|
市場調査レポート
商品コード
1170396
アデノシン受容体(A2a/A2b)拮抗薬-パイプラインアナリティクス:2022年Adenosine Receptors (A2a/A2b) Antagonists-Pipeline Analytics 2022 |
|||||||
| アデノシン受容体(A2a/A2b)拮抗薬-パイプラインアナリティクス:2022年 |
|
出版日: 2022年12月01日
発行: Mellalta Meets LLP
ページ情報: 英文 188 Pages
納期: 即納可能
|
- 全表示
- 概要
- 目次
当レポートでは、アデノシン受容体(A2a/A2b)拮抗薬の市場機会を提供し、主要競合分析、企業のパイプライン医薬品プロファイル、臨床試験、その他の発展動向などをまとめています。
サンプルビュー
目次
概要
A2aR/A2bR拮抗薬の標的の背景
- A2A/A2Bアデノシン受容体拮抗薬の概要
- 癌免疫療法におけるアデノシン-A2A受容体経路
- A2AR拮抗薬と臨床経過
- A2Bアデノシン受容体(A2BAR)と癌
- 今後の方向性
- アデノシン経路を標的とした臨床試験からの重要な教訓
A2aR/A2bR拮抗薬パイプライン分析、相別
- A2aR/A2bR拮抗薬の開発- 概要
- パイプライン製品、開発段階別
- A2aR/A2bR拮抗薬の競合情勢状況、相別
- パイプライン製品、企業別
- パイプライン製品、適応症および相別
- A2aR/A2bR拮抗薬臨床および規制単剤療法および併用療法
- A2aR/A2bR拮抗薬MOAタイプ別製品
- A2aR/A2bR拮抗薬- 適応症/相別資産
- A2aR/A2bR拮抗薬の臨床および規制のタイムライン
A2aR/A2bR拮抗薬のライセンシング供与、買収、およびコラボレーション契約
A2aR/A2bR拮抗薬パイプラインの状況
- プロファイル比較の概要
- A2aR/A2bR拮抗薬パイプラインの薬剤プロファイル
- 第II相
- PBF 999
- エトルマデナント
- イマラデナント
- タミナデナント
- イヌパデナント
- 第I/II相
- シフォラデナント
- TT 702
- PORT-6
- PORT-7
- 第I相
- CS 3005
- EXS21546
- INCB 106385
- INT 1B3
- PBF 1129
- M-1069
- ILB-2109
- DZD2269
- 前臨床
- ARX 001822
- ヴィパデナント
- PORT-9
- PORT-8
- CPI-395
- 創薬
- A2AR拮抗薬
- LNC-001
- LNC-002
- LNC-003
- RT-AR001
- 第II相
A2aR/A2bR拮抗薬製品のポジショニング
マトリクス分析
付録
The Adenosine Receptors (A2a/A2b) Antagonists report covers the Adenosine Receptors (A2a/A2b) Antagonists market opportunity providing Key Competitive Analysis, 24+ Companies with Pipeline Drug Profiles, Clinical Trials, Other Developments (Collaboration Details, Funding, etc.), Licensing and Agreements, Business Agreements, Business Partners as well as Clinical Partner. The report covers pipeline product analysis by stage of development, competitive landscape by phases, companies, therapy area, and indication by phases. The Adenosine Receptors (A2a/A2b) Antagonists report adds value in terms of describing clinical-stage products concerning their clinical & regulatory timelines as well as in terms of providing the current market opportunity, drivers, and challenges.
SAMPLE VIEW

Adenosine is involved in a range of physiological and pathological effects through membrane-bound receptors linked to G proteins. There are four subtypes of adenosine receptors, described as A1AR, A2AAR, A2BAR, and A3AR, which are the center of cAMP signal pathway-based drug development. Several types of agonists, partial agonists or antagonists, and allosteric substances have been synthesized from these receptors as new therapeutic drug candidates. AR agonists for treating inflammation, pain, cancer, non-alcoholic steatohepatitis, angina, sickle cell disease, ischemic conditions, and diabetes have been under development.
In this report, Mellalta Meets provides an in-depth analysis of Adenosine Receptors (A2a/A2b) Antagonists covering discovery, preclinical and clinical studies, details of partnerships and business deal values, targeted technologies and therapy areas, investments, and acquisition trends. Currently, there are more than 27 candidate Adenosine Receptors (A2a/A2b) Antagonists products currently under evaluation in clinical and preclinical studies. The major key players operating in the market are Palobiofarma, Arcus Biosciences/Gilead Sciences, AstraZeneca/Sosei Heptares, Novartis/Palobiofarma, iTeos Therapeutics, Corvus Pharmaceuticals/Angel Pharmaceuticals.
As per analysis, A2AR/A2B Receptor inhibition activates and enhances the proliferation of various immune cells, abrogates the adenosine-mediated immunosuppression in the tumor microenvironment (TME), and activates the immune system to exert anti-tumor immune responses against cancer cells, which leads to tumor cell killing with the development pipeline full of molecules like small molecules, and combination therapies.
Key Highlights of the Adenosine Receptors (A2a/A2b) Antagonists Report:
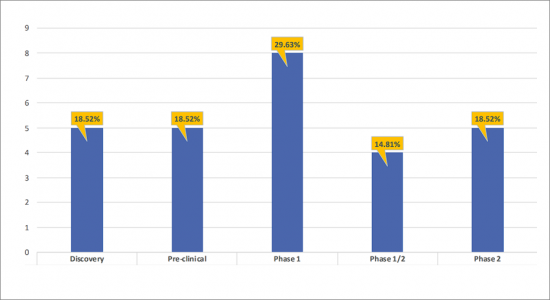
- There are ~8 products in the Phase 1 stage of development, representing 29.63% of the total share of the developing A2aR/A2bR Antagonist landscape. The remaining 70.37% has been contributed by Phase 2 (18.52%), Phase 1/2 (14.81), Pre-clinical (18.52%), and Discovery (18.52%) assets.
- The pipeline of Adenosine Receptors (A2a/A2b) Antagonists is dominated by biotech companies headquartered in the United States with American companies holding the top 9 positions. They represent ~37.50% of all the pipeline Adenosine Receptors (A2a/A2b) Antagonists in clinical stages.
- Adenosine Receptors (A2a/A2b) Antagonists pipeline landscape includes 67% of Monotherapy trials and 33% of Combination Trials
- The pipeline of Adenosine Receptors (A2a/A2b) Antagonists is dominated by Phase 1 (8), Phase 2 (5), Phase 1/2 (4), Pre-clinical (5), and Discovery (5) assets.
- To be continued.....
Report Coverage:
- Indication Prioritisation: Adenosine Receptors (A2a/A2b) Antagonists market potential based on Indications
- Business Transactions & Strategies: Key collaborations and deal values
- Adenosine Receptors (A2a/A2b) Antagonists Pipeline Development: Product Profiles, Clinical Trials & Results
- Adenosine Receptors (A2a/A2b) Antagonists Acquisition Targets
- Adenosine Receptors (A2a/A2b) Antagonists Competitive Intelligence
- Recent & Upcoming events
TABLE OF CONTENTS
OVERVIEW
The A2aR/A2bR Antagonist Target BACKGROUND
- A2A/A2B Adenosine Receptor Antagonist-Overview
- Adenosine-A2A Receptor Pathway in Cancer Immunotherapy
- A2AR Antagonist and Clinical Progress
- A2B Adenosine Receptor (A2BAR) and Cancer
- Future Directions
- Key Lessons from the clinical trials targeting the adenosine pathway
A2aR/A2bR Antagonists PIPELINE ANALYSIS by Phases
- A2aR/A2bR Antagonists Development - Overview
- Pipeline Products by Stage of Development
- A2aR/A2bR Antagonists Competitive Landscape by Phases
- Pipeline Products by Company
- Pipeline Products by Indication and Phases
- A2aR/A2bR Antagonists Clinical & Regulatory Monotherapy & Combinations
- A2aR/A2bR Antagonists Products by MOA type
- A2aR/A2bR Antagonists- Assets by Indication/Phase
- A2aR/A2bR Antagonists Clinical & Regulatory Timelines
A2aR/A2bR Antagonists LICENSING, ACQUISITION, AND COLLABORATION DEALS
- A2aR/A2bR Antagonist Licensing, Acquisition, and Deal values
- A2aR/A2bR Antagonist Licensing by Transaction type and total amount size by Phases
A2aR/A2bR Antagonists Pipeline Landscape
- Profile Comparisons At-a-glance
- A2aR/A2bR Antagonist Pipeline Drug Profiles
- Phase II
- PBF 999 (Palobiofarma)
- Product Profile & Description
- Clinical Trials
- Collaborations
- Other Developments
- Etrumadenant (Arcus Biosciences/Gilead Sciences)
- Product Profile & Description
- Clinical Trials
- Collaborations
- Other Developments
- Imaradenant (AstraZeneca/Sosei Heptares)
- Product Profile & Description
- Clinical Trials
- Collaborations
- Other Developments
- Taminadenant (Novartis; Palobiofarma)
- Product Profile & Description
- Clinical Trials
- Collaborations
- Other Developments
- Inupadenant (iTeos Therapeutics)
- Product Profile & Description
- Clinical Trials
- Collaborations
- Other Developments
- PBF 999 (Palobiofarma)
- Phase I/II
- Ciforadenant (Corvus Pharmaceuticals; Genentech; Vernalis)
- Product Profile & Description
- Clinical Trials
- Collaborations
- Other Developments
- TT 702 (Teon Therapeutics)
- Product Profile & Description
- Clinical Trials
- Collaborations
- Other Developments
- PORT-6 (Portage Biotech)
- Product Profile & Description
- Clinical Trials
- Collaborations
- Other Developments
- PORT-7 (Portage Biotech)
- Product Profile & Description
- Clinical Trials
- Collaborations
- Other Developments
- Ciforadenant (Corvus Pharmaceuticals; Genentech; Vernalis)
- Phase I
- CS 3005 (CStone Pharmaceuticals)
- Product Profile & Description
- Clinical Trials
- Other Developments
- EXS21546 (Exscientia, Evotec)
- Product Profile & Description
- Clinical Trials
- Collaborations
- Other Developments
- INCB 106385 (Incyte Corporation)
- Product Profile & Description
- Clinical Trials
- Other Developments
- INT 1B3 (InteRNA Technologies)
- Product Profile & Description
- Clinical Trials
- Other Developments
- PBF 1129 (Palobiofarma)
- Product Profile & Description
- Clinical Trials
- Collaborations
- Other Developments
- M-1069 (Domain Therapeutics/Merck)
- Product Profile & Description
- Clinical Trials
- Collaborations
- Other Developments
- ILB-2109 (Innolake Biopharma)
- Product Profile & Description
- Clinical Trials
- Collaborations
- Other Developments
- DZD2269 (Dizal Pharma)
- Product Profile & Description
- Clinical Trials
- Collaborations
- Other Developments
- CS 3005 (CStone Pharmaceuticals)
- Pre-clinical
- ARX 001822 (AdoRx Therapeutics)
- Product Profile & Description
- Collaborations
- Other Developments
- Vipadenant (BMS)
- Product Profile & Description
- Collaborations
- Other Developments
- PORT-9 (Portage Biotech)
- Product Profile & Description
- Other Developments
- PORT-8 (Portage Biotech)
- Product Profile & Description
- Other Developments
- CPI-395 (Corvus Pharmaceuticals)
- Product Profile & Description
- Other Developments
- ARX 001822 (AdoRx Therapeutics)
- Discovery
- A2AR antagonist (Hanmi Pharmaceuticals)
- Product Profile & Description
- Other Developments
- LNC-001 (Lewis & Clark Pharmaceuticals)
- Product Profile & Description
- Collaborations
- Other Developments
- LNC-002 (Lewis & Clark Pharmaceuticals)
- Product Profile & Description
- Collaborations
- Other Developments
- LNC-003 (Lewis & Clark Pharmaceuticals)
- Product Profile & Description
- Collaborations
- Other Developments
- RT-AR001 (Lewis & Clark Pharmaceuticals)
- Product Profile & Description
- Collaborations
- Other Developments
- A2aR/A2bR Antagonists SWOT Analysis
- A2AR antagonist (Hanmi Pharmaceuticals)
- Phase II
A2aR/A2bR Antagonists Products Positioning
Matrix Analysis
Appendix
- About us
