|
|
市場調査レポート
商品コード
1517292
CDK2阻害剤市場- パイプライン分析(2024年)CDK2 Inhibitor - Pipeline Analytics 2024 |
||||||
|
|||||||
| CDK2阻害剤市場- パイプライン分析(2024年) |
|
出版日: 2024年07月19日
発行: Mellalta Meets LLP
ページ情報: 英文 114 Pages
納期: 即納可能
|
全表示
- 概要
- 目次
サイクリン依存性キナーゼ2(CDK2)は、細胞分裂と細胞周期を制御する極めて重要なプロテインキナーゼです。CDK2の調節異常は様々ながんに関与しており、治療介入の魅力的なターゲットとなっています。
CDK2阻害剤は魅力的な投資機会となります。これらの阻害剤は重要な細胞周期の動態を標的とするため、乳がんや卵巣がんなど負担の大きい複数のがん種に治療の可能性を提供しています。
CDK2阻害剤市場は、Sino Biopharma、Tiziana Life Sciences、Incyclix Bio、Pfizer、Cyclacel Pharmaceuticals、Blueprint Medicinesなどの主要企業が36以上の製品を提供しており、非常にダイナミックで競争が激しくなっています。これらの企業は、ER+/HER2乳がん、非小細胞肺がん、脱分化脂肪肉腫、卵巣がん、切除不能/転移性肝細胞がん、CCNE1増幅がん等、CDK2阻害剤の様々な適応症を追求しています。
強固なパイプラインと有望な臨床データにより、これらの阻害剤は、がん領域において高収益の機会を示しています。CDK2阻害活性を持つ低分子阻害剤は数多く同定されています。しかしながら、これらの阻害剤の開発には、特にサイクリン依存性キナーゼ(CDK)間の構造的類似性に起因する特異性に関する課題が多くなっています。
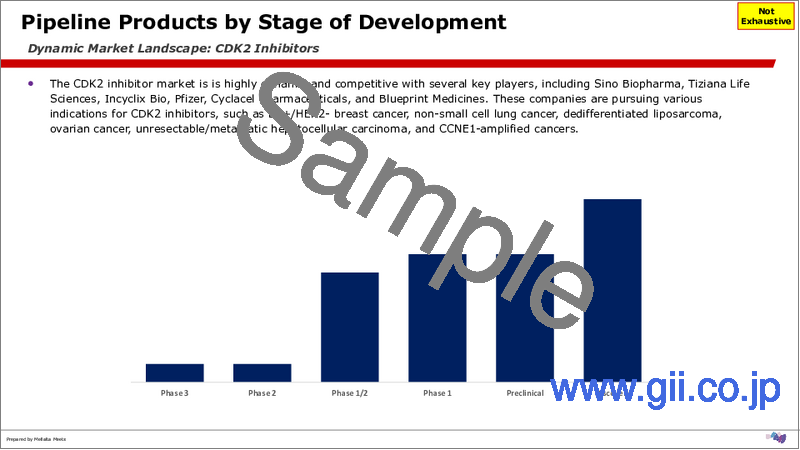
CDK2阻害剤市場は競争が激しく、36以上の阻害剤が存在します。大半は初期段階にあり、38.89%が探索段階、19.44%が前臨床段階、19.44%が第1相にあります第1/2相の製品は全体の16.67%を占め、第3相と第2相の製品はそれぞれ2.78%です。
主要参入企業の中では、Blueprint Medicinesとファイザーが最も資産数が多く、合計3つのポートフォリオを持っています。
当レポートでは、世界のCDK2阻害剤市場について調査し、市場の現状とともに、症例数の動向、患者動向、競合製品の市場における位置づけ、市場の機会などを提供しています。
目次
第1章 概要
第2章 CDK2阻害剤のターゲット背景
- CDK2阻害剤のイントロダクションと役割
- CDK4/6阻害剤に関する潜在的な懸念
- CDK2阻害剤:腫瘍学分野における非常に有望なターゲット
- CDK2阻害剤の臨床応用
- CDK4/6耐性の克服:CDK2阻害剤の戦略的役割
- CDK2阻害剤のアロステリックメカニズム
第3章 CDK2阻害剤パイプラインの相別分析
- CDK2阻害剤の開発- 概要
- 開発段階別のパイプライン製品
- 会社別パイプライン製品
- 適応症およびフェーズ別のパイプライン製品
- CDK2阻害剤単独療法および併用療法の臨床試験
- CDK2パイプライン:臨床試験の地理的分布
- CDK2阻害剤の臨床および規制タイムライン
- 後期段階のプロファイル比較の概要
第4章 CDK2阻害剤のライセンシング、買収、および提携契約
- CDK2阻害剤のライセンシング、買収、取引価値
- CDK2阻害剤ライセンシング取引タイプ別および相別総額規模
第5章 CDK2阻害剤パイプラインの情勢
- CDK2阻害剤パイプラインの薬剤プロファイル- 概要
- 第III相
- 第II相
- 第I/II相
- 第I相
- 前臨床
- 創薬
第6章 アロステリックCDK2阻害剤のメカニズム- 概要
第7章 CDK2阻害剤のSWOT分析
第8章 付録
The 2024 Mellalta Meets report analyzes the CDK2 inhibitor pipeline landscape, highlighting the market opportunities and competitive landscape with over 32 companies developing pipeline drug profiles.
Cyclin-dependent kinase 2 (CDK2) is a pivotal protein kinase that orchestrates cell division and the cell cycle. Dysregulation of CDK2 has been implicated in various cancers, making it an attractive target for therapeutic intervention.
CDK2 inhibitors present a compelling investment opportunity. These inhibitors target critical cell cycle dynamics, offering therapeutic potential across multiple high-burden cancer types such as Breast and Ovarian Cancer.
The CDK2 inhibitor market is highly dynamic and competitive with over 36 products with key players, including Sino Biopharma, Tiziana Life Sciences, Incyclix Bio, Pfizer, Cyclacel Pharmaceuticals, and Blueprint Medicines. These companies are pursuing various indications for CDK2 inhibitors, such as ER+/HER2- breast cancer, non-small cell lung cancer, dedifferentiated liposarcoma, ovarian cancer, unresectable/metastatic hepatocellular carcinoma, and CCNE1-amplified cancers.
With a robust pipeline and promising clinical data, these inhibitors represent a high-reward opportunity in the oncology landscape. Numerous small molecule inhibitors have been identified for their CDK2 inhibitory activity. However, the development of these inhibitors is fraught with challenges, particularly regarding specificity due to the structural similarities among cyclin-dependent kinases (CDKs).
Efforts are ongoing to engineer inhibitors that selectively target CDK2, thereby minimizing off-target effects and enhancing therapeutic efficacy. As research progresses, CDK2 inhibitors are poised to play a transformative role in overcoming CDK4/6 resistance, offering significant benefits for patients and lucrative opportunities for investors with the usage of different CDK2 targeting strategies such as protein-protein interaction, Allosteric, Orthosteric, and indirect inhibition.
The CDK2 Inhibitor - Pipeline Analytics -2024 report published by Mellalta Meets covers the Cell Therapies market opportunity providing Key Competitive Analysis, 32+ Companies and 36 Products with Pipeline Drug Profiles, Clinical Trials, Other Developments (Collaboration Details, Funding, etc.), Licensing and Agreements, Business Agreement, Business Partner as well as Clinical Partner. Reports cover pipeline product analysis by stage of development, competitive landscape by phases, companies, clinical trials by regions, and intervention type. CDK2 Inhibitor-Pipeline Analytics report adds value in terms of describing clinical-stage products for their clinical & regulatory timelines as well as in terms of providing the current market opportunity, drivers, and challenges.
Mellalta's CDK2 Inhibitor Pipeline Report - Key Highlights
The CDK2 inhibitors market is highly competitive, with over 36 inhibitors. The majority are in the early stage, with 38.89% in the Discovery stage, 19.44% in the pre-clinical stage, and 19.44% in Phase 1. Phase 1/2 products make up 16.67% of the total, while Phase 3 and Phase 2 products each represent 2.78%.
Among the key players, Blueprint Medicines and Pfizer stand out with the highest number of assets, totaling three in its portfolio.
Allosteric inhibitors are favored and preferred mechanisms due to their low toxicity profiles.
Cyclins are being directly targeted as a focused approach to avoid toxicity.
Report Coverage:
Indication Prioritization: CDK2 Inhibitor market potential
Business Transactions & Strategies: Key collaborations and deal values
CDK2 Inhibitor Pipeline Development: Product Profiles, Clinical Trials & Results
CDK2 Inhibitor Acquisition Targets
CDK2 Inhibitor Competitive Intelligence
Recent and upcoming events
Table of Content
1. OVERVIEW
2. The CDK2 Inhibitor Target BACKGROUND
- 2.1. Introduction and Role of CDK2 inhibitors
- 2.2. Potential Concern with CDK4/6 Inhibitors
- 2.3. CDK2 Inhibitors: A Highly-Potential Target in Oncology Space
- 2.4. Clinical Applications of CDK2 Inhibitors
- 2.5. Overcoming CDK4/6 Resistance: The Strategic Role of CDK2 Inhibitors
- 2.6. Allosteric Mechanism of CDK2 Inhibitors
3. CDK2 Inhibitor PIPELINE ANALYSIS by Phases
- 3.1. CDK2 Inhibitor Development - Overview
- 3.2. Pipeline Products by Stage of Development
- 3.3. Pipeline Products by Company
- 3.4. Pipeline Products by Indication and Phases
- 3.5. CDK2 Inhibitor Monotherapy & Combinations Clinical Trials
- 3.6. CDK2 Pipeline: Geographic Distribution of Clinical Trials
- 3.7. CDK2 Inhibitor Clinical & Regulatory Timelines
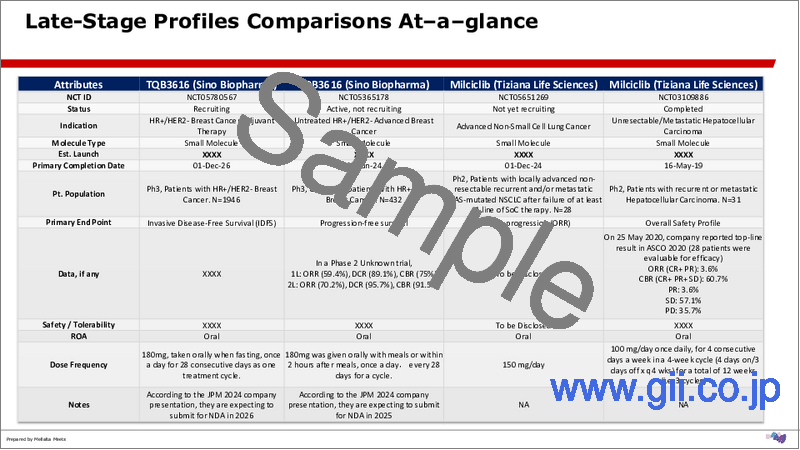
- 3.8. Late-Stage Profiles Comparisons At-a-glance
4. CDK2 Inhibitor LICENSING, ACQUISITION, AND COLLABORATION DEALS
- 4.1. CDK2 Inhibitor Licensing, Acquisition, and Deal values
- 4.2. CDK2 Inhibitor Licensing by Transaction type and total amount size by Phases
5. CDK2 Inhibitor Pipeline Landscape
- 5.1. CDK2 Inhibitor Pipeline Drug Profiles- at a glance
- 5.1.1. Phase III
- 5.1.1.1. TQB3616 (Sino Biopharma)
- 5.1.1.1.1. Product Profile & Description
- 5.1.1.1.2. Clinical Trials
- 5.1.1.1.3. Collaborations
- 5.1.1.1.4. Other Developments
- 5.1.1.1. TQB3616 (Sino Biopharma)
- 5.1.2. Phase II
- 5.1.2.1. Milciclib (Tiziana Life Sciences)
- 5.1.2.1.1. Product Profile & Description
- 5.1.2.1.2. Clinical Trials
- 5.1.2.1.3. Collaborations
- 5.1.2.1.4. Other Developments
- 5.1.2.1. Milciclib (Tiziana Life Sciences)
- 5.1.3. Phase I/II
- 5.1.3.1. INX-315 (Incyclix Bio)
- 5.1.3.1.1. Product Profile & Description
- 5.1.3.1.2. Clinical Trials
- 5.1.3.1.3. Collaborations
- 5.1.3.1.4. Other Developments
- 5.1.3.2. Fadraciclib (Cyclacel Pharmaceuticals)
- 5.1.3.2.1. Product Profile & Description
- 5.1.3.2.2. Clinical Trials
- 5.1.3.2.3. Collaborations
- 5.1.3.2.4. Other Developments
- 5.1.3.3. PF 07104091 (Pfizer)
- 5.1.3.3.1. Product Profile & Description
- 5.1.3.3.2. Clinical Trials
- 5.1.3.3.3. Collaborations
- 5.1.3.3.4. Other Developments
- 5.1.3.4. AVZO-021 (Avenzo Therapeutics/Allorion Therapeutics Inc)
- 5.1.3.4.1. Product Profile & Description
- 5.1.3.4.2. Clinical Trials
- 5.1.3.4.3. Collaborations
- 5.1.3.4.4. Other Developments
- 5.1.3.5. AZD 8421 (AstraZeneca)
- 5.1.3.5.1. Product Profile & Description
- 5.1.3.5.2. Clinical Trials
- 5.1.3.5.3. Collaborations
- 5.1.3.5.4. Other Developments
- 5.1.3.6. BLU 222 (Blueprint Medicines)
- 5.1.3.6.1. Product Profile & Description
- 5.1.3.6.2. Clinical Trials
- 5.1.3.6.3. Collaborations
- 5.1.3.6.4. Other Developments
- 5.1.3.1. INX-315 (Incyclix Bio)
- 5.1.4. Phase I
- 5.1.4.1. NKT 3447 (NiKang Therapeutics)
- 5.1.4.1.1. Product Profile & Description
- 5.1.4.1.2. Clinical Trials
- 5.1.4.1.3. Collaborations
- 5.1.4.1.4. Other Developments
- 5.1.4.2. BG-68501 (BeiGene)
- 5.1.4.2.1. Product Profile & Description
- 5.1.4.2.2. Clinical Trials
- 5.1.4.2.3. Collaborations
- 5.1.4.2.4. Other Developments
- 5.1.4.3. TYK 0540 (TYK Medicine)
- 5.1.4.3.1. Product Profile & Description
- 5.1.4.3.2. Clinical Trials
- 5.1.4.3.3. Collaborations
- 5.1.4.3.4. Other Developments
- 5.1.4.4. INCB123667 (Incyte)
- 5.1.4.4.1. Product Profile & Description
- 5.1.4.4.2. Clinical Trials
- 5.1.4.4.3. Collaborations
- 5.1.4.4.4. Other Developments
- 5.1.4.5. RGT-419B (REGOR THERAPEUTICS)
- 5.1.4.5.1. Product Profile & Description
- 5.1.4.5.2. Clinical Trials
- 5.1.4.5.3. Collaborations
- 5.1.4.5.4. Other Developments
- 5.1.4.6. SYH2043 (CSPC Ouyi Pharmaceutical)
- 5.1.4.6.1. Product Profile & Description
- 5.1.4.6.2. Clinical Trials
- 5.1.4.6.3. Collaborations
- 5.1.4.6.4. Other Developments
- 5.1.4.7. WXWH0240 (Cisen Pharmaceutical)
- 5.1.4.7.1. Product Profile & Description
- 5.1.4.7.2. Clinical Trials
- 5.1.4.7.3. Collaborations
- 5.1.4.7.4. Other Developments
- 5.1.4.1. NKT 3447 (NiKang Therapeutics)
- 5.1.5. Preclinical
- 5.1.5.1. IAM-C1 (Iambic Therapeutics)
- 5.1.5.1.1. Product Profile & Description
- 5.1.5.1.2. Collaborations
- 5.1.5.1.3. Other Developments
- 5.1.5.2. AU14-5 (AUCENTRA)
- 5.1.5.2.1. Product Profile & Description
- 5.1.5.2.2. Collaborations
- 5.1.5.2.3. Other Developments
- 5.1.5.3. CID-078 (Circle Pharma)
- 5.1.5.3.1. Product Profile & Description
- 5.1.5.3.2. Collaborations
- 5.1.5.3.3. Other Developments
- 5.1.5.4. CC002 (Chupu Chuangzhi (Wuhan) Pharmaceutical Technology)
- 5.1.5.4.1. Product Profile & Description
- 5.1.5.4.2. Collaborations
- 5.1.5.4.3. Other Developments
- 5.1.5.5. IpY.20 (Concarlo Therapeutics)
- 5.1.5.5.1. Product Profile & Description
- 5.1.5.5.2. Collaborations
- 5.1.5.5.3. Other Developments
- 5.1.5.6. CED-2368 (Bayer)
- 5.1.5.6.1. Product Profile & Description
- 5.1.5.6.2. Collaborations
- 5.1.5.6.3. Other Developments
- 5.1.5.7. TY-1210 (TYK Medicines)
- 5.1.5.7.1. Product Profile & Description
- 5.1.5.7.2. Collaborations
- 5.1.5.7.3. Other Developments
- 5.1.5.1. IAM-C1 (Iambic Therapeutics)
- 5.1.6. Discovery
- 5.1.6.1. CDK2 and CCNE1 (Kymera Therapeutics)
- 5.1.6.1.1. Product Profile & Description
- 5.1.6.1.2. Collaborations
- 5.1.6.1.3. Other Developments
- 5.1.6.2. CDK2 targeting therapeutics (Plexium)
- 5.1.6.2.1. Product Profile & Description
- 5.1.6.2.2. Collaborations
- 5.1.6.2.3. Other Developments
- 5.1.6.3. CDK2 Inhibitor (Bugworks)
- 5.1.6.3.1. Product Profile & Description
- 5.1.6.3.2. Collaborations
- 5.1.6.3.3. Other Developments
- 5.1.6.4. CDK2 degraders (Monte Rosa Therapeutics)
- 5.1.6.4.1. Product Profile & Description
- 5.1.6.4.2. Collaborations & Other Developments
- 5.1.6.5. BLU-956 (Blueprint Medicines)
- 5.1.6.5.1. Product Profile & Description
- 5.1.6.5.2. Collaborations
- 5.1.6.5.3. Other Developments
- 5.1.6.6. TPD: CDK2 (Blueprint Medicines)
- 5.1.6.6.1. Product Profile & Description
- 5.1.6.6.2. Collaborations
- 5.1.6.6.3. Other Developments
- 5.1.6.7. QR-6401 (REGOR THERAPEUTICS)
- 5.1.6.7.1. Product Profile & Description
- 5.1.6.7.2. Collaborations
- 5.1.6.7.3. Other Developments
- 5.1.6.8. CDK2 inhibitor (Calibr-Skaggs)
- 5.1.6.8.1. Product Profile & Description
- 5.1.6.8.2. Collaborations
- 5.1.6.8.3. Other Developments
- 5.1.6.9. Cyclin E/CDK2 inhibitor (Circle Pharma)
- 5.1.6.9.1. Product Profile & Description
- 5.1.6.9.2. Collaborations
- 5.1.6.9.3. Other Developments
- 5.1.6.10. TMX-2172 (Dana-Farber Cancer Institute)
- 5.1.6.10.1. Product Profile & Description
- 5.1.6.10.2. Collaborations
- 5.1.6.10.3. Other Developments
- 5.1.6.11. CDK2 Inhibitor (Acellera Therapeutics)
- 5.1.6.11.1. Product Profile & Description
- 5.1.6.11.2. Collaborations
- 5.1.6.11.3. Other Developments
- 5.1.6.12. FP19711 and FP24322 (Fog Pharma)
- 5.1.6.12.1. Product Profile & Description
- 5.1.6.12.2. Collaborations
- 5.1.6.12.3. Other Developments
- 5.1.6.13. I-125A and I-198 (Pfizer/ Belharra Therapeutics/ Magnet Biomedicine)
- 5.1.6.13.1. Product Profile & Description
- 5.1.6.13.2. Collaborations
- 5.1.6.13.3. Other Developments
- 5.1.6.1. CDK2 and CCNE1 (Kymera Therapeutics)
- 5.1.1. Phase III