|
市場調査レポート
商品コード
1170398
プロスタグランジンE2受容体4(EP4R)阻害剤-パイプラインアナリティクス:2022年Prostaglandin E2 Receptor 4 (EP4R) Inhibitors-Pipeline Analytics 2022 |
|||||||
| プロスタグランジンE2受容体4(EP4R)阻害剤-パイプラインアナリティクス:2022年 |
|
出版日: 2022年12月01日
発行: Mellalta Meets LLP
ページ情報: 英文 87 Pages
納期: 即納可能
|
- 全表示
- 概要
- 目次
当レポートでは、EP4R阻害剤の市場機会を提供する主要な競合分析、パイプライン医薬品のプロファイルを持つ企業、臨床試験、その他の発展動向などをまとめています。
サンプルビュー
目次
概要
EP4R阻害剤の標的の背景
- EP4R(プロスタグランジンE2受容体4)-概要
- C-ICにおけるEP4拮抗薬の作用機序
- EP4阻害剤の臨床応用
EP4R阻害剤パイプライン分析、相別
- EP4R阻害剤の開発- 概要
- パイプライン製品、開発段階別
- EP4R阻害剤の競合情勢環境、相別
- パイプライン製品、企業別
- パイプライン製品、適応症および相別
- EP4R阻害剤臨床および規制単剤療法および併用療法
- EP4R阻害剤- 適応症/相別資産
- EP4R阻害剤の臨床および規制のタイムライン
EP4R阻害剤のライセンス供与、買収、および提携契約
EP4R阻害剤パイプラインの展望
- プロファイル比較の概要
- EP4R阻害剤パイプラインの薬物プロファイル
- 第II相
- CR6086
- Grapiprant
- 第I/II相
- ONO 4578
- グラピプラント
- 第Ib相
- Palupiprant
- INV 1120
- TPST 1495
- 第I相
- KF-0210
- DT-9081
- 前臨床
- AAT-008
- YY001
- P001
- EP4R阻害剤SWOT分析
- EP4R阻害剤製品のポジショニング
- 付録
- 第II相
EP4R Inhibitors report covers the EP4R Inhibitors market opportunity providing Key Competitive Analysis, 16+ Companies with Pipeline Drug Profiles, Clinical Trials, Other Developments (Collaboration Details, Funding, etc.), Licensing and Agreements, Business Agreement, Business Partners as well as Clinical Partner. The report covers pipeline product analysis by stage of development, competitive landscape by phases, companies, therapy area, and indication by phases. EP4R inhibitor's reports add value in terms of describing clinical stage products concerning their clinical & regulatory timelines as well as in terms of providing the current market opportunity, drivers, and challenges.
SAMPLE VIEW

Given the multiple tumor-promoting roles of EP4 expression on tumor cells and host immune cells and endothelial cells, revealed that the combination of EP4 antagonists and other immunomodulating drugs should work synergistically, especially in TNBCs displaying a diverse microenvironment. This strongly suggests the potential benefit of EP4 antagonists in combination with other immunomodulating agents in cancer therapy.
In this report, Mellalta Meets provides an in-depth analysis of EP4R inhibitors covering preclinical and clinical studies, details of partnerships and business deal values, targeted technologies and therapy areas, investments, and acquisition trends. Currently, there are more than 12 candidate EP4R inhibitor products currently under evaluation in clinical and preclinical studies. The major key players operating in the market are Rottapharm Biotech, Haihe Biopharma/3D Medicine, Bristol-Myers Squibb/Ono Pharmaceuticals, Ikena Oncology, and many more which have robust clinical pipelines of EP4R inhibitor candidates.
As per analysis, the EP4 receptor is currently emerging as the most versatile and promising among PGE2 receptors with the development pipeline full of Molecules like small molecules and combination therapies.
Key Highlights of EP4R Inhibitors Report:
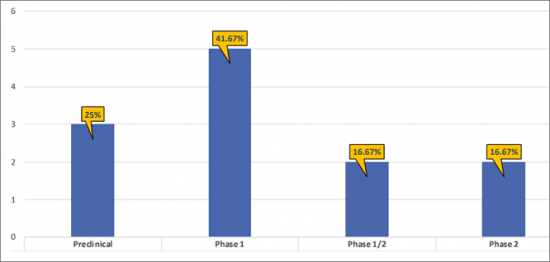
- There are ~5 products in the Phase 1 stage of development, representing 41.67% of the total share of the developing EP4R inhibitors landscape. The remaining 58.33% has been contributed by pre-clinical (25%); Phases 2 and 1/2 (16.67%).
- The pipeline of EP4R Inhibitors is dominated by biotech companies headquartered in China with Chinese companies holding the top 6 positions. They represent ~37.50% of all the pipeline EP4R inhibitors in clinical stages.
- EP4R Inhibitors pipeline landscape includes 47% of Monotherapy trials and 53% of Combination Trials
- The pipeline of EP4R inhibitors is dominated by Phase 1 (5); Pre-clinical (3); Phase 2 (2) assets and Phase 1/2 (2) assets.
- To be continued...
Report Coverage:
- Indication Prioritisation: EP4R Inhibitors market potential based on Indications
- Business Transactions & Strategies: Key collaborations and deal values
- EP4R Inhibitors Pipeline Development: Product Profiles, Clinical Trials & Results
- EP4R Inhibitors Acquisition Targets
- EP4R Inhibitors Competitive Intelligence
- Recent & Upcoming events
TABLE OF CONTENTS
OVERVIEW
The EP4R Inhibitors Target BACKGROUND
- EP4R (Prostaglandin E2 Receptor 4) -Overview
- Mechanism of Action of EP4 Antagonist in the C-IC
- Clinical Application of EP4 Inhibitors
EP4R Inhibitors PIPELINE ANALYSIS by Phases
- EP4R Inhibitors Development - Overview
- Pipeline Products by Stage of Development
- EP4R Inhibitors Competitive Landscape by Phases
- Pipeline Products by Company
- Pipeline Products by Indication and Phases
- EP4R Inhibitors Clinical & Regulatory Monotherapy & Combinations
- EP4R Inhibitors- Assets by Indication/Phase
- EP4R Inhibitors Clinical & Regulatory Timelines
EP4R Inhibitors LICENSING, ACQUISITION, AND COLLABORATION DEALS
- EP4R Inhibitors Licensing, Acquisition, and Deal values
- EP4R Inhibitors Licensing by Transaction type and total amount size by Phases
EP4R Inhibitors Pipeline Landscape
- Profile Comparisons At-a-glance
- EP4R Inhibitors Pipeline Drug Profiles
- Phase II
- CR6086 (Rottapharm Biotech)
- Product Profile & Description
- Clinical Trials
- Collaborations
- Other Developments
- Grapiprant (Haihe Biopharma/3D Medicine)
- Product Profile & Description
- Clinical Trials
- Collaborations
- Other Developments
- CR6086 (Rottapharm Biotech)
- Phase I/II
- ONO 4578 (Bristol-Myers Squibb/ Ono Pharmaceuticals)
- Product Profile & Description
- Clinical Trials
- Collaborations
- Other Developments
- Grapiprant (Ikena Oncology; AskAt; NewBay)
- Product Profile & Description
- Clinical Trials
- Collaborations
- Other Developments
- ONO 4578 (Bristol-Myers Squibb/ Ono Pharmaceuticals)
- Phase Ib
- Palupiprant (Adlai Nortye/Eisai Inc)
- Product Profile & Description
- Clinical Trials
- Collaborations
- Other Developments
- INV 1120 (Shenzhen Ionova Life Sciences Co., Ltd.)
- Product Profile & Description
- Clinical Trials
- Collaborations
- Other Developments
- TPST 1495 (Tempest Therapeutics)
- Product Profile & Description
- Clinical Trials
- Collaborations
- Other Developments
- Palupiprant (Adlai Nortye/Eisai Inc)
- Phase I
- KF-0210 (Keythera Pharmaceuticals)
- Product Profile & Description
- Clinical Trials
- Collaborations
- Other Developments
- DT-9081 (Domain Therapeutics)
- Product Profile & Description
- Clinical Trials
- Collaborations
- Other Developments
- KF-0210 (Keythera Pharmaceuticals)
- Preclinical
- AAT-008 (Ikena Oncology; AskAt; NewBay)
- Product Profile & Description
- Collaborations
- Other Developments
- YY001 (Bio Ray Lab)
- Product Profile & Description
- Collaborations
- Other Developments
- P 001 (NB Health Laboratory)
- Product Profile & Description
- Collaborations
- Other Developments
- AAT-008 (Ikena Oncology; AskAt; NewBay)
- EP4R Inhibitors SWOT Analysis
- EP4R Inhibitors Products Positioning
- Appendix
- About us
- Phase II
