysis by Trial Registration Year
- 7.8.2. Analysis by Trial Phase
- 7.8.3. Analysis by Trial Status
- 7.8.4. Analysis by Type of Therapy
- 7.8.5. Analysis by Therapeutic Area
- 7.8.6. Analysis by Type of Sponsor / Collaborator
- 7.8.7. Analysis by Geographical Location and Trial Status
- 7.8.8. Most Active Players: Analysis by Number of Registered Trials
- 7.8.9. Analysis by Patients Enrolled and Trial Phase
- 7.8.10. Analysis by Patients Enrolled and Type of Therapy
- 7.8.11. Analysis by Patients Enrolled and Therapeutic Area
- 7.8.12. Analysis by Patients Enrolled, Trial Status and Geographical Location
8. DEMAND ANALYSIS
- 8.1. Chapter Overview
- 8.2. Assumptions and Methodology
- 8.3. Global, Clinical Demand for Viral Vectors
- 8.3.1. Analysis by Type of Vector
- 8.3.2. Analysis by Type of Therapy
- 8.3.3. Analysis by Therapeutic Area
- 8.3.4. Analysis by Geographical Location
- 8.4. Global, Commercial Demand for Viral Vectors
- 8.4.1. Analysis by Type of Vector
- 8.4.2. Analysis by Type of Therapy
- 8.4.3. Analysis by Therapeutic Area
- 8.4.4. Analysis by Geographical Location
9. CASE STUDY: TANGENTIAL FLOW FILTRATION (TFF)
- 9.1. Chapter Overview
- 9.2. Role of TFF in Viral Vector Purification
- 9.2.1. Advantages of TFF
- 9.2.2. Disadvantages of TFF
- 9.3. TFF-related Product Suppliers
10. CASE STUDY: VIRAL VECTOR MANUFACTURERS
- 10.1. Chapter Overview
- 10.2. Commercial Scale Viral Vector Manufacturers
- 10.2.1. Analysis by Year of Establishment
- 10.2.2. Analysis by Company Size
- 10.2.3. Analysis by Type of Viral Vector
- 10.2.4. Analysis by Purpose of Production
- 10.2.5. Analysis by Geographical Location
11. MARKET SIZING AND OPPORTUNITY ANALYSIS
- 11.1. Chapter Overview
- 11.2. Forecast Methodology and Key Assumptions
- 11.3. Overall Viral Vector Purification Products Market, Till 2035
- 11.3.1. Viral Vector Purification Products Market: Distribution by Type of Viral Vector, Till 2035
- 11.3.2. Viral Vector Purification Products Market: Distribution by Type of Purification Technique, Till 2035
- 11.3.3. Viral Vector Purification Products Market: Distribution by Type of Therapy, Till 2035
- 11.3.4. Viral Vector Purification Products Market: Distribution by Therapeutic Area, Till 2035
- 11.3.5. Viral Vector Purification Products Market: Distribution by Scale of Operation, Till 2035
- 11.3.6. Viral Vector Purification Products Market: Distribution by Geographical Location, Till 2035
- 11.4. Viral Vector Purification Products Market for AAV Vectors, Till 2035
- 11.4.1. AAV Vector Purification Products Market: Distribution by Type of Purification Technique, Till 2035
- 11.4.2. AAV Vector Purification Products Market: Distribution by Type of Therapy, Till 2035
- 11.4.3. AAV Vector Purification Products Market: Distribution by Therapeutic Area, Till 2035
- 11.4.4. AAV Vector Purification Products Market: Distribution by Scale of Operation, Till 2035
- 11.4.5. AAV Vector Purification Products Market: Distribution by Geographical Location, Till 2035
- 11.5. Viral Vector Purification Products Market for Adenoviral Vectors, Till 2035
- 11.5.1. Adenoviral Vector Purification Products Market: Distribution by Type of Purification Technique, Till 2035
- 11.5.2. Adenoviral Vector Purification Products Market: Distribution by Type of Therapy, Till 2035
- 11.5.3. Adenoviral Vector Purification Products Market: Distribution by Therapeutic Area, Till 2035
- 11.5.4. Adenoviral Vector Purification Products Market: Distribution by Scale of Operation, Till 2035
- 11.5.5. Adenoviral Vector Purification Products Market: Distribution by Geographical Location, Till 2035
- 11.6. Viral Vector Purification Products Market for Lentiviral Vectors, Till 2035
- 11.6.1. Lentiviral Vector Purification Products Market: Distribution by Type of Purification Technique, Till 2035
- 11.6.2. Lentiviral Vector Purification Products Market: Distribution by Type of Therapy, Till 2035
- 11.6.3. Lentiviral Vector Purification Products Market: Distribution by Therapeutic Area, Till 2035
- 11.6.4. Lentiviral Vector Purification Products Market: Distribution by Scale of Operation, Till 2035
- 11.6.5. Lentiviral Vector Purification Products Market: Distribution by Geographical Location, Till 2035
- 11.7. Viral Vector Purification Products Market for Retroviral Vectors, Till 2035
- 11.7.1. Retroviral Vector Purification Products Market: Distribution by Type of Purification Technique, Till 2035
- 11.7.2. Retroviral Vector Purification Products Market: Distribution by Type of Therapy, Till 2035
- 11.7.3. Retroviral Vector Purification Products Market: Distribution by Therapeutic Area, Till 2035
- 11.7.4. Retroviral Vector Purification Products Market: Distribution by Scale of Operation, Till 2035
- 11.7.5. Retroviral Vector Purification Products Market: Distribution by Geographical Location, Till 2035
- 11.8. Viral Vector Purification Products Market for Other Viral Vectors, Till 2035
- 11.8.1. Other Viral Vector Purification Products Market: Distribution by Type of Purification Technique, Till 2035
- 11.8.2. Other Viral Vector Purification Products Market: Distribution by Type of Therapy, Till 2035
- 11.8.3. Other Viral Vector Purification Products Market: Distribution by Therapeutic Area, Till 2035
- 11.8.4. Other Viral Vector Purification Products Market: Distribution by Scale of Operation, Till 2035
- 11.8.5. Other Viral Vector Purification Products Market: Distribution by Geographical Location, Till 2035
12. CONCLUDING REMARKS
13. EXECUTIVE INSIGHTS
- 13.1. Chapter Overview
- 13.2. Company A
- 13.2.1. Company Snapshot
- 13.2.2. Interview Transcript: Chief Executive Officer
- 13.3. Company B
- 13.3.1. Company Snapshot
- 13.3.2. Interview Transcript: Chief Commercial Officer
- 13.4. Company C
- 13.4.1. Company Snapshot
- 13.4.2. Interview Transcript: Chief Scientific Officer


