
|
市場調査レポート
商品コード
1737059
臨床試験ソフトウェア市場:展開タイプ別、提供タイプ別、ソフトウェア機能別、エンドユーザー別、地域別Clinical Trial Software Market Distribution by Type of Deployment, Type of Delivery, Features of Software and Geographical Regions |
||||||
カスタマイズ可能
|
|||||||
| 臨床試験ソフトウェア市場:展開タイプ別、提供タイプ別、ソフトウェア機能別、エンドユーザー別、地域別 |
|
出版日: 2025年05月26日
発行: Roots Analysis
ページ情報: 英文 141 Pages
納期: 即日から翌営業日
|
全表示
- 概要
- 図表
- 目次
世界の臨床試験ソフトウェアの市場規模は、2035年までの予測期間中に14%のCAGRで拡大し、現在の6億9,000万米ドルから2035年までに36億8,000万米ドルに成長すると予測されています。
市場セグメンテーションでは、市場規模および市場機会を以下のパラメータで区分しています:
展開タイプ別
- オンクラウド
- オンプレミス
提供タイプ別
- ウェブベース
- リモートモニタリング
ソフトウェア機能別
- EDC
- eCOA/ePRO
- eConsent
エンドユーザー別
- 製薬/バイオテクノロジー産業
- 学術・研究機関
- その他の産業
地域別
- 北米
- 欧州
- アジア太平洋
臨床試験ソフトウェア市場:成長と動向
臨床試験とは、人に対する医学的、外科的、行動学的介入を評価し、疾病の診断と予防/治療のための新規アプローチを調査するためにデザインされた前向き生物医学研究です。新規の治療介入について規制当局から販売承認を得るためには、特定の適応症に対する薬剤の安全性と有効性を検証するために、非常に正確で精巧な臨床試験データが必要となります。しかし、この伝統的な臨床調査方法にはいくつかの課題があります。その中には、設備投資の高さ、患者の採用率の低さ、様々な治療介入の開発に携わる企業が直面する確実なデータの欠如などがあります。このため、十分な臨床エビデンスの創出が非効率となり、医薬品開発企業だけでなく、救命治療にアクセスする患者にとっても莫大な資本損失となります。実際、手作業による従来の臨床試験には、医薬品開発中のほぼ50%の時間が費やされています。このような状況の中、臨床試験ソフトウェアは、リアルタイムの分析、データ管理、薬剤の有害影響の追跡が可能であることから、大きな注目を集めています。
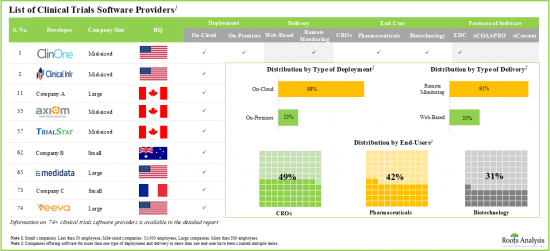
最近の動向では、様々な企業がEDC、eCOA/ePRO、eConsentを含む多くの臨床試験ソフトウェア/臨床試験管理システムを開発しています。これらのツールはリアルタイムの臨床試験を可能にし、薬物検査と分析のための患者のコンプライアンスを向上させる。その重要性から、複数の臨床試験ソフトウェア市場プレーヤーが現在この分野に参入し、革新的な技術を開発しています。特に、これらの技術は臨床試験の成果を促し、製薬会社の負担を軽減します。自動化された臨床試験ソフトウェアに対する需要が増加していることから、同市場は予測期間中に大幅な成長が見込まれます。
臨床試験ソフトウェア市場:主要インサイト
当レポートでは、臨床試験ソフトウェア市場の現状を掘り下げ、業界内の潜在的な成長機会を特定しています。主な調査結果は以下の通り:
- 世界の70以上の企業が、臨床調査方法の分散化を可能にするソフトウェアソリューションを開発し、臨床試験に費やす時間とコストを最適化していると主張しています。
- 現在の市場情勢は非常に断片化されており、先進的な機能を備えた臨床試験ソフトウェアを提供する既存企業と新規参入企業の両方が存在しています。実際、2000年以降、臨床試験ソフトウェアの開発に注力する新興企業が51社以上設立されています。
- 競合優位性を確立するため、各社は既存の機能を積極的に拡張し、それぞれの提供サービスを強化し、進化する業界ベンチマークに対応しています。
- 有利なリターンを期待して、多くの公的・私的投資家が多額の投資を行っており、資金調達活動が急増しています。特に北米では、この分野での資金調達事例の約90%となっています。
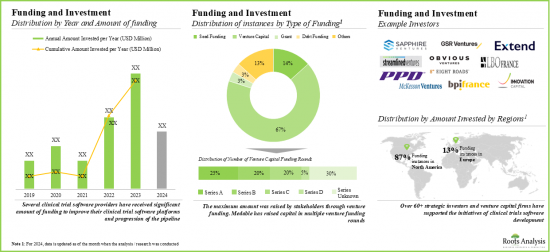
- この分野では過去数年間、パートナーシップやコラボレーションが大幅に増加しており、利害関係者の関心が高まっていることを示しています。
- 注目すべきは、M&A事例のほとんどが2023年に報告されていることで、徐々に統合へとシフトしていることを示しています。さらに、契約の大半(87%)は買収であり、次いで合併でした。
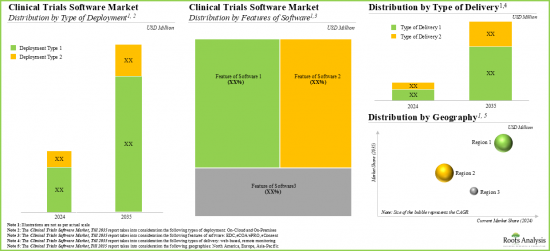
- 同市場は今後10年間、年率14%で成長すると予想されます。この機会は、展開のタイプ、ソフトウェア機能、提供のタイプ、地理的地域によってうまく分散される可能性が高いです。
臨床試験ソフトウェア市場:主要セグメント
eConsentソフトウェアセグメントが世界の臨床試験ソフトウェア市場で最大シェアを占める
ソフトウェア機能別では、臨床試験ソフトウェアの世界市場はEDC、eCOA/ePRO、eConsentソフトウェアに区分されます。現在、臨床試験ソフトウェア市場の大半はeConsentソフトウェアが占めています。eCOA/ePROセグメントの世界臨床試験ソフトウェア市場は、比較的高いCAGRで成長する可能性が高いことは注目に値します。
地域別に見ると、市場は北米、欧州、アジア太平洋に区分されます。現在のシナリオでは、北米が最大の市場シェアを獲得し、アジア太平洋の市場は予測期間中に有利な成長を示すと予想されています。
当レポートでは、世界の臨床試験ソフトウェア市場について調査し、市場の概要とともに、展開タイプ別、提供タイプ別、ソフトウェア機能別、エンドユーザー別、地域別の動向、および市場に参入する企業のプロファイルなどを提供しています。
目次
第1章 序文
第2章 エグゼクティブサマリー
第3章 イントロダクション
- 章の概要
- 臨床研究における既存の制約
- バーチャル臨床試験
- バーチャル臨床試験管理に関連する機会と課題
- 将来の展望
第4章 市場情勢:臨床試験ソフトウェア市場
- 章の概要
- 臨床試験ソフトウェア市場:製品リスト
- 臨床試験ソフトウェア市場:開発者の情勢
第5章 北米の臨床試験ソフトウェア開発会社:企業プロファイル
- 章の概要
- Advarra
- Arisglobal
- AssistRx
- Clario
- IBM
- IQVIA
- Medidata
- Oracle
- Signant Health
- Veeva
第6章 欧州の臨床試験ソフトウェア開発企業:企業プロファイル
- 章の概要
- Calyx
第7章 企業競争力分析
第8章 パートナーシップとコラボレーション
第9章 合併と買収
第10章 資金調達と投資分析
第11章 市場予測と機会分析
- 章の概要
- 予測調査手法と主要な前提条件
- 世界の臨床試験ソフトウェア市場、2021年~2035年
- 臨床試験ソフトウェア市場、2021年~2035年:展開タイプ別分布
- 臨床試験ソフトウェア市場、2021年~2035年:提供タイプ別分布
- 臨床試験ソフトウェア市場、2021年~2035年:ソフトウェア機能別分布
- 臨床試験ソフトウェア市場、2021年~2035年:地域別分布
第12章 結論
第13章 付録1:表形式のデータ
第14章 付録2:企業・団体一覧
List of Tables
- Table 4.1 Clinical Trials Software Market: Information on Type of Deployment, Type of Delivery, Type of End-User, Type of features of Software, Type of Trial Design and Type of Technology
- Table 4.2 Clinical Trials Software Market Developers: Information of Year of Establishment, Company Size and Location of Headquarters
- Table 4.3 Clinical Trials Software Market Developers: List of Software and Developers
- Table 6.1 Clinical Trials Software Market: List of Developers in North America
- Table 6.2 Advarra: Company Snapshot
- Table 6.3 Advarra: Product Portfolio
- Table 6.4 Advarra: Recent Developments and Future Outlook
- Table 6.5 ArisGlobal: Company Snapshot
- Table 6.6 ArisGlobal: Product Portfolio
- Table 6.7 ArisGlobal: Recent Developments and Future Outlook
- Table 6.8 AssistRx: Company Snapshot
- Table 6.9 AssistRx: Product Portfolio
- Table 6.10 AssistRx: Recent Developments and Future Outlook
- Table 6.11 Clario: Company Snapshot
- Table 6.12 Clario: Product Portfolio
- Table 6.13 Clario: Recent Developments and Future Outlook
- Table 6.14 IBM: Company Snapshot
- Table 6.15 IBM: Product Portfolio
- Table 6.16 IBM: Recent Developments and Future Outlook
- Table 6.17 IQVIA: Company Snapshot
- Table 6.18 IQVIA: Product Portfolio
- Table 6.19 IQVIA: Recent Developments and Future Outlook
- Table 6.20 Medidata: Company Snapshot
- Table 6.21 Medidata: Product Portfolio
- Table 6.22 Medidata: Recent Developments and Future Outlook
- Table 6.23 Oracle: Company Snapshot
- Table 6.24 Oracle: Product Portfolio
- Table 6.25 Oracle: Recent Developments and Future Outlook
- Table 6.26 Signant Health: Company Snapshot
- Table 6.27 Signant Health: Product Portfolio
- Table 6.28 Signant Health: Recent Developments and Future Outlook
- Table 6.29 Veeva: Company Snapshot
- Table 6.30 Veeva: Product Portfolio
- Table 6.31 Veeva: Recent Developments and Future Outlook
- Table 6.32 Oracle: Company Snapshot
- Table 6.33 Oracle: Product Portfolio
- Table 6.34 Oracle: Recent Developments and Future Outlook
- Table 7.1 Clinical Trials Software Market: List of developers in Europe
- Table 7.2 Calyx: Company Snapshot
- Table 7.3 Calyx: Product Portfolio
- Table 7.4 Calyx: Recent Developments and Future Outlook
- Table 9.1 Clinical Trials Software Market: List of Partnerships and Collaborations, 2016- 2021 (till September)
- Table 10.1 Clinical Trials Software Market: List of Mergers and Acquisitions, 2016-2021 (till September)
- Table 11.1 Clinical Trials Software Market: List of Funding Instances, 2016-2021 (till September)
List of Figures
- Figure 2.1 Executive Summary: Current Market Landscape of Clinical Trials Software Market
- Figure 2.2 Executive Summary: Partnerships and Collaborations
- Figure 2.3 Executive Summary: Funding and Investment
- Figure 2.4 Executive Summary: Market Forecast and Opportunity Analysis
- Figure 4.1 Clinical Trials Software: Distribution by Type of Deployment
- Figure 4.2 Clinical Trials Software: Distribution by Type of Delivery
- Figure 4.3 Clinical Trials Software: Distribution by Type of End-User
- Figure 4.4 Clinical Trials Software: Distribution by Features of Software
- Figure 4.5 Clinical Trials Software: Distribution by Trial Design
- Figure 4.6 Clinical Trials Software: Distribution by Type of Technology
- Figure 4.7 Clinical Trials Software Developers: Distribution by Year of Establishment
- Figure 4.8 Clinical Trials Software Developers: Distribution by Company Size
- Figure 4.9 Clinical Trials Software Developers: Distribution by Geography
- Figure 7.1 Company Competitiveness Analysis: Clinical Trials Software Developers in North America
- Figure 7.2 Company Competitiveness Analysis: Clinical Trials Software Developers in Europe
- Figure 7.3 Company Competitiveness Analysis: Clinical Trials Software Developers in Asia Pacific
- Figure 8.1 Partnerships and Collaborations: Distribution by Year of Partnership
- Figure 8.2 Partnerships and Collaborations: Distribution by Type of Partnership
- Figure 8.3 Partnerships and Collaborations: Distribution by Year and Type of Partnership
- Figure 8.4 Partnerships and Collaborations: Geographical Analysis
- Figure 8.5 Partnerships and Collaborations: Distribution by Intracontinental and Intercontinental Agreement
- Figure 8.6 Most Active Players: Distribution by Number of Partnerships
- Figure 9.1 Mergers and Acquisitions: Distribution by Type of Merger and Acquisition
- Figure 9.2 Mergers and Acquisitions: Distribution by Year of Merger and Acquisition
- Figure 9.3 Mergers and Acquisitions: Distribution by Geographical Activity
- Figure 9.4 Mergers and Acquisitions: Continent-wise Distribution
- Figure 9.5 Mergers and Acquisitions: Intercontinental and Intracontinental Deals
- Figure 9.6 Most Active Players: Distribution by Number of Instances
- Figure 10.1 Funding and Investments: Distribution by Year of Investment
- Figure 10.2 Funding and Investments: Distribution by Amount Invested
- Figure 10.3 Funding and Investments: Distribution by Year-wise Trend of Amount Invested, 2016-2021
- Figure 10.4 Funding and Investments: Distribution by Type of Funding
- Figure 10.5 Funding and Investments: Distribution of Amount Invested by Type of Funding
- Figure 10.6 Most Active Players: Distribution by Amount invested
- Figure 10.7 Funding and Investments: Geographical Distribution by Amount Invested, 2016-2021
- Figure 11.1 Clinical Trials Software Market, 2021-2035 (USD Million)
- Figure 11.2 Clinical Trials Software Market, 2021-2035: Distribution by Type of Deployment (USD Million)
- Figure 11.3 Clinical Trials Software Market, 2021-2035: Distribution by Type of Delivery (USD Million)
- Figure 11.4 Clinical Trials Software Market, 2021-2035: Distribution by Features of Software (USD Million)
CLINICAL TRIAL SOFTWARE MARKET: OVERVIEW
As per Roots Analysis, the global clinical trial software market is estimated to grow from USD 0.69 billion in the current year to USD 3.68 billion by 2035, at a CAGR of 14% during the forecast period, till 2035.
The market sizing and opportunity analysis has been segmented across the following parameters:
Type of Deployment
- On-Cloud
- On-Premises
Type of Delivery
- Web-Based
- Remote Monitoring
Features of Software
- EDC
- eCOA/ePRO
- eConsent
End Users
- Pharmaceutical / Biotechnology Industries
- Academic and Research Institutes
- Other Industries
Geographical Regions
- North America
- Europe
- Asia-Pacific
CLINICAL TRIAL SOFTWARE MARKET: GROWTH AND TRENDS
Clinical trials are prospective biomedical research studies designed to evaluate medical, surgical or behavioral interventions in people and investigate novel approaches for the diagnosis and prevention / treatment of diseases. In order to gain marketing approval from regulatory authorities for a novel therapeutic intervention, highly accurate and elaborate clinical trial data is required to validate the drug's safety and effectiveness towards a specific target indication. However, there are several challenges associated with this traditional way of clinical research. Some of these include high capital investment, low patient recruitment rates and lack of robust data faced by companies involved in the development of various therapeutic interventions. This leads to inefficiency in generating sufficient clinical evidence, resulting in massive capital losses for drug developers, as well as patients accessing these life-saving therapies. In fact, manual conventional clinical trials consume almost 50% of the time during drug development. In this context, clinical trial software has gained significant attention owing to its ability for real-time analysis, data management, and tracking of the adverse impact of drugs.

In recent years, various players have developed a number of clinical trial software / clinical trial management systems, including EDC, eCOA / ePRO, and eConsent. These tools enable real-time clinical studies and improve patient compliance for drug testing and analysis. Owing to its significance, several clinical trial software market players are currently engaged in this field to develop innovative technologies. Notably, these technologies encourage successful outcomes of clinical trials and reduce the burden of pharmaceutical companies. Given the increasing demand for automated clinical trial software, the market is expected to witness substantial growth during the forecast period.
CLINICAL TRIAL SOFTWARE MARKET: KEY INSIGHTS
The report delves into the current state of the clinical trial software market and identifies potential growth opportunities within the industry. Some key findings from the report include:
- 70+ companies worldwide claim to have developed software solutions allowing decentralization of the clinical research process, optimizing time and cost spent on clinical trials.
- The current market landscape is highly fragmented, with the presence of both established players and new entrants offering clinical trials software with advanced features. In fact, since 2000, over 51 start-ups focused on developing clinical trials software have been established.
- In pursuit of building a competitive edge, companies are actively expanding their existing capabilities to enhance their respective offerings and comply with evolving industry benchmarks.
- Foreseeing lucrative returns, many public and private investors have significant investments, marking a surge in funding activity. Notably, North America witnessed around 90% of funding instances in this domain.

- The domain has witnessed a considerable increase in partnerships and collaborations over the past few years, indicating the rising interest of stakeholders.
- Notably, most of the M&A instances were reported in 2023, indicating a gradual shift towards consolidation. Further, majority (87%) of agreements were acquisitions, followed by mergers.
- We expect the market to grow at an annualized rate of 14% in the coming decade; the opportunity is likely to be well distributed across type of deployment, features of software, type of delivery and geographical regions.

CLINICAL TRIAL SOFTWARE MARKET: KEY SEGMENTS
The eConsent Software Segment Holds the Largest Share of the Global Clinical Trial Software Market
Based on the features of software, the global market for clinical trial software is segmented into EDC, eCOA/ePRO and eConsent software. Currently, the majority of the clinical trial software market is captured by eConsent software. It is worth highlighting that the global clinical trial software market for eCOA / ePRO segment is likely to grow at a relatively higher CAGR.
North America Accounts for the Largest Share of the Market
Based on the geographical regions, the market is segmented into North America, Europe and Asia-Pacific. In the current scenario, North America is likely to capture the largest market share while the market in Asia-Pacific is anticipated to demonstrate lucrative growth during the forecast period.
Example Players in the Clinical Trial Software Market
- Advarra
- Arisglobal
- AssistRx
- Calyx
- Clario
- IBM
- IQVIA
- Medidata
- Oracle
- Signant Health
- Veeva
CLINICAL TRIAL SOFTWARE MARKET: RESEARCH COVERAGE
- Market Sizing and Opportunity Analysis: The report features an in-depth analysis of the global clinical trial software market, focusing on key market segments, including [A] type of deployment, [B] type of delivery, [C] features of software, [D] end users and [E] geographical regions.
- Clinical Trials Software Market Landscape: A comprehensive evaluation of clinical trials software market, based on several relevant parameters, such as [A] type of deployment, [B] type of delivery, [C] type of end-user, [D] features of software, [E] trial design and [F] type of technology. Additionally, a comprehensive evaluation of the companies engaged in developing clinical trials software, based on several relevant parameters, such as [G] year of establishment, [H] company and [I] location of headquarters.
- Company Profiles: In-depth profiles of key players engaged in the development of clinical trials software, focusing on [A] overview of the company, [B] product portfolio, and [C] recent developments and [D] an informed future outlook.
- Company Competitiveness Analysis: A comprehensive competitive analysis of clinical trials software developers, examining factors, such as [A] supplier strength, [B] product portfolio strength and [C] service applicability.
- Partnerships and Collaborations: An insightful analysis of the deals inked by stakeholders in the global clinical trials software market, based on several parameters, such as [A] year of partnership, [B] type of partnership, [C] geographical distribution of partnership activity and [D] most active players (in terms of the number of partnerships signed).
- Mergers and Acquisitions: An in-depth analysis of the mergers and acquisitions undertaken in this domain, based on relevant parameters, such as [A] type of agreement, [B] year of mergers and acquisitions, [C] geographical location and [D] most active players (in terms of the number of mergers and acquisitions).
- Funding and Investment Analysis: An in-depth analysis of the fundings raised by companies engaged in this domain, based on relevant parameters, such as [A] year of funding, [B] type of funding, [C] amount invested, [D] most active players and [F] most active investors.
KEY QUESTIONS ANSWERED IN THIS REPORT
- How many companies are currently engaged in this market?
- Which are the leading companies in this market?
- What factors are likely to influence the evolution of this market?
- What is the current and future market size?
- What is the CAGR of this market?
- How is the current and future market opportunity likely to be distributed across key market segments?
REASONS TO BUY THIS REPORT
- The report provides a comprehensive market analysis, offering detailed revenue projections of the overall market and its specific sub-segments. This information is valuable to both established market leaders and emerging entrants.
- Stakeholders can leverage the report to gain a deeper understanding of the competitive dynamics within the market. By analyzing the competitive landscape, businesses can make informed decisions to optimize their market positioning and develop effective go-to-market strategies.
- The report offers stakeholders a comprehensive overview of the market, including key drivers, barriers, opportunities, and challenges. This information empowers stakeholders to stay abreast of market trends and make data-driven decisions to capitalize on growth prospects.
ADDITIONAL BENEFITS
- Complimentary PPT Insights Packs
- Complimentary Excel Data Packs for all Analytical Modules in the Report
- 15% Free Content Customization
- Detailed Report Walkthrough Session with Research Team
- Free Updated report if the report is 6-12 months old or older
TABLE OF CONTENTS
1. PREFACE
- 1.1. Scope of the Report
- 1.2. Research Methodology
- 1.3. Key Questions Answered
- 1.4. Chapter Outlines
2. EXECUTIVE SUMMARY
3. INTRODUCTION
- 3.1. Chapter Overview
- 3.2. Existing Constraints in Clinical Research
- 3.2.1. Increasing Trial Costs and Complexity
- 3.2.2. Evolving Regulatory Standards
- 3.2.3. Patient Recruitment and Retention-Related Challenges
- 3.2.4. Inefficient Data Handling
- 3.3. Virtual Clinical Trials
- 3.3.1. Electronic Data Capture Solutions
- 3.3.2. Electronic Clinical Outcome Assessment and Electronic Patient Reported Outcome Solutions (eCOA / ePRO)
- 3.3.3. Electronic Consent Solutions
- 3.4. Opportunities and challenges associated with Virtual Clinical Trials Management
- 3.5 Future Perspectives
4 MARKET LANDSCAPE: CLINICAL TRIALS SOFTWARE MARKET
- 4.1. Chapter Overview
- 4.2. Clinical Trials Software Market: List of Products
- 4.2.1. Analysis by Type of Deployment
- 4.2.2. Analysis by Type of Delivery
- 4.2.3. Analysis by End-User
- 4.2.4. Analysis by Features of Software
- 4.2.5. Analysis by Trial Design
- 4.2.6. Analysis by Type of Technology
- 4.3. Clinical Trials Software Market: Developer Landscape
- 4.3.1. Analysis by Year of Establishment
- 4.3.2. Analysis by Company Size
- 4.3.3. Analysis by Geography
5 CLINICAL TRIALS SOFTWARE DEVELOPERS IN NORTH AMERICA: COMPANY PROFILES
- 5.1. Chapter Overview
- 5.2. Advarra
- 5.2.1. Company Overview
- 5.2.2. Product Portfolio: Clinical Trials Software
- 5.2.3. Recent Developments and Future Outlook
- 5.3. Arisglobal
- 5.3.1. Company Overview
- 5.3.2. Product Portfolio: Clinical Trials Software
- 5.3.3. Recent Developments and Future Outlook
- 5.4. AssistRx
- 5.4.1. Company Overview
- 5.4.2. Product Portfolio: Clinical Trials Software
- 5.4.3. Recent Developments and Future Outlook
- 5.5. Clario
- 5.5.1. Company Overview
- 5.5.2. Product Portfolio: Clinical Trials Software
- 5.5.3. Recent Developments and Future Outlook
- 5.6. IBM
- 5.6.1. Company Overview
- 5.6.2. Product Portfolio: Clinical Trials Software
- 5.6.3. Recent Developments and Future Outlook
- 5.7. IQVIA
- 5.7.1. Company Overview
- 5.7.2. Product Portfolio: Clinical Trials Software
- 5.7.3. Recent Developments and Future Outlook
- 5.8. Medidata
- 5.8.1. Company Overview
- 5.8.2. Product Portfolio: Clinical Trials Software
- 5.8.3. Recent Developments and Future Outlook
- 5.9. Oracle
- 5.9.1. Company Overview
- 5.9.2. Product Portfolio: Clinical Trials Software
- 5.9.3. Recent Developments and Future Outlook
- 5.10. Signant Health
- 5.10.1. Company Overview
- 5.10.2. Product Portfolio: Clinical Trials Software
- 5.10.3. Recent Developments and Future Outlook
- 5.11. Veeva
- 5.11.1. Company Overview
- 5.11.2. Product Portfolio: Clinical Trials Software
- 5.11.3. Recent Developments and Future Outlook
6 CLINICAL TRIALS SOFTWARE DEVELOPERS IN EUROPE: COMPANY PROFILES
- 6.1. Chapter overview
- 6.2. Calyx
- 6.2.1. Company Overview
- 6.2.2. Product Portfolio: Clinical Trials Software
- 6.2.3. Recent Developments and Future Outlook
7 COMPANY COMPETITIVENESS ANALYSIS
- 7.1. Chapter Overview
- 7.2. Key Parameters and Methodology
- 7.3. Competitiveness Analysis: Companies providing clinical trials software developers
- 7.4. Competitiveness Analysis: Companies providing clinical trials software in North America
- 7.5. Competitiveness Analysis: Companies providing clinical trials software in Europe
- 7.6. Competitiveness Analysis: Companies providing clinical trials software in Asia-Pacific
8 Partnerships and Collaborations
- 8.1. Chapter Overview
- 8.2. Partnership Models
- 8.3. Clinical Trials Software Market: Partnerships and Collaborations
- 8.3.1. Analysis by Year of Partnership
- 8.3.2. Analysis by Type of Partnership
- 8.3.3. Analysis by Type and Year of Partnership
- 8.4. Geographical Analysis
- 8.4.1. Analysis by Intracontinental and Intercontinental Agreements
- 8.4.2. Analysis by Local and International Agreements
- 8.5. Most Active Players: Analysis by Number of Partnerships
9 MERGERS AND ACQUISITIONS
- 9.1. Chapter Overview
- 9.2. Mergers and Acquisitions Models
- 9.3. Clinical Trials Software Market: Mergers and Acquisitions
- 9.3.1. Analysis by Type of Agreement
- 9.3.2. Analysis by Year of Mergers and Acquisitions
- 9.4. Analysis by Geographical Activity
- 9.4.1. Region-wise Analysis
- 9.4.2. Mergers and Acquisitions: Intercontinental and Intracontinental Deals
- 9.5. Most Active Players: Analysis by Number of Instances Acquisitions and Mergers
10 FUNDING AND INVESTMENT ANALYSIS
- 10.1. Chapter Overview
- 10.2. Types of Funding Instances
- 10.3. Clinical Trials Software Market: Recent Funding Instances
- 10.3.1. Analysis by Year of Investment
- 10.3.2. Analysis by Amount Invested
- 10.3.3. Analysis by Type of Funding
- 10.4. Most Active Players: Analysis by Number of Funding Instances
- 10.5. Regional Analysis by Amount Invested
- 10.6. Concluding Remarks
11 MARKET FORECAST AND OPPORTUNITY ANALYSIS
- 11.1. Chapter Overview
- 11.2. Forecast Methodology and Key Assumptions
- 11.3. Global Clinical Trials Software Market, 2021-2035
- 11.3.1. Clinical Trials Software Market, 2021-2035: Distribution by Type of Deployment
- 11.3.2. Clinical Trials Software Market, 2021-2035: Distribution by Type of Delivery
- 11.3.3. Clinical Trials Software Market, 2021-2035: Distribution by Features of Software
- 11.3.4. Clinical Trials Software Market, 2021-2035: Distribution by Geographical Region
- 11.3.4.1. Clinical Trials Software Market in North America, 2021-2035
- 11.3.4.1.1. Clinical Trials Software Market in North America, 2021-2035: Distribution by Type of Deployment
- 11.3.4.1.2. Clinical Trials Software Market in North America, 2021-2035: Distribution by Type of Delivery
- 11.3.4.1.3. Clinical Trials Software Market in North America, 2021-2035: Distribution by Features of Software
- 11.3.4.2. Clinical Trials Software Market in Europe, 2021-2035
- 11.3.4.2.1. Clinical Trials Software Market in Europe, 2021-2035: Distribution by Type of Deployment
- 11.3.4.2.2. Clinical Trials Software Market in Europe, 2021-2035: Distribution by Type of Delivery
- 11.3.4.2.3. Clinical Trials Software Market in Europe, 2021-2035: Distribution by Features of Software
- 11.3.4.3. Clinical Trials Software Market in Asia-Pacific, 2021-2035
- 11.3.4.3.1. Clinical Trials Software Market in Asia-Pacific, 2021-2035: Distribution by Type of Deployment
- 11.3.4.3.2. Clinical Trials Software Market Asia-Pacific, 2021-2035: Distribution by Type of Delivery
- 11.3.4.3.3. Clinical Trials Software Market Asia-Pacific, 2021-2035: Distribution by Features of Software
- 11.3.4.1. Clinical Trials Software Market in North America, 2021-2035
12. CONCLUSION
- 12.1. Chapter Overview


