
|
市場調査レポート
商品コード
1852010
ヒト胚性幹細胞:市場シェア分析、産業動向、統計、成長予測(2025年~2030年)Human Embryonic Stem Cells - Market Share Analysis, Industry Trends & Statistics, Growth Forecasts (2025 - 2030) |
||||||
カスタマイズ可能
適宜更新あり
|
|||||||
| ヒト胚性幹細胞:市場シェア分析、産業動向、統計、成長予測(2025年~2030年) |
|
出版日: 2025年08月29日
発行: Mordor Intelligence
ページ情報: 英文 110 Pages
納期: 2~3営業日
|
概要
ヒト胚性幹細胞市場規模は、2025年に12億2,000万米ドルと推定・予測され、予測期間中(2025-2030年)のCAGRは9.58%で、2030年には19億3,000万米ドルに達すると予測されます。
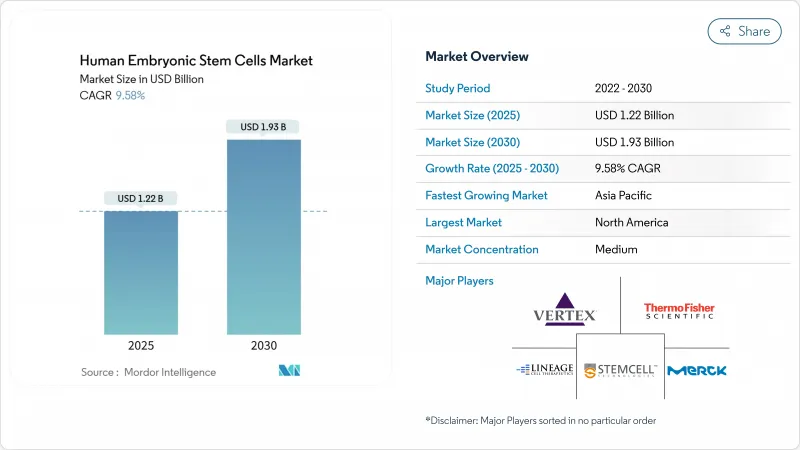
CRISPRを利用したライン・エンジニアリングの進歩、倫理的に入手可能な余剰体外受精胚の着実な増加、ゼノフリーGMP培養システムの商業的利用可能性が、心臓、網膜、内分泌疾患にわたる治療の可能性を拡大します。自動化されたクローズドシステム・バイオプロセシングがコンタミネーションリスクを削減し、バッチ処理能力を倍増させ、ヒト胚性幹細胞市場のアーリームーバーの競争力を研ぎ澄ますにつれて、産業界への浸透が高まる。FDAのRMATパスウェイや日本のファストトラック承認に代表される規制当局の支援は、臨床から市場へのタイムラインを早め、数十億米ドルの資金提供を約束させる。一方、遺伝子編集のパイオニアと製造のスペシャリストによる学際的なコラボレーションは、市場開拓のサイクルを短縮し、ヒト胚性幹細胞市場における知的財産の守備範囲を広げます。倫理的アクティヴィズムの高まりとコスト上昇圧力は依然として注視点であるが、技術主導の生産性向上は当面の逆風を相殺する勢いです。
世界のヒト胚性幹細胞市場の動向と洞察
心疾患と悪性疾患の高い有病率
心血管系疾患とがんは、世界的に死亡率の大半を占めており、多系統の修復ソリューションに対する持続的な臨床需要を生み出しています。ヒト胚性幹細胞由来の心筋スフェロイドは、ブタの梗塞モデルで収縮力を回復させ、現在、ヒト初の虚血性心筋症臨床試験に入っています。人口レベルでの心臓治療への支出が3,500億米ドルを超えるにつれ、商業的関心が高まっており、心臓代謝プログラムはヒト胚性幹細胞市場において主要な価値促進要因となっています。hESCを播種した3Dプリント心筋スキャフォールドのような付加製造のブレークスルーは、トランスレーショナル・パスウェイをさらに短縮します。これらのデータを総合すると、疾患修飾の可能性が検証され、2030年までのプレミアム価格設定の機会が強化されます。
再生医療への需要の高まり
米国では1,200以上の細胞・遺伝子治療の臨床試験が行われており、再生医療が縁の下の力持ちから最前線の治療へと移行しつつあることを示しています。ヒト胚性幹細胞に依存する同種療法プラットフォームは、既製の投与法を可能にし、自家療法の歴史的な規模の限界を解決しています。日本の60を超えるiPS細胞研究は、一貫した政策、償還の明確化、製造上のインセンティブがいかに採用を加速させるかを示しています。FDAによる間葉系間質細胞治療の画期的な承認は、臨床的に検証された製品を規制当局が承認する用意があることを示しており、間接的にhESC開発者に利益をもたらしています。hESC由来の角膜上皮を用いた視力回復の成功(90%以上の有効性)は、社会的信用を高め、患者募集や投資家への資金流入にプラスのフィードバックループを促進します。
高い治療費と生産コスト
現在のhESCベースの治療法は、クリーンルームでの手作業と低い歩留まりにより、1回の投与あたり10万米ドルを超えることが多いです。AIが誘導するロボット工学は、労働投入を50%削減し、ロット間の一貫性を実現し、5年以内に5万米ドル以下で損益分岐点の製造が可能になります。ニュージャージー州にあるサーモ・フィッシャーの4億7,500万米ドルのCDMOサイトは、コスト削減と規制遵守をターゲットとした業界規模の投資の例です。モジュール式ロボットクラスタは、拡張、収穫、最終充填仕上げを再現性よく処理し、キャンペーン時間を短縮し、バッチ不良リスクを低減します。ノースイースタン大学の予測AIモデルは、栄養供給と通過タイミングをさらに最適化し、歩留まりを産業用ベンチマークへと押し上げます。支払者の監視や価格に敏感な新興市場の需要を相殺するためには、こうした利益を持続させなければならないです。
セグメント分析
再生医療は、2024年のヒト胚性幹細胞市場シェアの58.57%を占め、地理的萎縮症患者におけるOpRegenの+5.5文字の視力向上などの臨床結果によって後押しされました。脊髄修復、膵島置換、心筋再筋肉化は現在、多施設臨床試験の先頭に立ち、このセグメントの圧倒的な収益軌道を強化しています。RMATやサキガケの指定を受けるプログラムが増えるにつれて、支払者は償還を正当化する実臨床エビデンスを獲得し、ヒト胚性幹細胞市場の好循環に拍車をかけています。
幹細胞生物学調査は、2030年までのCAGRが10.86%を記録し、自動化されたゲノム編集スクリーニングとヒト組織の複雑性を再現できるオルガノイド・プラットフォームから恩恵を受ける。CRISPRを利用した系統追跡とハイコンテントフェノミクスは標的同定のタイムラインを短縮し、新しいオルガノイド共培養システムは疾患モデルの精度を5倍に高める。アカデミックな中核施設がフィー・フォー・サービス・モデルに移行するにつれて、研究費は試薬やライセンシングのロイヤリティに還元され、サプライチェーン参加者の経常収益プールが拡大します。探索アプリケーションのヒト胚性幹細胞市場規模は、マルチプレックス・スクリーンが精密医療パイプラインに不可欠となるにつれて、着実に上昇すると予測されます。
地域分析
北米は、NIHとCIRMの旺盛な資金提供、RMATによる迅速な審査、CDMOの広範な能力を背景に、2024年の世界売上高の42.16%を維持します。スタンフォード主導の心臓試験、ノースウェスタンの脊髄イニシアティブ、サーモ・フィッシャーのプリンストン新拠点は、この地域のラボから上市までの統合を総体的に示しています。胚研究に対する連邦政府の資金提供をめぐる政治的不確実性は戦略的リスクとなるが、多様な民間投資が潜在的な公的予算の変動を緩和しています。
アジア太平洋は最も急成長しているクラスターであり、日本の条件付き承認制度と中国の国営リサーチパークを背景に、2030年までのCAGRが11.41%で進んでいます。日本では60以上の臨床試験が活発に行われており、規制当局の機敏性を浮き彫りにしている一方、住友製薬とニコン・ロンザの製造提携は多国籍パートナーからの資本流入を実証しています。政府補助金は施設建設や労働者訓練に充てられ、ヒト胚性幹細胞市場において現地のサプライチェーンの成熟度を高めています。
欧州の見通しは、2027年のSOHO規制の効果的な展開にかかっています。ドイツと英国は主要な学術クラスターを維持しています。英国の人工胚に関する実施規範は、大陸の標準を形成するかもしれない政策革新のシグナルです。フランスとイタリアは眼科と軟骨修復のニッチ分野に注力し、スカンジナビアのコンソーシアムは患者アクセスを広げるために低温物流に投資しています。西欧では償還のハードルが依然として高いが、国境を越えた協力体制とEU全体のHTA改革により、認証製品の市場アクセスが合理化されることが期待されます。
その他の特典:
- エクセル形式の市場予測(ME)シート
- 3ヶ月間のアナリストサポート
よくあるご質問
目次
第1章 イントロダクション
- 調査の前提条件と市場の定義
- 調査範囲
第2章 調査手法
第3章 エグゼクティブサマリー
第4章 市場情勢
- 市場概要
- 市場促進要因
- 心疾患と悪性疾患の高い有病率
- 再生医療需要の高まり
- 政府および民間資金提供プログラムの成長
- CRISPR対応hESC株工学
- ゼノフリーGMP培養システムが汚染リスクを低減
- 余剰体外受精胚が倫理的hESC供給を拡大する
- 市場抑制要因
- 高い治療費と生産コスト
- 厳格かつ異質なグローバル規制
- 急成長するiPSCの代替品が資金を食い尽くす
- ソーシャルメディア主導の倫理的アクティビズムが採用を抑制
- 規制情勢
- テクノロジーの展望
- ポーターのファイブフォース
- 新規参入業者の脅威
- 買い手の交渉力/消費者
- 供給企業の交渉力
- 代替品の脅威
- 競争企業間の敵対関係
第5章 市場規模と成長予測(金額-USD)
- 用途別
- 再生医療
- 幹細胞生物学研究
- 組織工学
- 毒性試験
- 製品タイプ別
- hESCライン
- 培地と試薬
- 器具・消耗品
- 地域別
- 北米
- 米国
- カナダ
- メキシコ
- 南米
- ブラジル
- アルゼンチン
- その他南米
- 欧州
- ドイツ
- 英国
- フランス
- イタリア
- スペイン
- その他欧州地域
- アジア太平洋
- 中国
- 日本
- インド
- オーストラリア
- 韓国
- その他アジア太平洋地域
- 中東・アフリカ
- GCC
- 南アフリカ
- その他中東・アフリカ地域
- 北米
第6章 競合情勢
- 市場集中度
- 戦略的動向
- 市場シェア分析
- 企業プロファイル
- Astellas Pharma Inc.
- Thermo Fisher Scientific Inc.
- Merck KGaA
- STEMCELL Technologies Inc.
- Takara Bio Inc.
- Lineage Cell Therapeutics Inc.
- PeproTech Inc.
- PromoCell GmbH
- Vertex Pharmaceuticals
- Lonza Group AG
- BD Biosciences
- GE HealthCare
- ViaCyte Inc.
- Fate Therapeutics
- BlueRock Therapeutics
- Cynata Therapeutics
- Pluri Inc.
- Organovo Holdings Inc.
- Samsung Biologics
- Cell Engineering Corp.
- Janssen(Cell Therapy Grp)


