|
市場調査レポート
商品コード
1114725
ER+/HER2-転移性乳癌(mBC)市場:1次調査(KOLの洞察) - 市場インテリジェンス - 疫学と2032年までの市場予測ER+/HER2- Metastatic Breast Cancer (mBC)| Primary Research (KOL's Insight) | Market Intelligence | Epidemiology & Market Forecast-2032 |
|||||||
| ER+/HER2-転移性乳癌(mBC)市場:1次調査(KOLの洞察) - 市場インテリジェンス - 疫学と2032年までの市場予測 |
|
出版日: 2022年08月15日
発行: Mellalta Meets LLP
ページ情報: 英文 356 Pages
納期: 即納可能
|
- 全表示
- 概要
- 目次
世界のER+/HER2-転移性乳癌(mBC)の市場規模は、2018年~2032年の調査期間中に6.6%のCAGRで拡大し、2032年までに158億米ドルになると予測されています。
当レポートでは、世界のER+/HER2-転移性乳癌(mBC)市場について調査し、市場の現状とともに、症例数の動向、患者動向、競合製品の市場における位置づけ、市場の機会などを提供しています。
目次
エグゼクティブサマリー
ER+/HER2-転移性乳癌(mBC)疾患の背景
- ER+/HER2-転移性乳癌の定義
- 症状と原因
- 病因
- 病期
ER+/HER2-転移性乳癌(mBC)の診断
疫学と患者集団
ER+/HER2-転移性乳癌(mBC)の疫学とモデルパラメータの主な情報源
- 米国
- ドイツ
- フランス
- イタリア
- スペイン
- 英国
- 日本
現在の治療法と医療行為
ER+/HER2-転移性乳癌(mBC)-治療研究
アンメットニーズ
ER+/HER2-転移性乳癌(mBC)-市販薬
新たな治療法
ER+/HER2-転移性乳癌(mBC)-価格と償還
将来の治療パラダイム
現在および新しい治療法の年間費用
市場の見通し
2032年までの国別市場予測
- 主要医薬品市場におけるER+/HER2-転移性乳癌(mBC)の治療薬売上、2018年~2032年
- ER+/HER2-転移性乳癌(mBC)治療の患者シェアと薬剤別
国別市場予測
- 米国
- ドイツ
- フランス
- イタリア
- スペイン
- 英国
- 日本
市場の促進要因と抑制要因
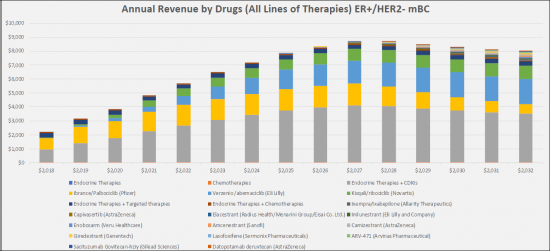
The ER + / HER2- metastatic breast cancer (mBC) market is relatively large with the highest market share holding CDK4 / 6 drugs, e.g. Pfizer's Ibrance (palbociclib), Novartis's Kisqali (ribociclib) and Eli Lilly's Verzenio (abemaciclib). By 2032, these drugs will continue to hold the largest share of the market, but we also expect the market to expand with the addition of new oral SERDs/SARMs/SERMs that will take a combined share of 19.1%, antibody drug conjugates with a share of the 2.9% and AKT inhibitors with a 2.6% share. The sales of the emerging therapies for the treatment of ER+/HER2- mBC in the study countries (United States, France, Germany, Italy, Spain, United Kingdom and Japan) will experience high growth over the 2022-2032 study period, adding a value estimated at a total market of $ 5.6 billion by 2032.
"Currently, -50% of ER+/HER2- mBC patients that are treated with ETs develop resistance or don't respond to initial ET. Endocrine therapies (Els) have advanced the care of patients with ER+/HER2- mBC, yet due to complex tumor mechanisms and endocrine resistance, progression still persists."
Breast cancer tumors are considered estrogen receptor (ER) and/or progesterone receptor (PR) positive if ≥1% of tumor cell nuclei are immunoreactive. Breast cancer cells that have receptors for either hormone are considered hormone receptor-positive (HR+), or just hormone-positive. Breast tumors may be positive for estrogen receptors (ER+), progesterone receptors (PR+) or both (ER/PR+). About 80 percent of all HR+ breast cancers are ER+ or ER/PR+. The average age at diagnosis for people with Breast cancer is 64, and most people are diagnosed between the ages of 62 and younger. ER+ breast cancer is not common in people younger than age 45.
Generally, the type of breast cancer a woman has governs her treatment choices. Tumors that express estrogen receptors (ER+) grow in the presence of estrogen. They are usually treated with endocrine therapy that blocks estrogen from attaching to estrogen receptors, thereby slowing tumor growth. Once the cancer has progressed to the metastatic setting, treatment is generally not curative and the goal is to prolong patients' lives, delay disease progression and minimize cancer-related symptoms.
Currently, overcoming acquired endocrine therapy resistance and improving efficacy outcomes in the 2L+ setting remains a key unmet need in the treatment of ER+/HER2- Metastatic Breast Cancer. A number of SERDs in development have shown improved anti-tumor activity compared to fulvestrant in tumors with ESR1 hotspot mutations that are resistant to AIs. Emerging Protein degraders may be well positioned to overcome resistance as they degrade the estrogen receptor rather than inhibit its action, and they only need to maintain transient binding
ER+/HER2- Metastatic Breast Cancer (mBC)- Epidemiology
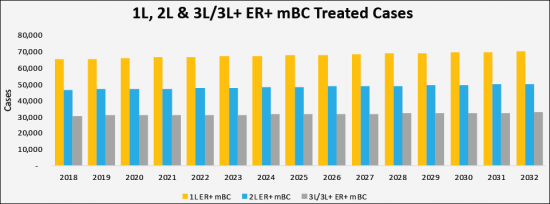
The total incidence cases in the G7 countries are anticipated to rise to 290,458 cases by 2032 for the study period (2018-2032). As per estimates, the the United States accounted for the highest incident of ER+/HER2- Metastatic Breast Cancer (mBC) cases in 2018 which was 49,239 cases and are expected to reach 53,540 cases by 2032 with a CAGR of 0.6% for the study period. Among the EU5, Germany had the highest ER+/HER2- Metastatic Breast Cancer (mBC) cases, followed by the France, UK, Italy and Spain. Japan is reported to have the highest number of cases after United States. Japan is reported to have the highest number of cases after the United States.

At the time of diagnosis, approximately 90% of breast cancers are not metastatic; however, in addition to the 10% metastatic at diagnosis, approximately 10-60% of localized breast cancers develop systemic relapse. Furthermore, the prognosis for ER+ mBC is a median five-year survival rate of 27%.
The 5-year relative survival rate is approximately 98.4% for women with localized breast cancer, although the rates decrease to approximately 90.7% for patients with regional involvement and 39.3% for patients with distant metastasis. Breast cancer management depends on biomarkers including estrogen receptor, progesterone receptor, and human epidermal growth factor receptor 2 (ER/PR/HER2). ER is an important prognostic biomarker in breast cancer. ER-positive tumors have a better prognosis than ER-negative tumours. Overall survival, disease free survival, recurrence/relapse-free survival, 5-year survival, time to treatment failure and time to recurrence have all been shown to be positively associated with ER expression.
ER+/HER2- Metastatic Breast Cancer (mBC)- Current Market Size & Forecast Trends
The estrogen receptor-positive and human epidermal growth factor receptor 2 negative (ER+/HER2-)metastatic breast cancer (mBC) therapeutics market is expected to experience high growth throughout our study period (i.e. 2018 to 2032) to USD 15.8 billion, representing compound annual growth (CAGR) of 6.6%.
The current therapies market to treat ER+/HER2- metastatic Breast Cancer (mBC) is large in the G7 Countries (United States, European 5 countries and Japan). The United States captured the highest market share in 2022 as compared to European 5 countries and Japan. We expect that with the launch of the new therapies, the current treatment landscape will grow, catering to the need of the metastatic patient's group in all lines.
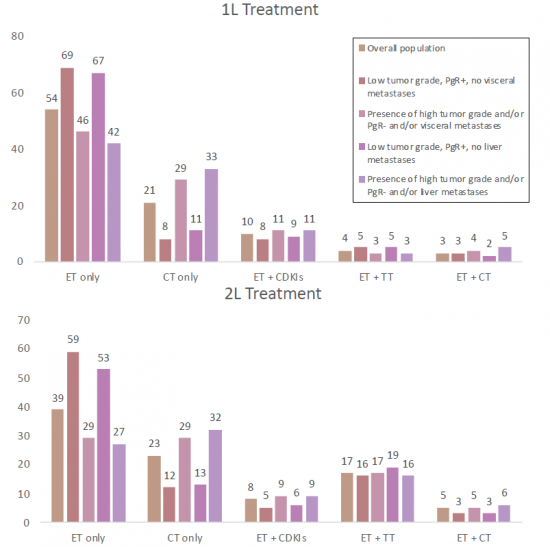
Endocrine therapy (ET) remains the backbone of treatment in these cases, improving survival and quality of life. However, treatment can lose effectiveness due to primary or acquired endocrine resistance. The ER+/HER2- cases are typically treated with endocrine therapy (ET), such as selective estrogen receptor inhibitors and aromatase inhibitors (AIs). Although ET remains the backbone of HR+ breast cancer treatment, resistance develops in up to 50% of patients with advanced breast cancer. To combat resistance, targeted therapies have been approved for HR+ breast cancer. ER+ tumors are fueled by the hormone estrogen, and they can be treated with endocrine drugs that inhibit that process. Over the years, an array of related medications has arisen, including aromatase inhibitors, ER degraders (such as Faslodex), ER modifiers, and gonadotropin-releasing hormone agonists. Notably, drugs known as CDK4/6 inhibitors have hit the scene in the past decade, including palbociclib, ribociclib, and abemaciclib; in combination with aromatase inhibitors, these can prolong survival and prevent recurrence far more effectively than aromatase inhibitors alone. Still, many ER+ tumors eventually develop resistance to those combinations. Fortunately, they can now be treated with, yet another class of drugs known as PI3K inhibitors, and additional drugs are continuously introduced in clinical trials.

In the 2018-2032 forecast period, we expect a greater uptake of the CDK4/6 inhibitors [Ibrance/Palbociclib (Pfizer); Verzenio /abemaciclib (Eli Lilly) and Kisqali/ribociclib (Novartis)], targeted therapies such as Piqray/ alpelisib (Novartis Pharmaceuticals), Lynparza/Olaparib (AstraZeneca) and also the launch of the new drugs i.e., Chemotherapies -Ixempra/Ixabepilone (Allarity Therapeutics), AKT Inhibitors-Capivasertib (AstraZeneca), Selective Estrogen/androgen Receptor Degraders/modulators (SERDs/SERMs/SARMs)-Elacestrant (Radius Health/Menarini Group/Eisai Co. Ltd.), Imlunestrant (Eli Lilly and Company), Enobosarm (Veru Healthcare), Amcenestrant (Sanofi), Camizestrant (AstraZeneca), Giredestrant (Genentech), Lasofoxifene (SermonixPharmaceuticals), ARV-471 (Arvinas Pharmaceutical), Antibody drug conjugates (ADC)-Sacituzumab Govitecan-hziy (Gilead Sciences) and Datopotamab deruxtecan (AstraZeneca).

Report Highlights:
- ER+/HER2-mBC - Current Market Trends
- ER+/HER2-mBC - Current & Forecasted Cases across the G7 Countries
- ER+/HER2-mBC- Market Opportunities And Sales Potential for Agents
- ER+/HER2-mBC - Patient-based Market Forecast to 2032
- ER+/HER2-mBC - Untapped Business Opportunities
- ER+/HER2-mBC - Product Positioning Vis-a-vis Competitors' Products
- ER+/HER2-mBC - KOLs Insight
Table of Contents
Executive Summary
- Key Findings
ER+/HER2- Metastatic Breast Cancer (mBC) Disease Background
- ER+/HER2- Metastatic Breast Cancer Definition
- Symptoms and Causes
- Pathogenesis
- Stages
ER+/HER2- Metastatic Breast Cancer (mBC) Diagnosis
- Diagnosis & Diagnostic Guidelines
- Biomarkers
- ESR1 Hotspot Mutations Lead to Resistance
Epidemiology and Patient Populations
- Key Findings
- Methods and data Sources
- Country Specific Incident Populations with ER+/HER2- Metastatic Breast Cancer (mBC) (US, Germany, France, Italy, Spain, UK, and Japan)
- Country Specific Incident Population with ER+/HER2- Metastatic Breast Cancer (mBC)
- Country Specific Treated Cases of ER+/HER2- Metastatic Breast Cancer (mBC) by Line of Therapies
Key Sources for ER+/HER2- Metastatic Breast Cancer (mBC) Epidemiology and Model Parameters
- United States
- United States Incident Populations with ER+/HER2- Metastatic Breast Cancer (mBC)
- United States Incident Populations with ER+/HER2- Metastatic Breast Cancer (mBC)
- United States Treated Cases of ER+/HER2- Metastatic Breast Cancer (mBC) by Line of Therapies
- Germany
- Germany Incident Populations with ER+/HER2- Metastatic Breast Cancer (mBC)
- Germany Incident Populations with ER+/HER2- Metastatic Breast Cancer (mBC)
- Germany Treated Cases of ER+/HER2- Metastatic Breast Cancer (mBC) by Line of Therapies
- France
- France Incident Populations with ER+/HER2- Metastatic Breast Cancer (mBC)
- France Incident Populations with ER+/HER2- Metastatic Breast Cancer (mBC)
- France Treated Cases of ER+/HER2- Metastatic Breast Cancer (mBC) by Line of Therapies
- Italy
- Italy Incident Populations with ER+/HER2- Metastatic Breast Cancer (mBC)
- Italy Incident Populations with ER+/HER2- Metastatic Breast Cancer (mBC)
- Italy Treated Cases of ER+/HER2- Metastatic Breast Cancer (mBC) by Line of Therapies
- Spain
- Spain Incident Populations with ER+/HER2- Metastatic Breast Cancer (mBC)
- Spain Incident Populations with ER+/HER2- Metastatic Breast Cancer (mBC)
- Spain Treated Cases of ER+/HER2- Metastatic Breast Cancer (mBC) by Line of Therapies
- United Kingdom
- United Kingdom Incident Populations with ER+/HER2- Metastatic Breast Cancer (mBC)
- United Kingdom Incident Populations with ER+/HER2- Metastatic Breast Cancer (mBC)
- United Kingdom Treated Cases of ER+/HER2- Metastatic Breast Cancer (mBC) by Line of Therapies
- Japan
- Japan Incident Populations with ER+/HER2- Metastatic Breast Cancer (mBC)
- Japan Incident Populations with ER+/HER2- Metastatic Breast Cancer (mBC)
- Japan Treated Cases of ER+/HER2- Metastatic Breast Cancer (mBC) by Line of Therapies
Current Therapies and Medical Practice
- Treatment & Medical Practices
- Benchmark Efficacy Data for CDK4/6 Inhibitors in 1L-3L ER+/HER2- mBC
- US Treatment Algorithm of Metastatic ER+ve Breast Cancer
- ER+/HER2- Metastatic Breast Cancer (mBC)-NCCN Treatment
- ER+/HER2+ Metastatic Breast Cancer (mBC)- NCCN Treatment Guidelines
- Treatment Algorithm of ER-positive/HER2-negative MBC- ESMO Guideline
- ER+/HER2- Metastatic Breast Cancer (mBC)- ESMO Guidelines
- KOLs Opinion on Current Treatment Option
ER+/HER2- Metastatic Breast Cancer (mBC)- Treatment Studies
- Endocrine therapy as a first-line treatment for ER+/HER2- Metastatic Breast Cancer in United States
- Real-world treatment patterns in a subset of HR+/HER2- advanced breast cancer patients with poor prognostic factors
- Preferred treatment sequences for HR+/HER2- Advanced Breast Cancer in Spain, France, Italy and Germany
- First-line treatment patterns in HR+/HER2- locally advanced or metastatic breast cancer in France, Germany, Italy, and Spain
Unmet Needs
ER+/HER2- Metastatic Breast Cancer (mBC)- Marketed Drug Chapters
- Benchmarking Studies
- Marketed Drug Chapters
- Ibrance/Palbociclib (Pfizer)
- Product Profile
- Clinical Development
- Sales & Market Opportunity by 2032
- Verzenio /abemaciclib (Eli Lilly)
- Product Profile
- Clinical Development
- Sales & Market Opportunity by 2032
- Kisqali/ribociclib (Novartis)
- Product Profile
- Clinical Development
- Sales & Market Opportunity by 2032
- Ibrance/Palbociclib (Pfizer)
Emerging Therapies
- Pipeline Overview
- Therapeutic Developments Pipeline for ER+/HER2- Metastatic Breast Cancer (mBC)
- Product Analysis
- Camizestrant (AstraZeneca)
- Product Profile
- Clinical Development
- Sales & Market Opportunity by 2032
- Giredestrant (Genentech)
- Product Profile
- Clinical Development
- Sales & Market Opportunity by 2032
- Elacestrant (Radius Health/Menarini Group/Eisai Co. Ltd.)
- Product Profile
- Clinical Development
- Sales & Market Opportunity by 2032
- Enobosarm (Veru Healthcare)
- Product Profile
- Clinical Development
- Sales & Market Opportunity by 2032
- Imlunestrant (Eli Lilly and Company)
- Product Profile
- Clinical Development
- Sales & Market Opportunity by 2032
- Amcenestrant (Sanofi)
- Product Profile
- Clinical Development
- Sales & Market Opportunity by 2032
- Capivasertib (AstraZeneca)
- Product Profile
- Clinical Development
- Datopotamab deruxtecan (AstraZeneca)
- Product Profile
- Clinical Development
- Lerociclib (G1 Therapeutics/EQRx/Genor Biopharma)
- Product Profile
- Clinical Development
- Sacituzumab govitecan (Gilead Sciences)
- Product Profile
- Clinical Development
- Lasofoxifene (Sermonix Pharmaceuticals)
- Product Profile
- Clinical Development
- ARV-471 (Arvinas Pharmaceutical)
- Product Profile
- Clinical Development
- Camizestrant (AstraZeneca)
- Product Analysis
ER+/HER2- Metastatic Breast Cancer (mBC)- Pricing & Reimbursement
Future Treatment Paradigm
- ER+/HER2- Metastatic Breast Cancer (mBC) Competitor Landscape and Approvals Anticipated
- Future Treatment Algorithms and Competitor Positioning
- Key Data Summary for Emerging Treatment
Annual Cost of Current & Emerging Therapies
Market Outlook
- Key Findings
Country Specific Market Forecast to 2032
- Sales of Drugs to Treat ER+/HER2- Metastatic Breast Cancer (mBC) in the Major Pharmaceutical Markets, 2018-2032
- Patient Share of ER+/HER2- Metastatic Breast Cancer (mBC) Therapies and by Drug
Market Forecast by Country
- United States
- United States Market for ER+/HER2- Metastatic Breast Cancer (mBC) 2018-2032 (USD Million)
- United States Market for ER+/HER2- Metastatic Breast Cancer (mBC) by Therapies 2018-2032 (USD Million)
- Germany
- Germany Market for ER+/HER2- Metastatic Breast Cancer (mBC) 2018-2032 (USD Million)
- Germany Market for ER+/HER2- Metastatic Breast Cancer (mBC) by Therapies 2018-2032 (USD Million)
- France
- France Market for ER+/HER2- Metastatic Breast Cancer (mBC) 2018-2032 (USD Million)
- France Market for ER+/HER2- Metastatic Breast Cancer (mBC) by Therapies 2018-2032 (USD Million)
- Italy
- Italy Market for ER+/HER2- Metastatic Breast Cancer (mBC) 2018-2032 (USD Million)
- Italy Market for ER+/HER2- Metastatic Breast Cancer (mBC) by Therapies 2018-2032 (USD Million)
- Spain
- Spain Market for ER+/HER2- Metastatic Breast Cancer (mBC) 2018-2032 (USD Million)
- Spain Market for ER+/HER2- Metastatic Breast Cancer (mBC) by Therapies 2020-2030 (USD Million)
- United Kingdom
- United Kingdom Market for ER+/HER2- Metastatic Breast Cancer (mBC) 2018-2032 (USD Million)
- United Kingdom Market for ER+/HER2- Metastatic Breast Cancer (mBC) by Therapies 2018-2032 (USD Million)
- Japan
- Japan Market for ER+/HER2- Metastatic Breast Cancer (mBC) 2018-2032 (USD Million)
- Japan Market for ER+/HER2- Metastatic Breast Cancer (mBC) by Therapies 2018-2032 (USD Million)
Market Drivers and Constraints
- What Factors Are Driving the Market for ER+/HER2- Metastatic Breast Cancer (mBC)?
- What Factors Are Constraining the Market for ER+/HER2- Metastatic Breast Cancer (mBC)?
