|
市場調査レポート
商品コード
1170393
視神経脊髄炎スペクトラム障害(NMOSD)市場:1次調査(KOLの洞察) - 市場インテリジェンス - 疫学と2032年までの市場予測Neuromyelitis Optica Spectrum Disorder (NMOSD) | Primary Research (KOL's Insight) | Market Intelligence | Epidemiology & Market Forecast-2032 |
|||||||
| 視神経脊髄炎スペクトラム障害(NMOSD)市場:1次調査(KOLの洞察) - 市場インテリジェンス - 疫学と2032年までの市場予測 |
|
出版日: 2022年12月07日
発行: Mellalta Meets LLP
ページ情報: 英文 196 Pages
納期: 即納可能
|
- 全表示
- 概要
- 目次
当レポートでは、世界の視神経脊髄炎スペクトラム障害(NMOSD)市場について調査し、市場の現状とともに、症例数の動向、患者動向、競合製品の市場における位置づけ、市場の機会などを提供しています。
目次
エグゼクティブサマリー
視神経脊髄炎スペクトラム障害(NMOSD)疾患の背景
- 視神経脊髄炎スペクトラム障害(NMOSD):主なハイライト
- 意味
- 症状と原因
- 分類
- 病態生理学
- NMOSDの病態生理学的メカニズムと治療標的
- 診断
疫学の推定と2032年までの予測
- 主な調査結果
- 手法とデータソース
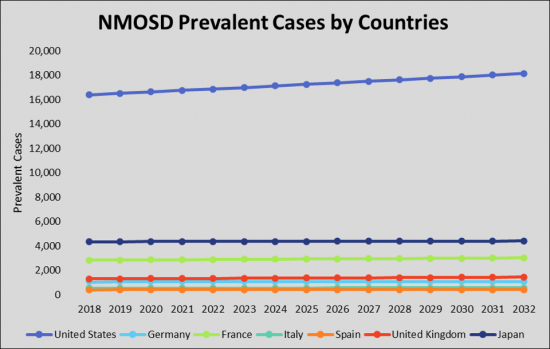
- 視神経脊髄炎スペクトラム障害(NMOSD)の国別有病件数(米国、ドイツ、フランス、イタリア、スペイン、英国、および日本)
- 国別の視神経脊髄炎スペクトラム障害(NMOSD)の急性および慢性治療件数
- 国別の慢性難治性/再発性(R/R)視神経脊髄炎スペクトラム障害(NMOSD)の有病件数
視神経脊髄炎スペクトラム障害(NMOSD)の疫学とモデルパラメータの主な情報源
- 米国
- ドイツ
- フランス
- イタリア
- スペイン
- 英国
- 日本
現在の治療法と医療行為
- 治療と医療行為
- NMOSD治療アルゴリズム
上市済み薬
- エクリズマブ
- サトラリズマブ
- イネビリズマブ
アンメットニーズ
新たな治療法
- 製品分析
- ラブリズマブ
- テリタシセプト
- SHR1459
- BAT4406F
- バトクリマブ
- XT-0528
- ウブリツキシマブ
- Lu AG06466
- Equecabtagene Autoleucel
- CRD1
視神経脊髄炎スペクトラム障害(NMOSD)- 費用負担と処方箋調査
将来の治療パラダイム
現在および新しい治療法の年間費用
市場の見通し
- 主な調査結果
- 2032年までの国別市場予測
国別市場予測
- 米国
- ドイツ
- フランス
- イタリア
- スペイン
- 英国
- 日本
市場促進要因と抑制要因
We see great future treatment opportunity for the NMOSD market because of the launch of the emerging therapies like Ravulizumab (Alexion Pharmaceuticals), Telitacicept (RC-Pharma), SHR1459 (Reistone Biopharma) and other potential therapies, providing the global solution for "optic neuritis with spinal cord manifestations and other neurologic disorders associated with the serum aquaporin-4 immunoglobulin G antibodies".
Neuromyelitis Optica Spectrum Disorder (NMOSD) is an inflammatory, demyelinating, antibody-mediated disease of the central nervous system (CNS) that predominantly targets the optic nerves, brainstem, and spinal cord. The NMOSD involves aquaporin 4 (AQP4) which is a membrane-bound protein with a high concentration in a certain region of the central nervous system, such as the optic nerve, and the spinal cord which is being attacked by the IgG antibody.
Neuromyelitis Optica Spectrum Disorder (NMOSD)- Epidemiology
NMOSD can be categorized into two pathophysiological entities depending on the presence or absence of AQP4-Ab. In approx. 80% of cases, AQP4-Ab can be detected, which causes a primarily astrocytopathic disease. Interestingly, in about 50% of the AQP4-Ab seronegative NMOSD patients autoantibodies against myelin-oligodendrocyte-glycoprotein (MOG-Ab) can be seen, whose pathomorphological correlate is primarily oligodendrocytopathic.AQP4-IgG testing in cases with good clinical reason to suspect NMOSD may be of value and that a positive AQP4-IgG test lessens the clinical and radiologic requirements for a diagnosis of NMOSD; these principles guided the development of the diagnostic criteria proposed by the IPND. The 2015 diagnostic criteria outline a stratified diagnosis based on the presence or absence of serum aquaporin-4 immunoglobulin G antibodies (AQP4-IgG), which are highly specific for NMOSD. Most-though not all-patients with NMO have detectable serum antibodies that target AQP4-IgG.
As per estimates, the United States accounted for the highest prevalence of Neuromyelitis Optica Spectrum Disorder (NMOSD) cases are expected to reach ~18,152 by 2032 during the study period.

Neuromyelitis Optica Spectrum Disorder (NMOSD)- Current Market Size & Forecast Trends
The current therapies market to treat Neuromyelitis Optica Spectrum Disorder (NMOSD) has transformed in recent years with new options for a disease that have the potential to significantly improve patient outcomes. In countries where all three of these new treatments are approved for clinical use, clinicians are now faced with a changing landscape of options to offer their patients with NMOSD, who might have different priorities among efficacy, safety, cost, monitoring burden, and logistics. The current therapies market to treat NMOSD is large as 100% of the affected population will be prescribed with immunosuppressive agents as the first line of therapy.
Currently, three medications have been approved for the treatment of NMOSD and these include satralizumab, eculizumab and inebilizumab. These medications have shown promising results concerning short-term efficacy. However, there is no data on long-term safety and efficacy in NMOSD patients and their efficacy in AQP4-Ab negative NMOSD patients is not yet known. At present, drugs such as azathioprine, rituximab and mycophenolate mofetil are being used to prevent relapse for patients with NMOSD. However, recurrent relapse in patients with NMOSD is still common which is due to the lack of specific therapeutic target. Additionally, some patients are intolerable to certain treatments due to their side effects, such as avascular necrosis of femoral head and hepatic impairment.
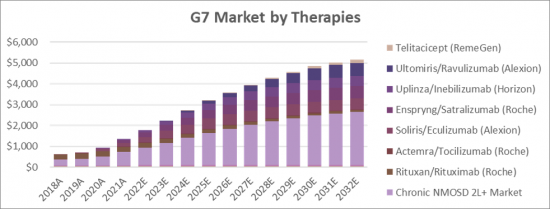
The Neuromyelitis Optica Spectrum Disorder (NMOSD) therapeutics market is expected to increase at a significant rate during the figure time frame, 2021 to 2032. In 2021, the market is developing at a consistent rate and the market is relied upon to ascend over the projected horizon with the rise in adoption strategies by key market players. The therapy market is expected to experience high growth throughout our study period, increasing from USD 362 million in 2018 to USD 2.64 billion in 2032, representing compound annual growth (CAGR) of 15.25%. Primary drivers of this growth will be the uptake of the existing drugs i.e., Soliris/Eculizumab (Alexion), Enspryng/Satralizumab (Roche), and Uplinza/Inebilizumab (Horizon) as well as the launch of the new drugs i.e., Ravulizumab (Alexion) Telitacicept (RemeGen).

Report Highlights:
- Neuromyelitis Optica Spectrum Disorder (NMOSD) - Current Market Trends
- Neuromyelitis Optica Spectrum Disorder (NMOSD) - Current & Forecasted Cases across the G7 Countries
- Neuromyelitis Optica Spectrum Disorder (NMOSD) - Market Opportunities And Sales Potential for Agents
- Neuromyelitis Optica Spectrum Disorder (NMOSD) - Patient-based Market Forecast to 2030
- Neuromyelitis Optica Spectrum Disorder (NMOSD) - Untapped Business Opportunities
- Neuromyelitis Optica Spectrum Disorder (NMOSD) - Product Positioning Vis-a-vis Competitors' Products
- Neuromyelitis Optica Spectrum Disorder (NMOSD) - KOLs Insight
Table of Contents
Executive Summary
- Key Findings
Neuromyelitis Optica Spectrum Disorder (NMOSD) Disease Background
- Neuromyelitis Optica Spectrum Disorder (NMOSD): Key Highlights
- Definition
- Symptoms & Causes
- Classifications
- Pathophysiology
- Pathophysiologic Mechanisms and Therapeutic Targets in NMOSD
- Diagnosis
Epidemiology Estimated and Forecast to 2032
- Key Findings
- Methods and Data Sources
- Country Specific Prevalent Cases of Neuromyelitis Optica Spectrum Disorder (NMOSD) (US, Germany, France, Italy, Spain, UK, and Japan)
- Country Specific Acute & Chronic Treated Cases of Neuromyelitis Optica Spectrum Disorder (NMOSD)
- Country Specific Chronic Refractory/Relapsed (R/R) Prevalent cases of Neuromyelitis Optica Spectrum Disorder (NMOSD)
Key Sources for Neuromyelitis Optica Spectrum Disorder (NMOSD) Epidemiology and Model Parameters
- United States
- United States Prevalent Cases of Neuromyelitis Optica Spectrum Disorder (NMOSD)
- United States Acute & Chronic Treated Cases of Neuromyelitis Optica Spectrum Disorder (NMOSD)
- United States Chronic Refractory/Relapsed (R/R) Prevalent cases of Neuromyelitis Optica Spectrum Disorder (NMOSD)
- Germany
- Germany Prevalent Cases of Neuromyelitis Optica Spectrum Disorder (NMOSD)
- Germany Acute & Chronic Treated Cases of Neuromyelitis Optica Spectrum Disorder (NMOSD)
- Germany Chronic Refractory/Relapsed (R/R) Prevalent cases of Neuromyelitis Optica Spectrum Disorder (NMOSD)
- France
- France Prevalent Cases of Neuromyelitis Optica Spectrum Disorder (NMOSD)
- France Acute & Chronic Treated Cases of Neuromyelitis Optica Spectrum Disorder (NMOSD)
- France Chronic Refractory/Relapsed (R/R) Prevalent cases of Neuromyelitis Optica Spectrum Disorder (NMOSD)
- Italy
- Italy Prevalent Cases of Neuromyelitis Optica Spectrum Disorder (NMOSD)
- Italy Acute & Chronic Treated Cases of Neuromyelitis Optica Spectrum Disorder (NMOSD)
- Italy Chronic Refractory/Relapsed (R/R) Prevalent cases of Neuromyelitis Optica Spectrum Disorder (NMOSD)
- Spain
- Spain Prevalent Cases of Neuromyelitis Optica Spectrum Disorder (NMOSD)
- Spain Acute & Chronic Treated Cases of Neuromyelitis Optica Spectrum Disorder (NMOSD)
- Spain Chronic Refractory/Relapsed (R/R) Prevalent cases of Neuromyelitis Optica Spectrum Disorder (NMOSD)
- United Kingdom
- United Kingdom Prevalent Cases of Neuromyelitis Optica Spectrum Disorder (NMOSD)
- United Kingdom Acute & Chronic Treated Cases of Neuromyelitis Optica Spectrum Disorder (NMOSD)
- United Kingdom Chronic Refractory/Relapsed (R/R) Prevalent cases of Neuromyelitis Optica Spectrum Disorder (NMOSD)
- Japan
- Japan Prevalent Cases of Neuromyelitis Optica Spectrum Disorder (NMOSD)
- Japan Acute & Chronic Treated Cases of Neuromyelitis Optica Spectrum Disorder (NMOSD)
- Japan Chronic Refractory/Relapsed (R/R) Prevalent cases of Neuromyelitis Optica Spectrum Disorder (NMOSD)
Current Therapies and Medical Practice
- Treatments & Medical Practices
- NMOSD Treatment Algorithm
Marketed Drug
- Eculizumab (Alexion Pharmaceuticals)
- Satralizumab (Roche)
- Inebilizumab (Horizon Therapeutics/Viela Bio)
Unmet Needs
Emerging Therapies
- Pipeline Overview
- Therapeutic Developments Pipeline for Neuromyelitis Optica Spectrum Disorder (NMOSD)
- Product Analysis
- Ravulizumab (Alexion Pharmaceuticals)
- Product Profile
- Clinical Development
- Sales & Market Opportunity by 2032
- Telitacicept (RC-Pharma)
- Product Profile
- Clinical Development
- Sales & Market Opportunity by 2032
- SHR1459 (Reistone Biopharma)
- Product Profile
- Clinical Development
- Sales & Market Opportunity by 2032
- BAT4406F (Bio-Thera Solutions)
- Product Profile
- Clinical Development
- Sales & Market Opportunity by 2032
- Batoclimab (Harbour BioMed)
- Product Profile
- Clinical Development
- XT-0528 (Xenter Therapeutics/Arrien Pharmaceuticals/Boston Pharmaceuticals)
- Product Profile
- Clinical Development
- Ublituximab (TG Therapeutics)
- Product Profile
- Clinical Development
- Lu AG06466 (Lundbeck/Abide Therapeutics)
- Product Profile
- Clinical Development
- Equecabtagene Autoleucel (Nanjing IASO Biotherapeutic Co.,Ltd/ Innovent Biologics, Inc.)
- Product Profile
- Clinical Development
- CRD1 (Merck)
- Product Profile
- Ravulizumab (Alexion Pharmaceuticals)
- Product Analysis
Neuromyelitis Optica Spectrum Disorder (NMOSD)- Cost Burden & Prescriptions survey
Future Treatment Paradigm
- Neuromyelitis Optica Spectrum Disorder (NMOSD) Competitor Landscape and Approvals Anticipated
- Future Treatment Algorithms and Competitor Positioning
- Key Data Summary for Emerging Treatment
Annual Cost of Current & Emerging Therapies
Market Outlook
- Key Findings
- Country Specific Market Forecast to 2032
- G7 total Market for Neuromyelitis Optica Spectrum Disorder (NMOSD) 2018-2032 (USD Million)
- G7 total Market for Neuromyelitis Optica Spectrum Disorder (NMOSD) by Therapies 2018-2032 (USD Million)
Market Forecast by Country
- United States
- United States Market for Neuromyelitis Optica Spectrum Disorder (NMOSD) 2018-2032 (USD Million)
- United States Market for Neuromyelitis Optica Spectrum Disorder (NMOSD) by Therapies 2018-2032 (USD Million)
- Germany
- Germany Market for Neuromyelitis Optica Spectrum Disorder (NMOSD) 2018-2032 (USD Million)
- Germany Market for Neuromyelitis Optica Spectrum Disorder (NMOSD) by Therapies 2018-2032 (USD Million)
- France
- France Market for Neuromyelitis Optica Spectrum Disorder (NMOSD) 2018-2032 (USD Million)
- France Market for Neuromyelitis Optica Spectrum Disorder (NMOSD) by Therapies 2018-2032 (USD Million)
- Italy
- Italy Market for Neuromyelitis Optica Spectrum Disorder (NMOSD) 2018-2032 (USD Million)
- Italy Market for Neuromyelitis Optica Spectrum Disorder (NMOSD) by Therapies 2018-2032 (USD Million)
- Spain
- Spain Market for Neuromyelitis Optica Spectrum Disorder (NMOSD) 2018-2032 (USD Million)
- Spain Market for Neuromyelitis Optica Spectrum Disorder (NMOSD) by Therapies 2018-2032 (USD Million)
- United Kingdom
- United Kingdom Market for Neuromyelitis Optica Spectrum Disorder (NMOSD) 2018-2032 (USD Million)
- United Kingdom Market for Neuromyelitis Optica Spectrum Disorder (NMOSD) by Therapies 2018-2032 (USD Million)
- Japan
- Japan Market for Neuromyelitis Optica Spectrum Disorder (NMOSD) 2018-2032 (USD Million)
- Japan Market for Neuromyelitis Optica Spectrum Disorder (NMOSD) by Therapies 2018-2032 (USD Million)
Market Drivers and Constraints
- What Factors Are Driving the Market for Neuromyelitis Optica Spectrum Disorder (NMOSD)?
- What Factors Are Constraining the Market for Neuromyelitis Optica Spectrum Disorder (NMOSD)?
