
|
市場調査レポート
商品コード
1762522
アンチセンスオリゴヌクレオチド市場:業界動向と世界の予測 - アンチセンス分子タイプ別、ASO世代タイプ別、標的適応疾患別、投与経路別、治療タイプ別、地域別Antisense Oligonucleotides Market: Industry Trends and Global Forecasts - Distribution by Type of Antisense Molecule, Type of ASO Generation, Target Disease Indication, Route of Administration, Type of Therapy, and Geography |
||||||
カスタマイズ可能
|
|||||||
| アンチセンスオリゴヌクレオチド市場:業界動向と世界の予測 - アンチセンス分子タイプ別、ASO世代タイプ別、標的適応疾患別、投与経路別、治療タイプ別、地域別 |
|
出版日: 2025年07月04日
発行: Roots Analysis
ページ情報: 英文 252 Pages
納期: 即日から翌営業日
|
全表示
- 概要
- 図表
- 目次
アンチセンスオリゴヌクレオチド市場:概要
今年度のアンチセンスオリゴヌクレオチドの市場規模は25億米ドルで、予測期間中に15%のCAGRで拡大すると予測されています。
この市場セグメンテーションでは、市場規模と機会を以下のパラメータで区分しています:
アンチセンス分子タイプ
- DNA分子
- RNA分子
ASO世代タイプ
- 第一世代製品
- 第二世代製品
- 第三世代製品
標的適応疾患
- 筋萎縮性側索硬化症
- デュシェンヌ型筋ジストロフィー
- 家族性カイロミクロン血症症候群
- 家族性部分的リポジストロフィー
- 遺伝性トランスサイレチン(hATTR)アミロイドーシス
- ハンチントン病
- リーバー先天性黒内障
- 脊髄性筋萎縮症
投与経路
- 髄腔内投与
- 静脈内投与
- 硝子体内療法
- 皮下療法
- 開口部内治療
治療タイプ
- 併用療法
- 単剤治療
地域
- 北米
- 欧州
- アジア太平洋
- その他の地域
アンチセンス・オリゴヌクレオチド市場:成長と動向
オリゴヌクレオチドは、15~20個のヌクレオチド残基からなる短い一本鎖DNAまたはRNA分子です。現代のバイオ医薬品において、これらのオリゴヌクレオチドの用途は、遺伝学的検査、基礎的な生体分子研究、法医学的分析(これらに限定されるものではない)を含む広大なものです。アンチセンスオリゴヌクレオチドは、多様なオリゴヌクレオチドの一種であり、標的mRNAに特異的に結合する短い一本鎖のRNA/DNA分子であり、様々なメカニズムを通じてタンパク質の発現を変化させる能力を有します。
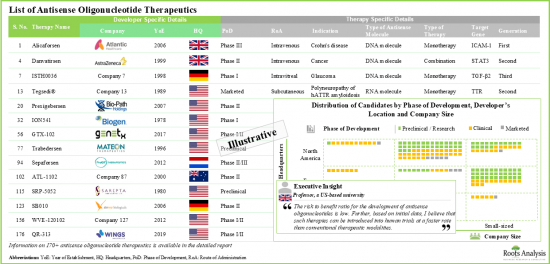
アンチセンス治療薬は、タンパク質の産生を損ない、ヒトゲノムの特定の標的遺伝子の機能を阻害する最も有望な薬剤のひとつと考えられています。現在、このメカニズムは、腫瘍性疾患、遺伝性疾患、肝疾患、呼吸器疾患、感染症など、さまざまな疾患の治療のために、さまざまな段階の臨床試験で研究されている多くの治療薬の基礎となっています。実際、最近の動向では、オリゴヌクレオチド医薬品の開発者は、コロナウイルス(COVID-19)に対するオリゴヌクレオチド介入の妥当性についても研究しています。アンチセンスオリゴヌクレオチド市場における技術革新と開発のペースを考えると、アンチセンスオリゴヌクレオチドは予測される将来において主要な治療手段となることが期待できます。
アンチセンスオリゴヌクレオチド市場:主要インサイト
当レポートでは、アンチセンスオリゴヌクレオチド市場の現状を調査し、潜在的な成長機会を特定しています。当レポートの主な調査結果は以下の通りです。
- アンチセンスオリゴヌクレオチドの潜在的な治療効果を評価するため、現在世界中で約30の企業が幅広い適応症の治療に取り組んでいます。
- パイプラインには開発段階の異なる170以上の治療薬候補があり、単剤または他の治療薬との併用で評価されています。
- 承認された治療薬や後期段階の候補品の大部分は、遺伝性疾患、神経疾患、腫瘍性疾患の治療を目的としています。
- アンチセンスオリゴヌクレオチドの利点を考慮すると、これらの介入は主に単剤療法として評価されています。単剤療法として研究されている後期段階の薬剤にはTofersenとPelacarsenがあります。
- 70近いアンチセンスオリゴヌクレオチドベースの治療候補を評価する臨床試験施設には18,000人以上の患者が登録されています。
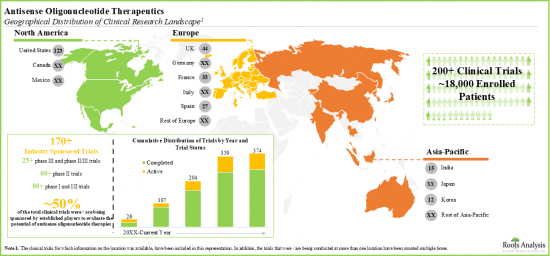
- アンチセンスオリゴヌクレオチド治療薬の大部分は皮下投与用にデザインされていますが、様々なドラッグデリバリーシステムを用いて患者が自己投与することも可能です。
- この分野の研究活動を支援するために、いくつかの組織が財政的支援を行っています。現在、主に神経疾患の治療薬の調査に重点が置かれています。
- この分野の利害関係者(米国)に授与された助成金の数は、ここ数年増加の一途をたどっており、総額の70%以上が研究プロジェクトに授与されています。
- この分野にはNIHの様々な管理機関が関与しているが、中でもNINDS、NHLBI、NCIの参加が比較的目立ちます。
- この分野への関心の高まりは、さまざまな利害関係者がさまざまな応用分野で結んだ提携の数にも表れています。
- 20近い分子が開発後期段階にあることから、企業は主に製品開発と商業化を目的として共同研究を行っています。
- 既存企業も新規参入企業も、過去には戦略的パートナーシップを結んでおり、これらの契約は主に遺伝性疾患と神経疾患について結ばれています。
- 市場化された治療薬や後期段階の治療薬の販売別収益という点で、将来的な機会はさまざまな疾患領域、分子の種類、主要な地理的地域にうまく分散されると予想されます。
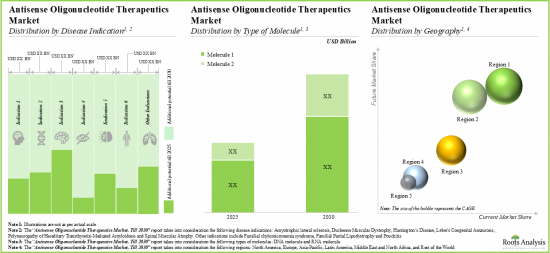
- 同市場は今後10年にわたって安定した成長を遂げる可能性が高く、その機会は異なる世代、投与経路、様々なタイプの治療薬に分散すると思われます。
アンチセンスオリゴヌクレオチド市場の参入企業例
- Antisense Therapeutics
- Biogen
- Bio-Path Holdings
- Ionis Pharmaceuticals
- ProQR Therapeutics
- Sarepta Therapeutics
- Sterna Biologicals
- Wave Life Sciences
目次
第1章 序文
第2章 エグゼクティブサマリー
第3章 イントロダクション
- 章の概要
- オリゴヌクレオチドおよび関連医薬品の概要
- オリゴヌクレオチドのタイプ
- オリゴヌクレオチド治療薬
- RNA干渉治療薬
- アンチセンスオリゴヌクレオチド治療薬
- 将来の展望
第4章 アンチセンスオリゴヌクレオチド治療薬:市場情勢
- 章の概要
- アンチセンスオリゴヌクレオチド治療薬:パイプラインレビュー
- アンチセンス分子タイプ別分析
- ASO世代別分析
- 開発段階別分析
- 標的遺伝子別解析
- 標的適応疾患別分析
- 治療領域別分析
- 投与経路別分析
- 治療法別の分析
- アンチセンスオリゴヌクレオチド治療薬:開発者一覧
- 設立年別分析
- 企業規模別分析
第4章 本社所在地別分析
第5章 企業プロファイル
- 章の概要
- Antisense Therapeutics
- Biogen
- Bio-Path Holdings
- Ionis Pharmaceuticals
- ProQR Therapeutics
- Sarepta Therapeutics
- Sterna Biologicals
- Wave Life Sciences
第6章 臨床試験の分析
- 章の概要
- 範囲と調査手法
- アンチセンスオリゴヌクレオチド治療薬:臨床試験分析
第7章 学術助成金分析
- 章の概要
- 範囲と調査手法
- アンチセンスオリゴヌクレオチド治療薬:学術助成金の分析
第8章 パートナーシップとコラボレーション
- 章の概要
- パートナーシップモデル
- アンチセンスオリゴヌクレオチド治療薬:提携・協力一覧
第9章 市場予測と機会分析
- 章の概要
- 予測調査手法と主要な前提条件
- 2035年までの世界のアンチセンスオリゴヌクレオチド治療薬市場
- 2035年までの世界のアンチセンスオリゴヌクレオチド治療薬市場:個別製品の売上予測
- 2035年までの世界のアンチセンスオリゴヌクレオチド治療薬市場:アンチセンス分子タイプ別
- 2035年までの世界のアンチセンスオリゴヌクレオチド治療薬市場:ASO世代別
- 2035年までの世界のアンチセンスオリゴヌクレオチド治療薬市場:標的適応疾患別
- 2035年までの世界のアンチセンスオリゴヌクレオチド治療薬市場:投与経路別
- 2035年までの世界のアンチセンスオリゴヌクレオチド治療薬市場:治療タイプ別
- 2035年までの世界のアンチセンスオリゴヌクレオチド市場:地域別
第10章 ケーススタディ:オリゴヌクレオチド製造業者と精製サービス
- 章の概要
- 調査および診断用途に特化したオリゴヌクレオチド製造業者のリスト
- 治療用途に特化したオリゴヌクレオチド製造業者一覧
第11章 結論
第12章 付録1表形式データ
第13章 付録2企業・団体一覧
List of Tables
- Table 4.1 Antisense Oligonucleotide Therapeutics: List of Drugs
- Table 4.2 Antisense Oligonucleotide Therapeutics: List of Developers
- Table 5.1 Antisense Oligonucleotide Therapeutics: List of Companies Profiled
- Table 5.2 Antisense Therapeutics: Company Snapshot
- Table 5.3 Drug Profile: ATL1102
- Table 5.4 Drug Profile: ATL1103
- Table 5.5 Antisense Therapeutics: Recent Developments and Future Outlook
- Table 5.6 Biogen: Company Snapshot
- Table 5.7 Drug Profile: Tofersen
- Table 5.8 Drug Profile: ION541
- Table 5.9 Biogen: Recent Developments and Future Outlook
- Table 5.10 Bio-Path Holdings: Company Snapshot
- Table 5.11 Drug Profile: Prexigebersen
- Table 5.12 Drug Profile: BP1002
- Table 5.13 Drug Profile: BP1003
- Table 5.14 Bio-Path Holdings: Recent Developments and Future Outlook
- Table 5.15 Ionis Pharmaceuticals: Company Snapshot
- Table 5.16 Drug Profile: Spinraza(R)
- Table 5.17 Drug Profile: Tegsedi(R)
- Table 5.18 Drug Profile: Waylivra(R)
- Table 5.19 Drug Profile: AKCEA-APOCIII-LRx
- Table 5.20 Drug Profile: IONIS-PKK-LRx
- Table 5.21 Drug Profile: IONIS-PKK-Rx
- Table 5.22 Drug Profile: IONIS-ENAC-2.5Rx
- Table 5.23 Drug Profile: IONIS-FB-LRx
- Table 5.24 Drug Profile: IONIS-AGT-LRx
- Table 5.25 Drug Profile: AKCEA-TTR-LRx
- Table 5.26 Drug Profile: IONIS-GHR-LRx
- Table 5.27 Drug Profile: ION253
- Table 5.28 Drug Profile: IONIS-TMPRSS6-LRx
- Table 5.29 Drug Profile: ION736
- Table 5.30 Drug Profile: IONIS-AR-2.5Rx
- Table 5.31 Drug Profile: ION224
- Table 5.32 Drug Profile: ISIS-FGFR4RX
- Table 5.33 Drug Profile: ISIS-GCCRRx
- Table 5.34 Drug Profile: IONIS-GCGRRx
- Table 5.35 Drug Profile: IONIS-HBV-LRx
- Table 5.36 Drug Profile: ION251
- Table 5.37 Drug Profile: ION 449
- Table 5.38 Drug Profile: BIIB078
- Table 5.39 Drug Profile: BIIB094
- Table 5.40 Drug Profile: BIIB101
- Table 5.41 Drug Profile: ISIS-EIF4ERx
- Table 5.42 Drug Profile: BIIB080
- Table 5.43 Drug Profile: ION663
- Table 5.44 Drug Profile: ION674
- Table 5.45 Drug Profile: ION537
- Table 5.46 Drug Profile: ION929
- Table 5.47 Drug Profile: ION363
- Table 5.48 Drug Profile: ION373
- Table 5.49 Drug Profile: ION283
- Table 5.50 Drug Profile: ION260
- Table 5.51 Drug Profile: ION581
- Table 5.52 Drug Profile: ION716
- Table 5.53 Drug Profile: ION904
- Table 5.54 Drug Profile: ION547
- Table 5.55 Ionis Pharmaceuticals: Recent Developments and Future Outlook
- Table 5.56 ProQR Therapeutics: Company Snapshot
- Table 5.57 Drug Profile: Sepofarsen
- Table 5.58 Drug Profile: QR-421a
- Table 5.59 Drug Profile: QR-1123
- Table 5.60 Drug Profile: QR-411
- Table 5.61 Drug Profile: QR-504a
- Table 5.62 Drug Profile: QRX-704
- Table 5.63 Drug Profile: QR-1011
- Table 5.64 Drug Profile: QRX-461
- Table 5.65 Drug Profile: QRX-136
- Table 5.66 Drug Profile: QRX-1204
- Table 5.67 ProQR Therapeutics: Recent Developments and Future Outlook
- Table 5.68 Sarepta Therapeutics: Company Snapshot
- Table 5.69 Drug Profile: Exondys 51
- Table 5.70 Drug Profile: Vyondys 53
- Table 5.71 Drug Profile: Amondys 45
- Table 5.72 Drug Profile: SRP-5051
- Table 5.73 Drug Profile: Exon 52
- Table 5.74 Drug Profile: Exon 43
- Table 5.75 Drug Profile: Exon 44
- Table 5.76 Drug Profile: Exon 50
- Table 5.77 Drug Profile: Exon 55
- Table 5.78 Drug Profile: SRP-5052
- Table 5.79 Drug Profile: SRP-5053
- Table 5.80 Drug Profile: SRP-5044
- Table 5.81 Drug Profile: SRP-5045
- Table 5.82 Drug Profile: SRP-5050
- Table 5.83 Sarepta Therapeutics: Recent Developments and Future Outlook
- Table 5.84 Sterna Biologicals: Company Snapshot
- Table 5.85 Drug Profile: SB010
- Table 5.86 Drug Profile: SB011
- Table 5.87 Drug Profile: SB012
- Table 5.88 Sterna Biologicals: Recent Developments and Future Outlook
- Table 5.89 Wave Life Sciences: Company Snapshot
- Table 5.90 Drug Profile: WVE-120102
- Table 5.91 Drug Profile: Suvodirsen
- Table 5.92 Drug Profile: WVE-004
- Table 5.93 Drug Profile: WVE-003
- Table 5.94 Drug Profile: WVE-N531
- Table 5.95 Drug Profile: Undisclosed Drug 1
- Table 5.96 Drug Profile: Undisclosed Drug 2
- Table 5.97 Drug Profile: Undisclosed Drug 3
- Table 5.98 Drug Profile: Undisclosed Drug 4
- Table 5.99 Drug Profile: Undisclosed Drug 5
- Table 5.100 Drug Profile: Undisclosed Drug 6
- Table 5.101 Wave Life Sciences: Recent Developments and Future Outlook
- Table 8.1 Antisense Oligonucleotide Therapeutics: List of Partnerships and Collaborations
- Table 9.1 Antisense Oligonucleotide Therapeutics: Summary of the Competitive Insights
- Table 10.1 Oligonucleotide Manufacturers Focused on Research and Diagnostic Applications: List of Industry Players
- Table 10.2 Oligonucleotide Manufacturers Focused on Therapeutic Applications: List of Industry Players
- Table 12.1 Antisense Oligonucleotide Therapeutics: Distribution by Type of Antisense Molecule
- Table 12.2 Antisense Oligonucleotide Therapeutics: Distribution by ASO Generation
- Table 12.3 Antisense Oligonucleotide Therapeutics: Distribution by Phase of Development
- Table 12.4 Antisense Oligonucleotide Therapeutics: Distribution by Target Genes
- Table 12.5 Antisense Oligonucleotide Therapeutics: Distribution by Target Indications
- Table 12.6 Antisense Oligonucleotide Therapeutics: Distribution by Therapeutic Areas
- Table 12.7 Antisense Oligonucleotide Therapeutics: Distribution by Route of Administration
- Table 12.8 Antisense Oligonucleotide Therapeutics: Distribution by Type of Therapy
- Table 12.9 Antisense Oligonucleotide Therapeutic Developers: Distribution by Year of Establishment
- Table 12.10 Antisense Oligonucleotide Therapeutic Developers: Distribution by Company Size
- Table 12.11 Antisense Oligonucleotide Therapeutic Developers: Distribution by Location of Headquarters
- Table 12.12 Biogen: Annual Revenues, Since 2015 (USD Million)
- Table 12.13 Ionis Pharmaceuticals: Annual Revenues, Since 2015 (USD Million)
- Table 12.14 Sarepta Therapeutics: Annual Revenues, Since 2015 (USD Million)
- Table 12.15 Wave Life Sciences: Annual Revenues, Since 2015 (USD Million)
- Table 12.16 Clinical Trial Analysis: Cumulative Distribution of Trials by Registration Year
- Table 12.17 Clinical Trial Analysis: Distribution by Trial Phase
- Table 12.18 Clinical Trial Analysis: Distribution by Trial Recruitment Status
- Table 12.19 Clinical Trial Analysis: Distribution by Trial Registration Year and Number of Patients Enrolled
- Table 12.20 Clinical Trial Analysis: Distribution by Study Design
- Table 12.21 Clinical Trial Analysis: Distribution by Type of Sponsor / Collaborator
- Table 12.22 Leading Players: Distribution by Number of Registered Trials
- Table 12.23 Clinical Trial Analysis: Distribution by Target Therapeutic Area
- Table 12.24 Clinical Trial Analysis: Distribution by Trial Registration Year and Target Gene
- Table 12.25 Popular Indications: Distribution by Number of Registered Trials
- Table 12.26 Popular Interventions: Distribution by Number of Registered Trials
- Table 12.27 Clinical Trial Analysis: Geographical Distribution by Number of Registered Trials
- Table 12.28 Clinical Trial Analysis: Geographical Distribution by Number of Patients Enrolled
- Table 12.29 Grant Analysis: Cumulative Trend by Year of Grant Award, Since 2017
- Table 12.30 Grant Analysis: Cumulative Distribution by Amount Awarded, Since 2017 (USD Million)
- Table 12.31 Grant Analysis: Distribution by Administering Institute Center
- Table 12.32 Grant Analysis: Distribution by Support Period
- Table 12.33 Grant Analysis: Distribution by Administering Institute Center and Support Period
- Table 12.34 Grant Analysis: Distribution by Type of Grant Application
- Table 12.35 Grant Analysis: Distribution by Purpose of Grant Award
- Table 12.36 Grant Analysis: Distribution by Activity Code
- Table 12.37 Grant Analysis: Distribution by Study Section Involved
- Table 12.38 Grant Analysis: Distribution by Type of Recipient Organization
- Table 12.39 Grant Analysis: Geographical Distribution of Recipient Organizations
- Table 12.40 Popular Therapeutic Areas: Distribution by Number of Grants
- Table 12.41 Popular NIH Departments: Distribution by Number of Grants
- Table 12.42 Prominent Program Officers: Distribution by Number of Grants
- Table 12.43 Popular Recipient Organizations: Distribution by Number of Grants
- Table 12.44 Partnerships and Collaborations: Cumulative Year-wise Trend, Since 2016
- Table 12.45 Partnerships and Collaborations: Distribution by Type of Partnership
- Table 12.46 Partnerships and Collaborations: Distribution by Type of Partnership and Generation of Antisense Molecule Involved
- Table 12.47 Partnerships and Collaborations: Distribution by Type of Partnership and Target Therapeutic Area
- Table 12.48 Partnerships and Collaborations: Distribution by Year of Partnership and Type of Partner
- Table 12.49 Partnerships and Collaborations: Distribution by Type of Partnership and Type of Partner
- Table 12.50 Most Active Players: Distribution by Number of Partnerships
- Table 12.51 Partnerships and Collaborations: Regional Distribution
- Table 12.52 Global Antisense Oligonucleotide Therapeutics Market, Till 2035 (USD Million)
- Table 12.53 Alicaforsen: Sales Forecast, Till 2035 (USD Million)
- Table 12.54 Eteplirsen: Sales Forecast, Till 2035 (USD Million)
- Table 12.55 Golodirsen: Sales Forecast, Till 2035 (USD Million)
- Table 12.56 Inotersen: Sales Forecast, Till 2035 (USD Million)
- Table 12.57 Sepofarsen: Sales Forecast, Till 2035 (USD Million)
- Table 12.58 Tofersen: Sales Forecast, Till 2035 (USD Million)
- Table 12.59 Tominersen: Sales Forecast, Till 2035 (USD Million)
- Table 12.60 Viltolarsen: Sales Forecast, Till 2035 (USD Million)
- Table 12.61 Volanesorsen: Sales Forecast, Till 2035 (USD Million)
- Table 12.62 Global Antisense Oligonucleotide Therapeutics Market, Till 2035: Distribution by Type of Antisense Molecule (USD Million)
- Table 12.63 Global Antisense Oligonucleotide Therapeutics Market for RNA Molecules, Till 2035 (USD Million)
- Table 12.64 Global Antisense Oligonucleotide Therapeutics Market for DNA Molecules, Till 2035 (USD Million)
- Table 12.65 Global Antisense Oligonucleotide Therapeutics Market, Till 2035: Distribution by ASO Generation (USD Million)
- Table 12.66 Global Antisense Oligonucleotide Therapeutics Market for First-generation Products, Till 2035 (USD Million)
- Table 12.67 Global Antisense Oligonucleotide Therapeutics Market for Second-generation Products, Till 2035 (USD Million)
- Table 12.68 Global Antisense Oligonucleotide Therapeutics Market for Third-generation Products, Till 2035 (USD Million)
- Table 12.69 Global Antisense Oligonucleotide Therapeutics Market, Till 2035: Distribution by Target Disease Indication (USD Million)
- Table 12.70 Global Antisense Oligonucleotide Therapeutics Market for Amyotrophic Lateral Sclerosis, Till 2035 (USD Million)
- Table 12.71 Global Antisense Oligonucleotide Therapeutics Market for Duchenne Muscular Dystrophy, Till 2035 (USD Million)
- Table 12.72 Global Antisense Oligonucleotide Therapeutics Market for Familial Chylomicronemia Syndrome, Till 2035 (USD Million)
- Table 12.73 Global Antisense Oligonucleotide Therapeutics Market for Familial Partial Lipodystrophy, Till 2035 (USD Million)
- Table 12.74 Global Antisense Oligonucleotide Therapeutics Market for Hereditary Transthyretin-Mediated (hATTR) Amyloidosis, Till 2035 (USD Million)
- Table 12.75 Global Antisense Oligonucleotide Therapeutics Market for Huntington's Disease, Till 2035 (USD Million)
- Table 12.76 Global Antisense Oligonucleotide Therapeutics Market for Leber's Congenital Amaurosis, Till 2035 (USD Million)
- Table 12.77 Global Antisense Oligonucleotide Therapeutics Market for Pouchitis, Till 2035 (USD Million)
- Table 12.78 Global Antisense Oligonucleotide Therapeutics Market for Spinal Muscular Atrophy, Till 2035 (USD Million)
- Table 12.79 Global Antisense Oligonucleotide Therapeutics Market, Till 2035: Distribution by Route of Administration (USD Million)
- Table 12.80 Global Antisense Oligonucleotide Therapeutics Market for Intrathecal Therapies, Till 2035 (USD Million)
- Table 12.81 Global Antisense Oligonucleotide Therapeutics Market for Intravenous Therapies, Till 2035 (USD Million)
- Table 12.82 Global Antisense Oligonucleotide Therapeutics Market for Intravitreal Therapies, Till 2035 (USD Million)
- Table 12.83 Global Antisense Oligonucleotide Therapeutics Market for Subcutaneous Therapies, Till 2035 (USD Million)
- Table 12.84 Global Antisense Oligonucleotide Therapeutics Market for Intraorifice Therapies, Till 2035 (USD Million)
- Table 12.85 Global Antisense Oligonucleotide Therapeutics Market, Till 2035: Distribution by Type of Therapy (USD Million)
- Table 12.86 Global Antisense Oligonucleotide Therapeutics Market for Combination Therapies Till 2035 (USD Million)
- Table 12.87 Global Antisense Oligonucleotide Therapeutics Market for Monotherapies, Till 2035 (USD Million)
- Table 12.88 Global Antisense Oligonucleotide Market, Till 2035: Distribution by Geography (USD Million)
- Table 12.89 Antisense Oligonucleotide Market in the US, Till 2035 (USD Million)
- Table 12.90 Antisense Oligonucleotide Market in Canada, Till 2035 (USD Million)
- Table 12.91 Antisense Oligonucleotide Market in the UK, Till 2035 (USD Million)
- Table 12.92 Antisense Oligonucleotide Market in Germany, Till 2035 (USD Million)
- Table 12.93 Antisense Oligonucleotide Market in France, Till 2035 (USD Million)
- Table 12.94 Antisense Oligonucleotide Market in Italy, Till 2035 (USD Million)
- Table 12.95 Antisense Oligonucleotide Market in Spain, Till 2035 (USD Million)
- Table 12.96 Antisense Oligonucleotide Market in Australia, Till 2035 (USD Million)
- Table 12.97 Antisense Oligonucleotide Market in Japan, Till 2035 (USD Million)
- Table 12.98 Antisense Oligonucleotide Market in Korea, Till 2035 (USD Million)
- Table 12.99 Antisense Oligonucleotide Market in Brazil, Till 2035 (USD Million)
- Table 12.100 Antisense Oligonucleotide Market in Israel, Till 2035 (USD Million)
- Table 12.101 Oligonucleotide Manufacturers Focused on Research and Diagnostic Applications: Distribution by Year of Establishment
- Table 12.102 Oligonucleotide Manufacturers Focused on Research and Diagnostic Applications: Distribution by Company Size
- Table 12.103 Oligonucleotide Manufacturers Focused on Research and Diagnostic Applications: Distribution by Scale of Operation
- Table 12.104 Oligonucleotide Manufacturers Focused on Research and Diagnostic Applications: Distribution by Location of Headquarters
- Table 12.105 Oligonucleotide Manufacturers Focused on Research and Diagnostic Applications: Distribution by Type of Purification Method Used
- Table 12.106 Oligonucleotide Manufacturers Focused on Therapeutic Applications: Distribution by Year of Establishment
- Table 12.107 Oligonucleotide Manufacturers Focused on Therapeutic Applications: Distribution by Company Size
- Table 12.108 Oligonucleotide Manufacturers Focused on Therapeutic Applications: Distribution by Scale of Operation
- Table 12.109 Oligonucleotide Manufacturers Focused on Therapeutic Applications: Distribution by Location of Headquarters
- Table 12.110 Oligonucleotide Manufacturers Focused on Therapeutic Applications: Distribution by Type of Purification Method Used
List of Figures
- Figure 3.1 Classification of Oligonucleotides
- Figure 3.2 Key Components of RNA interference
- Figure 3.3 Mechanism of RNA interference
- Figure 3.4 Key Features of Antisense Oligonucleotides
- Figure 3.5 Mechanism of Antisense Oligonucleotides
- Figure 4.1 Antisense Oligonucleotide Therapeutics: Distribution by Type of Antisense Molecule
- Figure 4.2 Antisense Oligonucleotide Therapeutics: Distribution by ASO Generation
- Figure 4.3 Antisense Oligonucleotide Therapeutics: Distribution by Phase of Development
- Figure 4.4 Antisense Oligonucleotide Therapeutics: Distribution by Target Genes
- Figure 4.5 Antisense Oligonucleotide Therapeutics: Distribution by Target Indications
- Figure 4.6 Antisense Oligonucleotide Therapeutics: Distribution by Therapeutic Areas
- Figure 4.7 Antisense Oligonucleotide Therapeutics: Distribution by Route of Administration
- Figure 4.8 Antisense Oligonucleotide Therapeutics: Distribution by Type of Therapy
- Figure 4.9 Antisense Oligonucleotide Therapeutic Developers: Distribution by Year of Establishment
- Figure 4.10 Antisense Oligonucleotide Therapeutic Developers: Distribution by Company Size
- Figure 4.11 Antisense Oligonucleotide Therapeutic Developers: Distribution by Location of Headquarters
- Figure 4.12 Grid Analysis: Distribution by Phase of Development of Therapeutics, Company Size and Location of Headquarters
- Figure 5.1 Biogen: Annual Revenues, Since 2015 (USD Million)
- Figure 5.2 Ionis Pharmaceuticals: Annual Revenues, Since 2015 (USD Million)
- Figure 5.3 Sarepta Therapeutics: Annual Revenues, Since 2015 (USD Million)
- Figure 5.4 Wave Life Sciences: Annual Revenues, Since 2015 (USD Million)
- Figure 6.1 Clinical Trial Analysis: Cumulative Distribution of Trials by Registration Year
- Figure 6.2 Clinical Trial Analysis: Distribution by Trial Phase
- Figure 6.3 Clinical Trial Analysis: Distribution by Trial Recruitment Status
- Figure 6.4 Clinical Trial Analysis: Distribution by Trial Registration Year and Number of Patients Enrolled
- Figure 6.5 Clinical Trial Analysis: Distribution by Study Design
- Figure 6.6 Clinical Trial Analysis: Distribution by Type of Sponsor / Collaborator
- Figure 6.7 Leading Players: Distribution by Number of Registered Trials
- Figure 6.8 Word Cloud: Key Focus Areas
- Figure 6.9 Clinical Trial Analysis: Distribution by Target Therapeutic Area
- Figure 6.10 Clinical Trial Analysis: Distribution by Trial Registration Year and Target Gene
- Figure 6.11 Popular Indications: Distribution by Number of Registered Trials
- Figure 6.12 Popular Interventions: Distribution by Number of Registered Trials
- Figure 6.13 Clinical Trial Analysis: Geographical Distribution by Number of Registered Trials
- Figure 6.14 Clinical Trial Analysis: Geographical Distribution by Number of Patients Enrolled
- Figure 7.1 Grant Analysis: Cumulative Trend by Year of Grant Award, Since 2017
- Figure 7.2 Grant Analysis: Cumulative Distribution by Amount Awarded (USD Million), Since 2017
- Figure 7.3 Grant Analysis: Distribution by Administering Institute Center
- Figure 7.4 Grant Analysis: Distribution by Support Period
- Figure 7.5 Grant Analysis: Distribution by Administering Institute Center and Support Period
- Figure 7.6 Grant Analysis: Distribution by Type of Grant Application
- Figure 7.7 Grant Analysis: Distribution by Purpose of Grant Award
- Figure 7.8 Grant Analysis: Distribution by Activity Code
- Figure 7.9 Grant Analysis: Distribution by Study Section Involved
- Figure 7.10 Grant Analysis: Distribution by Type of Recipient Organization
- Figure 7.11 Word Cloud Analysis: Emerging Focus Areas
- Figure 7.12 Grant Analysis: Geographical Distribution of Recipient Organizations
- Figure 7.13 Popular Therapeutic Areas: Distribution by Number of Grants
- Figure 7.14 Popular NIH Departments: Distribution by Number of Grants
- Figure 7.15 Prominent Program Officers: Distribution by Number of Grants
- Figure 7.16 Popular Recipient Organizations: Distribution by Number of Grants
- Figure 8.1 Partnerships and Collaborations: Cumulative Year-wise Trend, Since 2016
- Figure 8.2 Partnerships and Collaborations: Distribution by Type of Partnership
- Figure 8.3 Partnerships and Collaborations: Distribution by Type of Partnership and Generation of Antisense Molecule Involved
- Figure 8.4 Partnerships and Collaborations: Distribution by Type of Partnership and Target Therapeutic Area
- Figure 8.5 Partnerships and Collaborations: Distribution by Year of Partnership and Type of Partner
- Figure 8.6 Partnerships and Collaborations: Distribution by Type of Partnership and Type of Partner
- Figure 8.7 Most Active Players: Distribution by Number of Partnerships
- Figure 8.8 Partnerships and Collaborations: Regional Distribution
- Figure 8.9 Partnerships and Collaborations: Intercontinental and Intracontinental Agreements
- Figure 9.1 Global Antisense Oligonucleotide Therapeutics Market, Till 2035 (USD Million)
- Figure 9.2 Alicaforsen: Sales Forecast, Till 2035 (USD Million)
- Figure 9.3 Eteplirsen: Sales Forecast, Till 2035 (USD Million)
- Figure 9.4 Golodirsen: Sales Forecast, Till 2035 (USD Million)
- Figure 9.5 Inotersen: Sales Forecast, Till 2035 (USD Million)
- Figure 9.6 Sepofarsen: Sales Forecast, Till 2035 (USD Million)
- Figure 9.7 Tofersen: Sales Forecast, Till 2035 (USD Million)
- Figure 9.8 Tominersen: Sales Forecast, Till 2035 (USD Million)
- Figure 9.9 Viltolarsen: Sales Forecast, Till 2035 (USD Million)
- Figure 9.10 Volanesorsen: Sales Forecast, Till 2035 (USD Million)
- Figure 9.11 Global Antisense Oligonucleotide Therapeutics Market, Till 2035: Distribution by Type of Antisense Molecule (USD Million)
- Figure 9.12 Global Antisense Oligonucleotide Therapeutics Market for RNA Molecules, Till 2035 (USD Million)
- Figure 9.13 Global Antisense Oligonucleotide Therapeutics Market for DNA Molecules, Till 2035 (USD Million)
- Figure 9.14 Global Antisense Oligonucleotide Therapeutics Market, Till 2035: Distribution by ASO Generation (USD Million)
- Figure 9.15 Global Antisense Oligonucleotide Therapeutics Market for First-generation Products, Till 2035 (USD Million)
- Figure 9.16 Global Antisense Oligonucleotide Therapeutics Market for Second-generation Products, Till 2035 (USD Million)
- Figure 9.17 Global Antisense Oligonucleotide Therapeutics Market for Third-generation Products, Till 2035 (USD Million)
- Figure 9.18 Global Antisense Oligonucleotide Therapeutics Market, Till 2035: Distribution by Target Disease Indication (USD Million)
- Figure 9.19 Global Antisense Oligonucleotide Therapeutics Market for Amyotrophic Lateral Sclerosis, Till 2035 (USD Million)
- Figure 9.20 Global Antisense Oligonucleotide Therapeutics Market for Duchenne Muscular Dystrophy, Till 2035 (USD Million)
- Figure 9.21 Global Antisense Oligonucleotide Therapeutics Market for Familial Chylomicronemia Syndrome, Till 2035 (USD Million)
- Figure 9.22 Global Antisense Oligonucleotide Therapeutics Market for Familial Partial Lipodystrophy, Till 2035 (USD Million)
- Figure 9.23 Global Antisense Oligonucleotide Therapeutics Market for Hereditary Transthyretin-Mediated (hATTR) Amyloidosis, Till 2035 (USD Million)
- Figure 9.24 Global Antisense Oligonucleotide Therapeutics Market for Huntington's Disease, Till 2035 (USD Million)
- Figure 9.25 Global Antisense Oligonucleotide Therapeutics Market for Leber's Congenital Amaurosis, Till 2035 (USD Million)
- Figure 9.26 Global Antisense Oligonucleotide Therapeutics Market for Pouchitis, Till 2035 (USD Million)
- Figure 9.27 Global Antisense Oligonucleotide Therapeutics Market for Spinal Muscular Atrophy, Till 2035 (USD Million)
- Figure 9.28 Global Antisense Oligonucleotide Therapeutics Market, Till 2035: Distribution by Route of Administration (USD Million)
- Figure 9.29 Global Antisense Oligonucleotide Therapeutics Market for Intrathecal Therapies, Till 2035 (USD Million)
- Figure 9.30 Global Antisense Oligonucleotide Therapeutics Market for Intravenous Therapies, Till 2035 (USD Million)
- Figure 9.31 Global Antisense Oligonucleotide Therapeutics Market for Intravitreal Therapies, Till 2035 (USD Million)
- Figure 9.32 Global Antisense Oligonucleotide Therapeutics Market for Subcutaneous Therapies, Till 2035 (USD Million)
- Figure 9.33 Global Antisense Oligonucleotide Therapeutics Market for Intraorifice Therapies, Till 2035 (USD Million)
- Figure 9.34 Global Antisense Oligonucleotide Therapeutics Market, Till 2035: Distribution by Type of Therapy (USD Million)
- Figure 9.35 Global Antisense Oligonucleotide Therapeutics Market for Combination Therapies Till 2035 (USD Million)
- Figure 9.36 Global Antisense Oligonucleotide Therapeutics Market for Monotherapies, Till 2035 (USD Million)
- Figure 9.37 Global Antisense Oligonucleotide Market, Till 2035: Distribution by Geography (USD Million)
- Figure 9.38 Antisense Oligonucleotide Market in the US, Till 2035 (USD Million)
- Figure 9.39 Antisense Oligonucleotide Market in Canada, Till 2035 (USD Million)
- Figure 9.40 Antisense Oligonucleotide Market in the UK, Till 2035 (USD Million)
- Figure 9.41 Antisense Oligonucleotide Market in Germany, Till 2035 (USD Million)
- Figure 9.42 Antisense Oligonucleotide Market in France, Till 2035 (USD Million)
- Figure 9.43 Antisense Oligonucleotide Market in Italy, Till 2035 (USD Million)
- Figure 9.44 Antisense Oligonucleotide Market in Spain, Till 2035 (USD Million)
- Figure 9.45 Antisense Oligonucleotide Market in Australia, Till 2035 (USD Million)
- Figure 9.46 Antisense Oligonucleotide Market in Japan, Till 2035 (USD Million)
- Figure 9.47 Antisense Oligonucleotide Market in Korea, Till 2035 (USD Million)
- Figure 9.48 Antisense Oligonucleotide Market in Brazil, Till 2035 (USD Million)
- Figure 9.49 Antisense Oligonucleotide Market in Israel, Till 2035 (USD Million)
- Figure 10.1 Oligonucleotide Manufacturers Focused on Research and Diagnostic Applications: Distribution by Year of Establishment
- Figure 10.2 Oligonucleotide Manufacturers Focused on Research and Diagnostic Applications: Distribution by Company Size
- Figure 10.3 Oligonucleotide Manufacturers Focused on Research and Diagnostic Applications: Distribution by Scale of Operation
- Figure 10.4 Oligonucleotide Manufacturers Focused on Research and Diagnostic Applications: Distribution by Location of Headquarters
- Figure 10.5 Oligonucleotide Manufacturers Focused on Research and Diagnostic Applications: Distribution by Type of Purification Method Used
- Figure 10.6 Oligonucleotide Manufacturers Focused on Therapeutic Applications: Distribution by Year of Establishment
- Figure 10.7 Oligonucleotide Manufacturers Focused on Therapeutic Applications: Distribution by Company Size
- Figure 10.8 Oligonucleotide Manufacturers Focused on Therapeutic Applications: Distribution by Scale of Operation
- Figure 10.9 Oligonucleotide Manufacturers Focused on Therapeutic Applications: Distribution by Location of Headquarters
- Figure 10.10 Oligonucleotide Manufacturers Focused on Therapeutic Applications: Distribution by Type of Purification Method Used
ANTISENSE OLIGONUCLEOTIDES MARKET: OVERVIEW
As per Roots Analysis, the global antisense oligonucleotides market valued at USD 2.5 billion in the current year is anticipated to grow at a lucrative CAGR of 15% during the forecast period.
The market sizing and opportunity analysis has been segmented across the following parameters:
Type of Antisense Molecule
- DNA Molecules
- RNA Molecules
Type of ASO Generation
- First-Generation Products
- Second-Generation Products
- Third-Generation Products
Target Disease Indication
- Amyotrophic Lateral Sclerosis
- Duchenne Muscular Dystrophy
- Familial Chylomicronemia Syndrome
- Familial Partial Lipodystrophy
- Hereditary Transthyretin-Mediated (hATTR) Amyloidosis
- Huntington's Disease
- Leber's Congenital Amaurosis
- Spinal Muscular Atrophy
Route of Administration
- Intrathecal Therapies
- Intravenous Therapies
- Intravitreal Therapies
- Subcutaneous Therapies
- Intraorifice Therapies
Type of Therapy
- Combination Therapies
- Monotherapies
Geography
- North America
- Europe
- Asia-Pacific
- Rest of the World
ANTISENSE OLIGONUCLEOTIDES MARKET: GROWTH AND TRENDS
Oligonucleotides are short single stranded DNA or RNA molecules, that comprise 15-20 nucleotide residues. In modern biopharmaceuticals, the applications of these oligonucleotides are vast, including (but not limited to) genetic testing, fundamental biomolecular research, and forensic analysis. Antisense oligonucleotides, a diverse class of oligonucleotides are short, single-stranded RNA / DNA molecules specifically binding to the target mRNA and have the ability to modify protein expression through a variety of mechanisms.

Antisense therapeutics are considered to be one of the most promising agents for impairing protein production and blocking the function of the specific target gene of interest in the human genome. Presently, this mechanism forms the basis for many therapeutics being investigated in different stages of clinical trials for treatment of a variety of disorders, including oncological disorders, genetic diseases, hepatic diseases, respiratory disorders and infectious diseases. In fact, in the recent past, the oligonucleotide drug developers had also investigated the relevance of these interventions against the Coronavirus (COVID-19). Given the pace of innovation and developments in the antisense oligonucleotides market, we can expect antisense oligonucleotides to become a major therapeutic modality in the foreseen future.
ANTISENSE OLIGONUCLEOTIDES MARKET: KEY INSIGHTS
The report delves into the current state of the antisense oligonucleotides market and identifies potential growth opportunities within industry. Some key findings from the report include:
- Around 30 players from are presently engaged in evaluating the potential therapeutic benefits of antisense oligonucleotides for the treatment of a wide range of disease indications, worldwide.
- The pipeline features 170+ candidate therapies in different stages of development, being evaluated either as monotherapies or in combination with other interventions; most of these products are administered parenterally.
- Majority of the approved therapies and late-stage candidates are intended for the treatment of genetic disorders, neurological disorders and oncological disorders.
- Given the advantages of antisense oligonucleotides, these interventions are primarily evaluated as monotherapy. Late-stage drugs being investigated as monotherapy include Tofersen and Pelacarsen.
- Over 18,000 patients have been enrolled in clinical trial sites evaluating close to 70 antisense oligonucleotide-based therapy candidates.

- Majority of the antisense oligonucleotide therapeutics are designed for subcutaneous administration; these can be self-administered by the patients using different drug delivery systems.
- Several organizations have extended financial support to aid research efforts in this domain; currently, the focus, in terms of funds disbursed, is primarily in support of investigations of drugs for treating neurological conditions.
- The number of grants awarded to stakeholders in this domain (in the US) has continuously increased in the past few years; more than 70% of the total amount was awarded for research projects.
- The field has witnessed the involvement of various administering institutes of the NIH; of all the institutes, participation of the NINDS, NHLBI, and NCI has been relatively more prominent.
- The rising interest in this field is reflected in the number of partnerships inked by the various stakeholders across different application areas.
- Given that nearly 20 molecules are in the late stages of development, companies have primarily collaborated for product development and commercialization purposes.
- Both established players and the new entrants have forged strategic partnerships in the recent past; these deals have primarily been inked for genetic and neurological disorders.
- The future opportunity, in terms of revenues from the sales of marketed and late-stage therapies, is anticipated to be well distributed across different disease areas, types of molecules and key geographical regions.

- The market is likely to witness steady growth over the coming decade; the opportunity will be dispersed across different generations, routes of administration and various types of therapies.
Example Players in the Antisense Oligonucleotides Market
- Antisense Therapeutics
- Biogen
- Bio-Path Holdings
- Ionis Pharmaceuticals
- ProQR Therapeutics
- Sarepta Therapeutics
- Sterna Biologicals
- Wave Life Sciences
ANTISENSE OLIGONUCLEOTIDES MARKET: RESEARCH COVERAGE
- Market Sizing and Opportunity Analysis: The report features an in-depth analysis of the global antisense oligonucleotides market, focusing on key market segments, including [A] type of antisense molecule, [B] type of ASO generation, [C] target disease indication, [D] route of administration, [E] type of therapy and [F] geography.
- Market Landscape: A comprehensive evaluation of antisense oligonucleotide therapeutics, based on several relevant parameters, such as [A] type of antisense molecule, [B] ASO generation, [C] phase of development of lead candidates, [D] target genes, [E] target disease indications, [F] target therapeutic areas, [G] route of administration and [H] type of therapy. Additionally, a comprehensive evaluation of drug developers, based on several relevant parameters, such as [A] year of establishment, [B] company size and [C] location of headquarters.
- Company Profiles: In-depth profiles of antisense oligonucleotide therapeutic developers, focusing on [A] overview of the company, [B] financial information (if available), [C] product portfolio and [D] recent developments and an informed future outlook.
- Clinical Trial Analysis: An insightful analysis of clinical trials related to antisense oligonucleotide therapeutics, based on several parameters, such as [A] trial registration year, [B] trial phase, [C] trial recruitment status, [D] enrolled patient population, [E] study design, leading industry sponsors / collaborators (in terms of number of trials conducted), [F] trial focus, [G] target therapeutic area and [H] target genes.
- Grants Analysis: A comprehensive assessment of grants that have been awarded to research institutes for antisense oligonucleotide therapeutic projects, based on various relevant parameters, such as [A] year of grant award, [B] amount awarded, [C] administering institute center, [D] support period, [E] type of grant application, [F] purpose of grant award, [G] activity code, [H] study section involved, [I] type of recipient organizations and [J] focus area. Additionally, a comprehensive assessment of grants focusing on, [A] geographical distribution of recipient organizations, [B] popular therapeutic areas, [C] popular funding institute centers, [D] prominent program officers and [E] popular recipient organizations.
- Partnerships and Collaborations: An insightful analysis of the deals inked by stakeholders in the antisense oligonucleotide market, based on several parameters, such as [A] year of partnership, [B] type of partnership, [C] most active players (in terms of number of partnerships signed) and [D] regional analysis.
- Case Study: A detailed discussion on the oligonucleotide CMOs and purification service providers, highlighting information on the [A] year of establishment, [B] company size, [C] scale of operation, [D] location of headquarters and [E] type of purification method used.
KEY QUESTIONS ANSWERED IN THIS REPORT
- How many companies are currently engaged in this market?
- Which are the leading companies in this market?
- What factors are likely to influence the evolution of this market?
- What is the current and future market size?
- What is the CAGR of this market?
- How is the current and future market opportunity likely to be distributed across key market segments?
REASONS TO BUY THIS REPORT
- The report provides a comprehensive market analysis, offering detailed revenue projections of the overall market and its specific sub-segments. This information is valuable to both established market leaders and emerging entrants.
- Stakeholders can leverage the report to gain a deeper understanding of the competitive dynamics within the market. By analyzing the competitive landscape, businesses can make informed decisions to optimize their market positioning and develop effective go-to-market strategies.
- The report offers stakeholders a comprehensive overview of the market, including key drivers, barriers, opportunities, and challenges. This information empowers stakeholders to stay abreast of market trends and make data-driven decisions to capitalize on growth prospects.
ADDITIONAL BENEFITS
- Complimentary PPT Insights Packs
- Complimentary Excel Data Packs for all Analytical Modules in the Report
- 15% Free Content Customization
- Detailed Report Walkthrough Session with Research Team
- Free Updated report if the report is 6-12 months old or older
TABLE OF CONTENTS
1. PREFACE
- 1.1. Scope of the Report
- 1.2. Research Methodology
- 1.2.1. Research Assumptions
- 1.2.2. Project Methodology
- 1.2.3. Forecast Methodology
- 1.2.4. Robust Quality Control
- 1.2.5. Key Considerations
- 1.2.5.1. Demographics
- 1.2.5.2. Economic Factors
- 1.2.5.3. Government Regulations
- 1.2.5.4. Supply Chain
- 1.2.5.5. COVID Impact / Related Factors
- 1.2.5.6. Market Access
- 1.2.5.7. Healthcare Policies
- 1.2.5.8. Industry Consolidation
- 1.3 Key Questions Answered
- 1.4. Chapter Outlines
2. EXECUTIVE SUMMARY
3. INTRODUCTION
- 3.1. Chapter Overview
- 3.2. Overview on Oligonucleotides and Affiliated Medical Products
- 3.2.1. Types of Oligonucleotides
- 3.2.1.1. Antisense Oligonucleotides (ASOs)
- 3.2.1.2. Aptamers
- 3.2.1.3. miRNA
- 3.2.1.4. shRNA
- 3.2.1.5. siRNA
- 3.2.1.6. Other Oligonucleotides
- 3.2.1. Types of Oligonucleotides
- 3.3. Oligonucleotide Therapeutics
- 3.3.1. RNA-Interference Therapeutics
- 3.3.1.1. Components of RNA-Interference Therapeutics
- 3.3.1.2. Mechanism of RNA-Interference Therapeutics
- 3.3.2. Antisense Oligonucleotide Therapeutics
- 3.3.2.1. Mechanism of Antisense Oligonucleotide Therapeutics
- 3.3.2.2. Types of Antisense Oligonucleotide Therapeutics
- 3.3.1. RNA-Interference Therapeutics
- 3.4. Future Perspectives
4. ANTISENSE OLIGONUCLEOTIDE THERAPEUTICS: MARKET LANDSCAPE
- 4.1. Chapter Overview
- 4.2. Antisense Oligonucleotide Therapeutics: Pipeline Review
- 4.2.1. Analysis by Type of Antisense Molecule
- 4.2.2. Analysis by ASO Generation
- 4.2.3. Analysis by Phase of Development
- 4.2.4. Analysis by Target Genes
- 4.2.5. Analysis by Target Indications
- 4.2.6. Analysis by Therapeutic Areas
- 4.2.7. Analysis by Route of Administration
- 4.2.8. Analysis by Type of Therapy
- 4.3. Antisense Oligonucleotide Therapeutics: List of Developers
- 4.3.1. Analysis by Year of Establishment
- 4.3.2. Analysis by Company Size
4. 3.3. Analysis by Location of Headquarters
- 4.4. Grid Analysis: Distribution by Phase of Development, Company Size and Location of Headquarters
5. COMPANY PROFILES
- 5.1. Chapter Overview
- 5.2. Antisense Therapeutics
- 5.2.1. Company Overview
- 5.2.2. Antisense Oligonucleotide Therapeutics Portfolio
- 5.2.3. Recent Developments and Future Outlook
- 5.3. Biogen
- 5.3.1. Company Overview
- 5.3.2. Financial Information
- 5.3.3. Antisense Oligonucleotide Therapeutics Portfolio
- 5.3.4. Recent Developments and Future Outlook
- 5.4. Bio-Path Holdings
- 5.4.1. Company Overview
- 5.4.2. Antisense Oligonucleotide Therapeutics Portfolio
- 5.4.3. Recent Developments and Future Outlook
- 5.5. Ionis Pharmaceuticals
- 5.5.1. Company Overview
- 5.5.2. Financial Information
- 5.5.3. Antisense Oligonucleotide Therapeutics Portfolio
- 5.5.4. Recent Developments and Future Outlook
- 5.6. ProQR Therapeutics
- 5.6.1. Company Overview
- 5.6.2. Antisense Oligonucleotide Therapeutics Portfolio
- 5.6.3. Recent Developments and Future Outlook
- 5.7. Sarepta Therapeutics
- 5.7.1. Company Overview
- 5.7.2. Financial Information
- 5.7.3. Antisense Oligonucleotide Therapeutics Portfolio
- 5.7.4. Recent Developments and Future Outlook
- 5.8. Sterna Biologicals
- 5.8.1. Company Overview
- 5.8.2. Antisense Oligonucleotide Therapeutics Portfolio
- 5.8.3. Recent Developments and Future Outlook
- 5.9. Wave Life Sciences
- 5.9.1. Company Overview
- 5.9.2. Financial Information
- 5.9.3. Antisense Oligonucleotide Therapeutics Portfolio
- 5.9.4. Recent Developments and Future Outlook
6. CLINICAL TRIAL ANALYSIS
- 6.1. Chapter Overview
- 6.2. Scope and Methodology
- 6.3. Antisense Oligonucleotide Therapeutics: Clinical Trial Analysis
- 6.3.1. Analysis by Trial Registration Year
- 6.3.2. Analysis by Trial Phase
- 6.3.3. Analysis by Trial Recruitment Status
- 6.3.4. Analysis by Trial Registration Year and Number of Patients Enrolled
- 6.3.5. Analysis by Study Design
- 6.3.6. Analysis by Type of Sponsor / Collaborator
- 6.3.7. Leading Players: Analysis by Number of Registered Trials
- 6.3.8. Word Cloud: Key Focus Areas
- 6.3.9. Analysis by Target Therapeutic Area
- 6.3.10. Analysis by Trial Registration Year and Target Gene
- 6.3.11. Popular Indications: Analysis by Number of Registered Trials
- 6.3.12. Popular Interventions: Analysis by Number of Registered Trials
- 6.3.13. Geographical Analysis by Number of Registered Trials
- 6.3.14. Geographical Analysis by Number of Patients Enrolled
7. ACADEMIC GRANTS ANALYSIS
- 7.1. Chapter Overview
- 7.2. Scope and Methodology
- 7.3. Antisense Oligonucleotide Therapeutics: Analysis of Academic Grants
- 7.3.1. Analysis by Year of Grant Award
- 7.3.2. Analysis by Amount Awarded
- 7.3.3. Analysis by Administering Institute Center
- 7.3.4. Analysis by Support Period
- 7.3.5. Analysis by Administering Institute Center and Support Period
- 7.3.6. Analysis by Type of Grant Application
- 7.3.7. Analysis by Purpose of Grant Award
- 7.3.8. Analysis by Activity Code
- 7.3.9. Analysis by Study Section Involved
- 7.3.10. Analysis by Type of Recipient Organization
- 7.3.11. Word Cloud Analysis: Emerging Focus Areas
- 7.3.12. Geographical Distribution of Recipient Organizations
- 7.3.13. Popular Therapeutic Areas: Analysis by Number of Grants
- 7.3.14. Popular NIH Departments: Analysis by Number of Grants
- 7.3.15. Prominent Program Officers: Analysis by Number of Grants
- 7.3.16. Popular Recipient Organizations: Analysis by Number of Grants
8. PARTNERSHIPS AND COLLABORATIONS
- 8.1. Chapter Overview
- 8.2. Partnership Models
- 8.3. Antisense Oligonucleotide Therapeutics: List of Partnerships and Collaborations
- 8.3.1. Analysis by Year of Partnership
- 8.3.2. Analysis by Type of Partnership
- 8.3.3. Analysis by Type of Partnership and Generation of Antisense Molecule Involved
- 8.3.4. Analysis by Type of Partnership and Target Therapeutic Area
- 8.3.5. Analysis by Year of Partnership and Type of Partner
- 8.3.6. Analysis by Type of Partnership and Type of Partner
- 8.3.7. Most Active Players: Analysis by Number of Partnerships
- 8.3.8. Regional Analysis
- 8.3.8.1. Intercontinental and Intracontinental Agreements
9. MARKET FORECAST AND OPPORTUNITY ANALYSIS
- 9.1. Chapter Overview
- 9.2. Forecast Methodology and Key Assumptions
- 9.3. Global Antisense Oligonucleotide Therapeutics Market, Till 2035
- 9.4. Global Antisense Oligonucleotide Therapeutics Market, Till 2035: Individual Product Sales Forecasts
- 9.4.1. Alicaforsen (Atlantic Healthcare)
- 9.4.1.1. Target Patient Population
- 9.4.1.2. Sales Forecast
- 9.4.2. Eteplirsen (Sarepta Therapeutics)
- 9.4.2.1. Target Patient Population
- 9.4.2.2. Sales Forecast
- 9.4.3. Golodirsen (Sarepta Therapeutics)
- 9.4.3.1. Target Patient Population
- 9.4.3.2. Sales Forecast
- 9.4.4. Inotersen (Ionis Pharmaceuticals)
- 9.4.4.1. Target Patient Population
- 9.4.4.2. Sales Forecast
- 9.4.5. Sepofarsen (ProQR Therapeutics)
- 9.4.5.1. Target Patient Population
- 9.4.5.2. Sales Forecast
- 9.4.6. Tofersen (Biogen)
- 9.4.6.1. Target Patient Population
- 9.4.6.2. Sales Forecast
- 9.4.7. Tominersen (Roche)
- 9.4.7.1. Target Patient Population
- 9.4.7.2. Sales Forecast
- 9.4.8. Viltolarsen (Nippon Shinyaku)
- 9.4.8.1. Target Patient Population
- 9.4.8.2. Sales Forecast
- 9.4.9. Volanesorsen (Ionis Pharmaceuticals)
- 9.4.9.1. Target Patient Population
- 9.4.9.2. Sales Forecast
- 9.4.1. Alicaforsen (Atlantic Healthcare)
- 9.5. Global Antisense Oligonucleotide Therapeutics Market, Till 2035: Distribution by Type of Antisense Molecule
- 9.5.1. Global Antisense Oligonucleotide Therapeutics Market for RNA Molecules, Till 2035
- 9.5.2. Global Antisense Oligonucleotide Therapeutics Market for DNA Molecules, Till 2035
- 9.6. Global Antisense Oligonucleotide Therapeutics Market, Till 2035: Distribution by ASO Generation
- 9.6.1. Global Antisense Oligonucleotide Therapeutics Market for First-generation Products, Till 2035
- 9.6.2. Global Antisense Oligonucleotide Therapeutics Market for Second-generation Products, Till 2035
- 9.6.3. Global Antisense Oligonucleotide Therapeutics Market for Third-generation Products, Till 2035
- 9.7. Global Antisense Oligonucleotide Therapeutics Market, Till 2035: Distribution by Target Disease Indication
- 9.7.1. Global Antisense Oligonucleotide Therapeutics Market for Amyotrophic Lateral Sclerosis, Till 2035
- 9.7.2. Global Antisense Oligonucleotide Therapeutics Market for Duchenne Muscular Dystrophy, Till 2035
- 9.7.3. Global Antisense Oligonucleotide Therapeutics Market for Familial Chylomicronemia Syndrome, Till 2035
- 9.7.4. Global Antisense Oligonucleotide Therapeutics Market for Familial Partial Lipodystrophy, Till 2035
- 9.7.5. Global Antisense Oligonucleotide Therapeutics Market for Hereditary Transthyretin-Mediated (hATTR) Amyloidosis, Till 2035
- 9.7.6. Global Antisense Oligonucleotide Therapeutics Market for Huntington's Disease, Till 2035
- 9.7.7. Global Antisense Oligonucleotide Therapeutics Market for Leber's Congenital Amaurosis, Till 2035
- 9.7.8. Global Antisense Oligonucleotide Therapeutics Market for Pouchitis, Till 2035
- 9.7.9. Global Antisense Oligonucleotide Therapeutics Market for Spinal Muscular Atrophy, Till 2035
- 9.8. Global Antisense Oligonucleotide Therapeutics Market, Till 2035: Distribution by Route of Administration
- 9.8.1. Global Antisense Oligonucleotide Therapeutics Market for Intrathecal Therapies, Till 2035
- 9.8.2. Global Antisense Oligonucleotide Therapeutics Market for Intravenous Therapies, Till 2035
- 9.8.3. Global Antisense Oligonucleotide Therapeutics Market for Intravitreal Therapies, Till 2035
- 9.8.4. Global Antisense Oligonucleotide Therapeutics Market for Subcutaneous Therapies, Till 2035
- 9.8.5. Global Antisense Oligonucleotide Therapeutics Market for Intraorifice Therapies, Till 2035
- 9.9. Global Antisense Oligonucleotide Therapeutics Market, Till 2035: Distribution by Type of Therapy
- 9.9.1. Global Antisense Oligonucleotide Therapeutics Market for Combination Therapies Till 2035
- 9.9.2. Global Antisense Oligonucleotide Therapeutics Market for Monotherapies, Till 2035
- 9.10. Global Antisense Oligonucleotide Market, Till 2035: Geographical Distribution
- 9.10.1. Antisense Oligonucleotide Market in the US, Till 2035
- 9.10.2. Antisense Oligonucleotide Market in Canada, Till 2035
- 9.10.3. Antisense Oligonucleotide Market in the UK, Till 2035
- 9.10.4. Antisense Oligonucleotide Market in Germany, Till 2035
- 9.10.5. Antisense Oligonucleotide Market in France, Till 2035
- 9.10.6. Antisense Oligonucleotide Market in Italy, Till 2035
- 9.10.7. Antisense Oligonucleotide Market in Spain, Till 2035
- 9.10.8. Antisense Oligonucleotide Market in Australia, Till 2035
- 9.10.9. Antisense Oligonucleotide Market in Japan, Till 2035
- 9.10.10. Antisense Oligonucleotide Market in Korea, Till 2035
- 9.10.11. Antisense Oligonucleotide Market in Brazil, Till 2035
- 9.10.12. Antisense Oligonucleotide Market in Israel, Till 2035
10. CASE STUDY: OLIGONUCLEOTIDE MANUFACTURERS AND PURIFICATION SERVICES
- 10.1. Chapter Overview
- 10.2. List of Oligonucleotide Manufacturers Focused on Research and Diagnostic Applications
- 10.2.1. Analysis by Year of Establishment
- 10.2.2. Analysis by Company Size
- 10.2.3. Analysis by Scale of Operation
- 10.2.4. Analysis by Location of Headquarters
- 10.2.5. Analysis by Type of Purification Method Used
- 10.3. List of Oligonucleotide Manufacturers Focused on Therapeutic Applications
- 10.3.1. Analysis by Year of Establishment
- 10.3.2. Analysis by Company Size
- 10.3.3. Analysis by Scale of Operation
- 10.3.4. Analysis by Location of Headquarters
- 10.3.5. Analysis by Type of Purification Method Used


