|
|
市場調査レポート
商品コード
1347336
患者由来異種移植片/PDXモデルの世界市場:タイプ別、移植方法別、腫瘍タイプ別、用途別、エンドユーザー別-2028年までの予測Patient-Derived Xenograft / PDX Model Market by Type, Implantation Method, Tumor Type, Application, End User - Global Forecast to 2028 |
||||||
|
● お客様のご希望に応じて、既存データの加工や未掲載情報(例:国別セグメント)の追加などの対応が可能です。 詳細はお問い合わせください。 |
|||||||
| 患者由来異種移植片/PDXモデルの世界市場:タイプ別、移植方法別、腫瘍タイプ別、用途別、エンドユーザー別-2028年までの予測 |
|
出版日: 2023年08月30日
発行: MarketsandMarkets
ページ情報: 英文 237 Pages
納期: 即納可能
|
- 全表示
- 概要
- 目次
患者由来異種移植片/PDXモデルの市場規模は、予測期間中に13.4%のCAGRで拡大し、2023年の3億6,600万米ドルから2028年には6億8,700万米ドルに達すると予測されています。
患者由来異種移植片/PDXモデル市場の世界の成長を促進する主な要因は、前臨床の予測可能性の向上、個別化医療の進展、癌の有病率の上昇です。しかし、動物モデルの使用をめぐる倫理的な懸念は、市場の成長をある程度抑制すると予想されます。
タイプ別では、患者由来異種移植片/PDXモデル市場はマウスモデルとラットモデルに分類されます。マウスモデルは、操作や調達が容易であること、ラットと比較して施設での観察下での飼育が必要最小限であることから、患者由来異種移植片/PDXモデル市場における製品の成長率が高くなっており、2023年の市場規模はマウスモデルが最大であり、急成長しています。
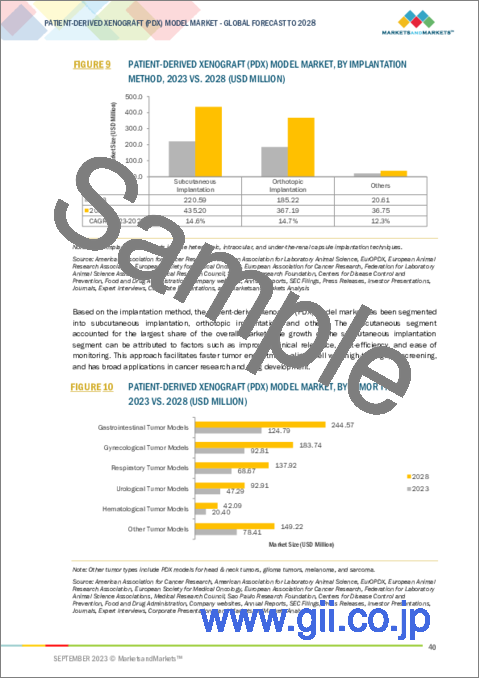
移植方法別では、市場は皮下移植、同所移植、その他の移植方法(異所移植、眼内移植、腎被膜下移植)に区分されます。2023年には、皮下移植が患者由来異種移植/PDXモデル市場で最大のシェアを占め、急成長しています。皮下移植では、患者由来の腫瘍組織を宿主動物(多くはマウス)の皮下に移植します。この方法は、他の移植方法に比べて比較的簡単で侵襲が少ないため、研究者にとって実用的な選択肢となっています。
北米は患者由来異種移植片/PDXモデル市場で最大のシェアを占めています。この地域は、高度に発達した強固なバイオ医薬品産業を有し、高度な研究・臨床インフラを備えています。このような環境は、学界、研究機関、製薬企業間の効率的な協力を促進し、PDXモデルの効果的な利用と採用を可能にしています。
第二に、北米の規制の枠組みは、前臨床および臨床研究におけるPDXモデルを含む革新的技術の統合をサポートしています。厳格な規制基準により、PDXモデルが検証され、医薬品開発プロセスに適用されることが保証されるため、投資家と業界の信頼が高まっています。
さらに、アジア太平洋はPDXモデル市場にとって有利な成長の可能性を秘めています。この地域は、強固なヘルスケアインフラ、急成長するバイオ医薬品産業、患者数の多さなどの恩恵を受け、PDXモデルの利用に理想的な環境を育んでいます。
当レポートでは、世界の患者由来異種移植片/PDXモデル市場について調査し、タイプ別、移植方法別、腫瘍タイプ別、用途別、エンドユーザー別、地域別動向、および市場に参入する企業のプロファイルなどをまとめています。
目次
第1章 イントロダクション
第2章 調査手法
第3章 エグゼクティブサマリー
第4章 重要考察
第5章 市場概要
- イントロダクション
- 市場力学
- 技術分析
- 顧客のビジネスに影響を与える動向/混乱
- サプライチェーン分析
- バリューチェーン分析
- エコシステム分析
- ポーターのファイブフォース分析
- 規制分析
- 価格分析
- 特許分析
- 2023年の主な会議とイベント
- 主要な利害関係者と購入基準
第6章 患者由来異種移植片/PDXモデル市場、移植方法別
- イントロダクション
- 皮下移植
- 同所性移植
- その他
第7章 患者由来異種移植片/PDXモデル市場、タイプ別
- イントロダクション
- マウスモデル
- ラットモデル
第8章 患者由来異種移植片/PDXモデル市場、用途別
- イントロダクション
- 前臨床薬の開発
- バイオマーカー分析
- トランスレーショナル研究
- バイオバンク
第9章 患者由来異種移植片/PDXモデル市場、エンドユーザー別
- イントロダクション
- 製薬会社およびバイオテクノロジー企業
- CRO
- 学術研究機関
第10章 患者由来異種移植片/PDXモデル市場、腫瘍タイプ別
- イントロダクション
- 消化管腫瘍モデル
- 婦人科腫瘍モデル
- 呼吸器腫瘍モデル
- 泌尿器腫瘍モデル
- 血液腫瘍モデル
- その他
第11章 患者由来異種移植片/PDXモデル市場、地域別
- イントロダクション
- 北米
- 欧州
- アジア太平洋
- ラテンアメリカ
- 中東・アフリカ
第12章 競合情勢
- 概要
- 大手企業が採用した主な戦略
- トップ4プレーヤーの収益シェア分析、2022年
- 上位4社の市場シェア分析、2020年~2022年
- 主要企業の企業評価マトリックス、2022年
- スタートアップ/中小企業(SMES)の企業評価マトリックス、2022年
- 競合ベンチマーキング
- 競争シナリオと動向
第13章 企業プロファイル
- 主要参入企業
- JSR CORPORATION
- WUXI APPTEC
- THE JACKSON LABORATORY
- CHARLES RIVER LABORATORIES
- TACONIC BIOSCIENCES, INC.
- ONCODESIGN PRECISION MEDICINE
- INOTIV, INC.
- PHARMATEST SERVICES
- HERA BIOLABS
- EPO BERLIN-BUCH GMBH
- XENTECH
- UROSPHERE
- ALTOGEN LABS
- ABNOVA CORPORATION
- GENESIS BIOTECHNOLOGY GROUP
- その他の企業
- BIOCYTOGEN PHARMACEUTICALS CO., LTD.
- CREATIVE ANIMODEL
- BIODURO-SUNDIA INC.
- ARAGEN LIFE SCIENCES
- SHANGHAI LIDE BIOTECH CO., LTD.
- CERTIS ONCOLOGY SOLUTIONS
- INNOSER
- IVRS AB
- BEIJING IDMO CO. LTD.
- SHANGHAI CHEM PARTNER CO. LTD.
第14章 付録
The patient-derived xenografts/ PDX model market is projected to reach USD 687 million by 2028 from USD 366 million in 2023, at a CAGR of 13.4% during the forecast period. The key factors driving the growth of the global patient-derived xenograft/ PDX model market are the enhanced preclinical predictability, advancement of personalized medicine, and rise in the prevalence of cancer. However, ethical concerns surrounding the use of animal models are expected to restrain market growth to a certain extent.
The patient-derived xenografts/ PDX models market has been segmented based on type, implantation method, tumor type, application, end-user, and region.
"By type, the mouse model segment accounted for the largest share of the patient-derived xenografts/ PDX models market."
Based on type, the patient-derived xenografts/ PDX models market is categorized into mouse model and rat model. The mouse model segment is the largest and fastest-growing segment in the market in 2023, owing to ease of manipulation and procurement, and the minimum requirement for keeping them under observation compared to rats in the facilities has led to a higher growth rate for products in the patient-derived xenografts/ PDX models market.
"By implantation method, subcutaneous implantation segment accounted for the largest share in the patient-derived xenografts/ PDX models market."
Based on implantation method, the market is segmented into subcutaneous implantation, orthotopic implantation, and other implantation methods (heterotopic implantation, intraocular implantation, and under the renal capsule). In 2023, the subcutaneous segment accounted for the largest share and the fastest-growing segment of the patient-derived xenografts/ PDX models market. Subcutaneous implantation involves implanting patient-derived tumor tissue under the skin of a host animal (often a mouse). This method is relatively simple and minimally invasive compared to other implantation methods, making it a practical choice for researchers.
"North America: the largest share of the patient-derived xenografts/ PDX models market."
North America accounted for the largest share of the patient-derived xenografts/ PDX models market. The region possesses a highly developed and robust biopharmaceutical industry, with advanced research and clinical infrastructure. This environment facilitates efficient collaboration between academia, research institutions, and pharmaceutical companies, enabling the effective utilization and adoption of PDX models.
Secondly, North America's regulatory framework supports the integration of innovative technologies, including PDX models, in preclinical and clinical research. The stringent regulatory standards ensure that PDX models are validated and applicable for drug development processes, thereby bolstering investor and industry confidence.
Moreover, the APAC region offers lucrative growth potential for the PDX models market. This region benefits from a robust healthcare infrastructure, a burgeoning biopharmaceutical industry, and a large patient population, fostering an ideal setting for PDX model utilization.
The primary interviews conducted for this report can be categorized as follows:
- By Respondent: Supply Side- 63% and Demand Side- 37%
- By Designation: Executives - 45%, Research Scientists- 30%, and Managers - 25%
- By Country: North America- 40%, Europe- 25%, Asia Pacific- 20%, Latin America - 10%, and Middle East Africa- 5%
Prominent Players
- JSR Corporation (Japan)
- Wuxi Apptec (China)
- The Jackson Laboratory (US)
- Charles River Laboratories International, Inc. (US)
- Taconic Biosciences, Inc. (US)
- Oncodesign Precision Medicine (France)
- Inotiv, Inc. (US)
- Pharmatest Services (Finland)
- Hera BioLabs (US)
- EPO Berlin-Buch GmbH (Germany)
- Xentech (France)
- Urosphere (France)
- Altogen Labs (US)
- Abnova Corporation (US)
- Genesis Biotechnology Group (US)
- Biocytogen Pharmaceuticals Co., Ltd. (China)
- Creative Animodel (US)
- BioDuro-Sundia (US)
- Aragen Life Sciences (India)
- Shanghai LIDE Biotech Co., Ltd. (China)
- Certis Oncology Solutions (US)
- InnoSer (Netherlands)
- IVRS AB (Sweden)
- Beijing IDMO Co. Ltd. (China)
- Shanghai Chempartner Co. Ltd. (China)
Research Coverage:
This report provides a detailed picture of the patient-derived xenografts/ PDX models market. It aims at estimating the size and future growth potential of the market across different segments, such as type, implantation method, tumor type, application, and end-user, and region (North America, Europe, Asia Pacific, Latin America, and Middle East Africa). The report also includes an in-depth competitive analysis of the key market players, along with their company profiles, recent developments, and key market strategies.
Key Benefits of Buying the Report:
The report will help market leaders/new entrants by providing them with the closest approximations of the revenue numbers for the overall patient-derived xenografts/ PDX models market and its segments. It will also help stakeholders better understand the competitive landscape and gain more insights to better position their business and make suitable go-to-market strategies. This report will enable stakeholders to understand the market's pulse and provide them with information on the key market drivers, restraints, trends, opportunities, and challenges.
The report provides insights on the following pointers:
- Analysis of key drivers (growing demand for personalized medicine, technological advancements in PDX technology, and rising investments for cancer research), restraints (FDA's announcement to discontinue animal models for clinical trials), opportunities (emergence of CRISPR in biomedical research), and challenges (development of alternative animal testing methods) influencing the growth of the patient-derived xenografts/ PDX models market.
- Product Development/ Innovation: Detailed insights on upcoming technologies, research and development activities, and new product launches in the patient-derived xenografts/ PDX models market.
- Market Development: Comprehensive information about lucrative markets- the report analyses the patient-derived xenografts/ PDX models market across varied regions.
- Market Diversification: Exhaustive information about new products, untapped geographies, recent developments, and investments in the patient-derived xenografts/ PDX models market.
- Competitive Assessment: In-depth assessment of market shares, growth strategies, and service offerings of leading players like JSR Corporation (Japan), The Jackson Laboratory (US), and Charles River Laboratories International, Inc. (US), among others, in the patient-derived xenografts/ PDX models market strategies. The report also helps stakeholders understand the pulse of the patient-derived xenografts/ PDX models market and provides them with information on key market drivers, restraints, challenges, and opportunities.
TABLE OF CONTENTS
1 INTRODUCTION
- 1.1 STUDY OBJECTIVES
- 1.2 MARKET DEFINITION
- 1.3 INCLUSIONS AND EXCLUSIONS
- 1.4 STUDY SCOPE
- 1.4.1 MARKETS COVERED
- 1.4.2 YEARS CONSIDERED
- 1.4.3 CURRENCY CONSIDERED
- 1.5 LIMITATIONS
- 1.6 STAKEHOLDERS
- 1.7 SUMMARY OF CHANGES
- 1.8 RECESSION IMPACT
2 RESEARCH METHODOLOGY
- 2.1 RESEARCH DATA
- FIGURE 1 PATIENT-DERIVED XENOGRAFT (PDX) MODEL MARKET: RESEARCH DESIGN
- 2.1.1 SECONDARY DATA
- 2.1.2 PRIMARY DATA
- FIGURE 2 PATIENT-DERIVED XENOGRAFT (PDX) MODEL MARKET: BREAKDOWN OF PRIMARIES
- 2.2 MARKET ESTIMATION METHODOLOGY
- FIGURE 3 MARKET SIZE ESTIMATION: COMPANY REVENUE ANALYSIS-BASED ESTIMATION, 2023
- FIGURE 4 PATIENT-DERIVED XENOGRAFT (PDX) MODEL MARKET SIZE, 2023 (USD MILLION)
- FIGURE 5 PATIENT-DERIVED XENOGRAFT (PDX) MODEL MARKET: FINAL CAGR PROJECTIONS, 2023-2028
- FIGURE 6 PATIENT-DERIVED XENOGRAFT (PDX) MODEL MARKET: CAGR PROJECTIONS FROM ANALYSIS OF DRIVERS, RESTRAINTS, OPPORTUNITIES, AND CHALLENGES
- 2.3 DATA TRIANGULATION METHODOLOGY
- FIGURE 7 DATA TRIANGULATION
- 2.4 INDUSTRY INSIGHTS
- 2.5 RESEARCH ASSUMPTIONS
- 2.6 RECESSION IMPACT
- TABLE 1 GLOBAL INFLATION RATE PROJECTIONS, 2021-2027 (%)
- TABLE 2 US HEALTHCARE EXPENDITURE, 2019-2022 (USD MILLION)
- TABLE 3 US HEALTHCARE EXPENDITURE, 2023-2027 (USD MILLION)
3 EXECUTIVE SUMMARY
- FIGURE 8 PATIENT-DERIVED XENOGRAFT (PDX) MODEL MARKET, BY TYPE, 2023 VS. 2028 (USD MILLION)
- FIGURE 9 PATIENT-DERIVED XENOGRAFT (PDX) MODEL MARKET, BY IMPLANTATION METHOD, 2023 VS. 2028 (USD MILLION)
- FIGURE 10 PATIENT-DERIVED XENOGRAFT (PDX) MODEL MARKET, BY TUMOR TYPE, 2023 VS. 2028 (USD MILLION)
- FIGURE 11 PATIENT-DERIVED XENOGRAFT (PDX) MODEL MARKET, BY REGION
4 PREMIUM INSIGHTS
- 4.1 ATTRACTIVE OPPORTUNITIES FOR PLAYERS IN PATIENT-DERIVED XENOGRAFT (PDX) MODEL MARKET
- FIGURE 12 RISING INVESTMENTS IN CANCER RESEARCH AND ADVANCEMENTS IN PDX TECHNOLOGY
- 4.2 NORTH AMERICAN PATIENT-DERIVED XENOGRAFT (PDX) MODEL MARKET, BY END USER AND COUNTRY (2023)
- FIGURE 13 PHARMACEUTICAL & BIOTECHNOLOGY COMPANIES SEGMENT AND US TO ACCOUNT FOR LARGEST SHARES OF NORTH AMERICAN PATIENT-DERIVED XENOGRAFT (PDX) MODEL MARKET IN 2023
- 4.3 PATIENT-DERIVED XENOGRAFT (PDX) MODEL MARKET, BY APPLICATION
- FIGURE 14 PRECLINICAL DRUG DEVELOPMENT SEGMENT TO HOLD LARGEST MARKET SHARE IN 2028
5 MARKET OVERVIEW
- 5.1 INTRODUCTION
- 5.2 MARKET DYNAMICS
- FIGURE 15 PATIENT-DERIVED XENOGRAFT (PDX) MODEL MARKET: DRIVERS, RESTRAINTS, OPPORTUNITIES, AND CHALLENGES
- TABLE 4 PATIENT-DERIVED XENOGRAFT (PDX) MODEL MARKET: IMPACT ANALYSIS
- 5.2.1 DRIVERS
- 5.2.1.1 Growing demand for personalized medicine
- 5.2.1.2 Technological advancements in patient-derived xenograft (PDX) models
- 5.2.1.3 Rising investments in cancer research
- 5.2.2 RESTRAINTS
- 5.2.2.1 Discontinuation of animal models for clinical trials by FDA
- 5.2.3 OPPORTUNITIES
- 5.2.3.1 Emergence of Clustered Regularly Interspaced Short Palindromic Repeats (CRISPR) in biomedical research
- 5.2.4 CHALLENGES
- 5.2.4.1 Development of alternative animal testing methods
- 5.3 TECHNOLOGY ANALYSIS
- 5.4 TRENDS/DISRUPTIONS IMPACTING CUSTOMERS' BUSINESSES
- FIGURE 16 REVENUE SHIFT AND NEW REVENUE POCKETS FOR PLAYERS IN PATIENT-DERIVED XENOGRAFT (PDX) MODEL MARKET
- 5.5 SUPPLY CHAIN ANALYSIS
- FIGURE 17 PATIENT-DERIVED XENOGRAFT (PDX) MODEL MARKET: SUPPLY CHAIN ANALYSIS
- TABLE 5 COMPANIES AND THEIR ROLE IN PATIENT-DERIVED XENOGRAFT (PDX) MODEL SUPPLY CHAIN
- 5.6 VALUE CHAIN ANALYSIS
- FIGURE 18 PATIENT-DERIVED XENOGRAFT (PDX) MODEL MARKET: VALUE CHAIN ANALYSIS
- 5.7 ECOSYSTEM ANALYSIS
- FIGURE 19 PATIENT-DERIVED XENOGRAFT (PDX) MODEL MARKET: ECOSYSTEM ANALYSIS
- 5.8 PORTER'S FIVE FORCES ANALYSIS
- TABLE 6 PATIENT-DERIVED XENOGRAFT (PDX) MODEL MARKET: PORTER'S FIVE FORCES ANALYSIS
- 5.8.1 INTENSITY OF COMPETITIVE RIVALRY
- 5.8.2 BARGAINING POWER OF SUPPLIERS
- 5.8.3 BARGAINING POWER OF BUYERS
- 5.8.4 THREAT OF SUBSTITUTES
- 5.8.5 THREAT OF NEW ENTRANTS
- 5.9 REGULATORY ANALYSIS
- 5.9.1 NORTH AMERICA
- 5.9.2 EUROPE
- 5.9.3 ASIA PACIFIC
- 5.9.3.1 China
- 5.9.3.2 Japan
- 5.9.3.3 India
- 5.9.3.4 Australia
- 5.9.4 LATIN AMERICA
- 5.9.5 MIDDLE EAST & AFRICA
- 5.10 PRICING ANALYSIS
- 5.10.1 AVERAGE SELLING PRICE (ASP) OF PRODUCTS OFFERED BY THREE KEY PLAYERS, BY TYPE
- TABLE 7 AVERAGE SELLING PRICE (ASP) OF PRODUCTS OFFERED BY THREE KEY PLAYERS, BY TYPE (USD)
- 5.10.2 AVERAGE SELLING PRICE (ASP) TREND
- 5.11 PATENT ANALYSIS
- FIGURE 20 GRANTED PATENTS FOR PDX MODELS, 2011-2023
- 5.12 KEY CONFERENCES AND EVENTS IN 2023
- TABLE 8 PATIENT-DERIVED XENOGRAFT (PDX) MODEL MARKET: KEY CONFERENCES AND EVENTS
- 5.13 KEY STAKEHOLDERS AND BUYING CRITERIA
- 5.13.1 KEY STAKEHOLDERS IN BUYING PROCESS
- FIGURE 21 INFLUENCE OF STAKEHOLDERS ON BUYING PROCESS
- 5.13.2 BUYING CRITERIA
- FIGURE 22 KEY BUYING CRITERIA FOR THREE KEY END USERS
6 PATIENT-DERIVED XENOGRAFT (PDX) MODEL MARKET, BY IMPLANTATION METHOD
- 6.1 INTRODUCTION
- TABLE 9 PATIENT-DERIVED XENOGRAFT (PDX) MODEL MARKET, BY IMPLANTATION METHOD, 2021-2028 (USD MILLION)
- 6.2 SUBCUTANEOUS IMPLANTATION
- 6.2.1 FACILITATES REAL-TIME ASSESSMENT OF TUMOR GROWTH
- TABLE 10 SUBCUTANEOUS IMPLANTATION: PATIENT-DERIVED XENOGRAFT (PDX) MODEL MARKET, BY REGION, 2021-2028 (USD MILLION)
- TABLE 11 SUBCUTANEOUS IMPLANTATION: NORTH AMERICA PATIENT-DERIVED XENOGRAFT (PDX) MODEL MARKET, BY COUNTRY, 2021-2028 (USD MILLION)
- TABLE 12 SUBCUTANEOUS IMPLANTATION: EUROPE PATIENT-DERIVED XENOGRAFT (PDX) MODEL MARKET, BY COUNTRY, 2021-2028 (USD MILLION)
- TABLE 13 SUBCUTANEOUS IMPLANTATION: ASIA PACIFIC PATIENT-DERIVED XENOGRAFT (PDX) MODEL MARKET, BY COUNTRY, 2021-2028 (USD MILLION)
- TABLE 14 SUBCUTANEOUS IMPLANTATION: LATIN AMERICA PATIENT-DERIVED XENOGRAFT (PDX) MODEL MARKET, BY COUNTRY, 2021-2028 (USD MILLION)
- TABLE 15 SUBCUTANEOUS IMPLANTATION: MIDDLE EAST & AFRICA PATIENT-DERIVED XENOGRAFT (PDX) MODEL MARKET, BY COUNTRY, 2021-2028 (USD MILLION)
- 6.3 ORTHOTOPIC IMPLANTATION
- 6.3.1 PROVIDES HIGHLY ACCURATE REPRESENTATION OF TUMOR BEHAVIOR
- TABLE 16 ORTHOTOPIC IMPLANTATION: PATIENT-DERIVED XENOGRAFT (PDX) MODEL MARKET, BY REGION, 2021-2028 (USD MILLION)
- TABLE 17 ORTHOTOPIC IMPLANTATION: NORTH AMERICA PATIENT-DERIVED XENOGRAFT (PDX) MODEL MARKET, BY COUNTRY, 2021-2028 (USD MILLION)
- TABLE 18 ORTHOTOPIC IMPLANTATION: EUROPE PATIENT-DERIVED XENOGRAFT (PDX) MODEL MARKET, BY COUNTRY, 2021-2028 (USD MILLION)
- TABLE 19 ORTHOTOPIC IMPLANTATION: ASIA PACIFIC PATIENT-DERIVED XENOGRAFT (PDX) MODEL MARKET, BY COUNTRY, 2021-2028 (USD MILLION)
- TABLE 20 ORTHOTOPIC IMPLANTATION: LATIN AMERICA PATIENT-DERIVED XENOGRAFT (PDX) MODEL MARKET, BY COUNTRY, 2021-2028 (USD MILLION)
- TABLE 21 ORTHOTOPIC IMPLANTATION: MIDDLE EAST & AFRICA PATIENT-DERIVED XENOGRAFT (PDX) MODEL MARKET, BY COUNTRY, 2021-2028 (USD MILLION)
- 6.4 OTHERS
- TABLE 22 OTHERS: PATIENT-DERIVED XENOGRAFT (PDX) MODEL MARKET, BY REGION, 2021-2028 (USD MILLION)
- TABLE 23 OTHERS: NORTH AMERICA PATIENT-DERIVED XENOGRAFT (PDX) MODEL MARKET, BY COUNTRY, 2021-2028 (USD MILLION)
- TABLE 24 OTHERS: EUROPE PATIENT-DERIVED XENOGRAFT (PDX) MODEL MARKET, BY COUNTRY, 2021-2028 (USD MILLION)
- TABLE 25 OTHERS: ASIA PACIFIC PATIENT-DERIVED XENOGRAFT (PDX) MODEL MARKET, BY COUNTRY, 2021-2028 (USD MILLION)
- TABLE 26 OTHERS: LATIN AMERICA PATIENT-DERIVED XENOGRAFT (PDX) MODEL MARKET, BY COUNTRY, 2021-2028 (USD MILLION)
- TABLE 27 OTHERS: MIDDLE EAST & AFRICA PATIENT-DERIVED XENOGRAFT (PDX) MODEL MARKET, BY COUNTRY, 2021-2028 (USD MILLION)
7 PATIENT-DERIVED XENOGRAFT (PDX) MODEL MARKET, BY TYPE
- 7.1 INTRODUCTION
- TABLE 28 PATIENT-DERIVED XENOGRAFT (PDX) MODEL MARKET, BY TYPE, 2021-2028 (USD MILLION)
- 7.2 MOUSE MODEL
- 7.2.1 GROWING USE IN CANCER RESEARCH AND DRUG DEVELOPMENT
- TABLE 29 MOUSE MODEL: PATIENT-DERIVED XENOGRAFT (PDX) MODEL MARKET, BY REGION, 2021-2028 (USD MILLION)
- TABLE 30 MOUSE MODEL: NORTH AMERICA PATIENT-DERIVED XENOGRAFT (PDX) MODEL MARKET, BY COUNTRY, 2021-2028 (USD MILLION)
- TABLE 31 MOUSE MODEL: EUROPE PATIENT-DERIVED XENOGRAFT (PDX) MODEL MARKET, BY COUNTRY, 2021-2028 (USD MILLION)
- TABLE 32 MOUSE MODEL: ASIA PACIFIC PATIENT-DERIVED XENOGRAFT (PDX) MODEL MARKET, BY COUNTRY, 2021-2028 (USD MILLION)
- TABLE 33 MOUSE MODEL: LATIN AMERICA PATIENT-DERIVED XENOGRAFT (PDX) MODEL MARKET, BY COUNTRY, 2021-2028 (USD MILLION)
- TABLE 34 MOUSE MODEL: MIDDLE EAST & AFRICA PATIENT-DERIVED XENOGRAFT (PDX) MODEL MARKET, BY COUNTRY, 2021-2028 (USD MILLION)
- 7.3 RAT MODEL
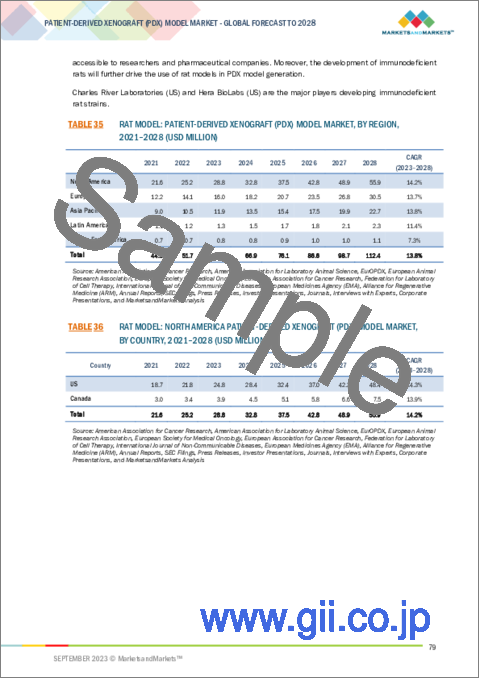
- 7.3.1 DEVELOPMENT OF NEW TECHNOLOGIES TO CREATE PDX RAT MODELS AT FASTER RATE
- TABLE 35 RAT MODEL: PATIENT-DERIVED XENOGRAFT (PDX) MODEL MARKET, BY REGION, 2021-2028 (USD MILLION)
- TABLE 36 RAT MODEL: NORTH AMERICA PATIENT-DERIVED XENOGRAFT (PDX) MODEL MARKET, BY COUNTRY, 2021-2028 (USD MILLION)
- TABLE 37 RAT MODEL: EUROPE PATIENT-DERIVED XENOGRAFT (PDX) MODEL MARKET, BY COUNTRY, 2021-2028 (USD MILLION)
- TABLE 38 RAT MODEL: ASIA PACIFIC PATIENT-DERIVED XENOGRAFT (PDX) MODEL MARKET, BY COUNTRY, 2021-2028 (USD MILLION)
- TABLE 39 RAT MODEL: LATIN AMERICA PATIENT-DERIVED XENOGRAFT (PDX) MODEL MARKET, BY COUNTRY, 2021-2028 (USD MILLION)
- TABLE 40 RAT MODEL: MIDDLE EAST & AFRICA PATIENT-DERIVED XENOGRAFT (PDX) MODEL MARKET, BY COUNTRY, 2021-2028 (USD MILLION)
8 PATIENT-DERIVED XENOGRAFT (PDX) MODEL MARKET, BY APPLICATION
- 8.1 INTRODUCTION
- TABLE 41 PATIENT-DERIVED XENOGRAFT (PDX) MODEL MARKET, BY APPLICATION, 2021-2028 (USD MILLION)
- 8.2 PRECLINICAL DRUG DEVELOPMENT
- 8.2.1 INCREASED NUMBER OF CLINICAL TRIALS
- TABLE 42 PRECLINICAL DRUG DEVELOPMENT: PATIENT-DERIVED XENOGRAFT (PDX) MODEL MARKET, BY REGION, 2021-2028 (USD MILLION)
- TABLE 43 PRECLINICAL DRUG DEVELOPMENT: NORTH AMERICA PATIENT-DERIVED XENOGRAFT (PDX) MODEL MARKET, BY COUNTRY, 2021-2028 (USD MILLION)
- TABLE 44 PRECLINICAL DRUG DEVELOPMENT: EUROPE PATIENT-DERIVED XENOGRAFT (PDX) MODEL MARKET, BY COUNTRY, 2021-2028 (USD MILLION)
- TABLE 45 PRECLINICAL DRUG DEVELOPMENT: ASIA PACIFIC PATIENT-DERIVED XENOGRAFT (PDX) MODEL MARKET, BY COUNTRY, 2021-2028 (USD MILLION)
- TABLE 46 PRECLINICAL DRUG DEVELOPMENT: LATIN AMERICA PATIENT-DERIVED XENOGRAFT (PDX) MODEL MARKET, BY COUNTRY, 2021-2028 (USD MILLION)
- TABLE 47 PRECLINICAL DRUG DEVELOPMENT: MIDDLE EAST & AFRICA PATIENT-DERIVED XENOGRAFT (PDX) MODEL MARKET, BY COUNTRY, 2021-2028 (USD MILLION)
- 8.3 BIOMARKER ANALYSIS
- 8.3.1 GROWING DEMAND FOR BIOMARKER-BASED PERSONALIZED CANCER THERAPY
- TABLE 48 BIOMARKER ANALYSIS: PATIENT-DERIVED XENOGRAFT (PDX) MODEL MARKET, BY REGION, 2021-2028 (USD MILLION)
- TABLE 49 BIOMARKER ANALYSIS: NORTH AMERICA PATIENT-DERIVED XENOGRAFT (PDX) MODEL MARKET, BY COUNTRY, 2021-2028 (USD MILLION)
- TABLE 50 BIOMARKER ANALYSIS: EUROPE PATIENT-DERIVED XENOGRAFT (PDX) MODEL MARKET, BY COUNTRY, 2021-2028 (USD MILLION)
- TABLE 51 BIOMARKER ANALYSIS: ASIA PACIFIC PATIENT-DERIVED XENOGRAFT (PDX) MODEL MARKET, BY COUNTRY, 2021-2028 (USD MILLION)
- TABLE 52 BIOMARKER ANALYSIS: LATIN AMERICA PATIENT-DERIVED XENOGRAFT (PDX) MODEL MARKET, BY COUNTRY, 2021-2028 (USD MILLION)
- TABLE 53 BIOMARKER ANALYSIS: MIDDLE EAST & AFRICA PATIENT-DERIVED XENOGRAFT (PDX) MODEL MARKET, BY COUNTRY, 2021-2028 (USD MILLION)
- 8.4 TRANSLATIONAL RESEARCH
- 8.4.1 BOOMING PERSONALIZED MEDICINE MARKET
- TABLE 54 TRANSLATIONAL RESEARCH: PATIENT-DERIVED XENOGRAFT (PDX) MODEL MARKET, BY REGION, 2021-2028 (USD MILLION)
- TABLE 55 TRANSLATIONAL RESEARCH: NORTH AMERICA PATIENT-DERIVED XENOGRAFT (PDX) MODEL MARKET, BY COUNTRY, 2021-2028 (USD MILLION)
- TABLE 56 TRANSLATIONAL RESEARCH: EUROPE PATIENT-DERIVED XENOGRAFT (PDX) MODEL MARKET, BY COUNTRY, 2021-2028 (USD MILLION)
- TABLE 57 TRANSLATIONAL RESEARCH: ASIA PACIFIC PATIENT-DERIVED XENOGRAFT (PDX) MODEL MARKET, BY COUNTRY, 2021-2028 (USD MILLION)
- TABLE 58 TRANSLATIONAL RESEARCH: LATIN AMERICA PATIENT-DERIVED XENOGRAFT (PDX) MODEL MARKET, BY COUNTRY, 2021-2028 (USD MILLION)
- TABLE 59 TRANSLATIONAL RESEARCH: MIDDLE EAST & AFRICA PATIENT-DERIVED XENOGRAFT (PDX) MODEL MARKET, BY COUNTRY, 2021-2028 (USD MILLION)
- 8.5 BIOBANKS
- 8.5.1 RISING DRUG DISCOVERY AND DEVELOPMENT PROJECTS
- TABLE 60 BIOBANKS: PATIENT-DERIVED XENOGRAFT (PDX) MODEL MARKET, BY REGION, 2021-2028 (USD MILLION)
- TABLE 61 BIOBANKS: NORTH AMERICA PATIENT-DERIVED XENOGRAFT (PDX) MODEL MARKET, BY COUNTRY, 2021-2028 (USD MILLION)
- TABLE 62 BIOBANKS: EUROPE PATIENT-DERIVED XENOGRAFT (PDX) MODEL MARKET, BY COUNTRY, 2021-2028 (USD MILLION)
- TABLE 63 BIOBANKS: ASIA PACIFIC PATIENT-DERIVED XENOGRAFT (PDX) MODEL MARKET, BY COUNTRY, 2021-2028 (USD MILLION)
- TABLE 64 BIOBANKS: LATIN AMERICA PATIENT-DERIVED XENOGRAFT (PDX) MODEL MARKET, BY COUNTRY, 2021-2028 (USD MILLION)
- TABLE 65 BIOBANKS: MIDDLE EAST & AFRICA PATIENT-DERIVED XENOGRAFT (PDX) MODEL MARKET, BY COUNTRY, 2021-2028 (USD MILLION)
9 PATIENT-DERIVED XENOGRAFT (PDX) MODEL MARKET, BY END USER
- 9.1 INTRODUCTION
- TABLE 66 PATIENT-DERIVED XENOGRAFT (PDX) MODEL MARKET, BY END USER, 2021-2028 (USD MILLION)
- 9.2 PHARMACEUTICAL & BIOTECHNOLOGY COMPANIES
- 9.2.1 ADVANCEMENTS IN TUMOR MICROENVIRONMENT RESEARCH
- TABLE 67 PHARMACEUTICAL & BIOTECHNOLOGY COMPANIES: PATIENT-DERIVED XENOGRAFT (PDX) MODEL MARKET, BY REGION, 2021-2028 (USD MILLION)
- TABLE 68 PHARMACEUTICAL & BIOTECHNOLOGY COMPANIES: NORTH AMERICA PATIENT-DERIVED XENOGRAFT (PDX) MODEL MARKET, BY COUNTRY, 2021-2028 (USD MILLION)
- TABLE 69 PHARMACEUTICAL & BIOTECHNOLOGY COMPANIES: EUROPE PATIENT-DERIVED XENOGRAFT (PDX) MODEL MARKET, 2021-2028 (USD MILLION)
- TABLE 70 PHARMACEUTICAL & BIOTECHNOLOGY COMPANIES: ASIA PACIFIC PATIENT-DERIVED XENOGRAFT (PDX) MODEL MARKET, BY COUNTRY, 2021-2028 (USD MILLION)
- TABLE 71 PHARMACEUTICAL & BIOTECHNOLOGY COMPANIES: LATIN AMERICA PATIENT-DERIVED XENOGRAFT (PDX) MODEL MARKET, BY COUNTRY, 2021-2028 (USD MILLION)
- TABLE 72 PHARMACEUTICAL & BIOTECHNOLOGY COMPANIES: MIDDLE EAST & AFRICA PATIENT-DERIVED XENOGRAFT (PDX) MODEL MARKET, BY COUNTRY, 2021-2028 (USD MILLION)
- 9.3 CONTRACT RESEARCH ORGANIZATIONS (CROS)
- 9.3.1 INCREASING OUTSOURCING OF DRUG DISCOVERY SERVICES
- TABLE 73 CONTRACT RESEARCH ORGANIZATIONS: PATIENT-DERIVED XENOGRAFT (PDX) MODEL MARKET, BY REGION, 2021-2028 (USD MILLION)
- TABLE 74 CONTRACT RESEARCH ORGANIZATIONS: NORTH AMERICA PATIENT-DERIVED XENOGRAFT (PDX) MODEL MARKET, BY COUNTRY, 2021-2028 (USD MILLION)
- TABLE 75 CONTRACT RESEARCH ORGANIZATIONS: EUROPE PATIENT-DERIVED XENOGRAFT (PDX) MODEL MARKET, BY COUNTRY, 2021-2028 (USD MILLION)
- TABLE 76 CONTRACT RESEARCH ORGANIZATIONS: ASIA PACIFIC PATIENT-DERIVED XENOGRAFT (PDX) MODEL MARKET, BY COUNTRY, 2021-2028 (USD MILLION)
- TABLE 77 CONTRACT RESEARCH ORGANIZATIONS: LATIN AMERICA PATIENT-DERIVED XENOGRAFT (PDX) MODEL MARKET, BY COUNTRY, 2021-2028 (USD MILLION)
- TABLE 78 CONTRACT RESEARCH ORGANIZATIONS: MIDDLE EAST & AFRICA PATIENT-DERIVED XENOGRAFT (PDX) MODEL MARKET, BY COUNTRY, 2021-2028 (USD MILLION)
- 9.4 ACADEMIC & RESEARCH INSTITUTIONS
- 9.4.1 RISING R&D INVESTMENTS IN LIFE SCIENCES
- TABLE 79 ACADEMIC & RESEARCH INSTITUTIONS: PATIENT-DERIVED XENOGRAFT (PDX) MODEL MARKET, BY REGION, 2021-2028 (USD MILLION)
- TABLE 80 ACADEMIC & RESEARCH INSTITUTIONS: NORTH AMERICA PATIENT-DERIVED XENOGRAFT (PDX) MODEL MARKET, BY COUNTRY, 2021-2028 (USD MILLION)
- TABLE 81 ACADEMIC & RESEARCH INSTITUTIONS: EUROPE PATIENT-DERIVED XENOGRAFT (PDX) MODEL MARKET, BY COUNTRY, 2021-2028 (USD MILLION)
- TABLE 82 ACADEMIC & RESEARCH INSTITUTIONS: ASIA PACIFIC PATIENT-DERIVED XENOGRAFT (PDX) MODEL MARKET, BY COUNTRY, 2021-2028 (USD MILLION)
- TABLE 83 ACADEMIC & RESEARCH INSTITUTIONS: LATIN AMERICA PATIENT-DERIVED XENOGRAFT (PDX) MODEL MARKET, BY COUNTRY, 2021-2028 (USD MILLION)
- TABLE 84 ACADEMIC & RESEARCH INSTITUTIONS: MIDDLE EAST & AFRICA PATIENT-DERIVED XENOGRAFT (PDX) MODEL MARKET, BY COUNTRY, 2021-2028 (USD MILLION)
10 PATIENT-DERIVED XENOGRAFT (PDX) MODEL MARKET, BY TUMOR TYPE
- 10.1 INTRODUCTION
- TABLE 85 PATIENT-DERIVED XENOGRAFT (PDX) MODEL MARKET, BY TUMOR TYPE, 2021-2028 (USD MILLION)
- 10.2 GASTROINTESTINAL TUMOR MODEL
- 10.2.1 RISING DEMAND FOR EFFECTIVE CANCER THERAPEUTICS
- TABLE 86 GASTROINTESTINAL TUMOR MODEL: PATIENT-DERIVED XENOGRAFT (PDX) MODEL MARKET, BY REGION, 2021-2028 (USD MILLION)
- TABLE 87 GASTROINTESTINAL TUMOR MODEL: NORTH AMERICA PATIENT-DERIVED XENOGRAFT (PDX) MODEL MARKET, BY COUNTRY, 2021-2028 (USD MILLION)
- TABLE 88 GASTROINTESTINAL TUMOR MODEL: EUROPE PATIENT-DERIVED XENOGRAFT (PDX) MODEL MARKET, BY COUNTRY, 2021-2028 (USD MILLION)
- TABLE 89 GASTROINTESTINAL TUMOR MODEL: ASIA PACIFIC PATIENT-DERIVED XENOGRAFT (PDX) MODEL MARKET, BY COUNTRY, 2021-2028 (USD MILLION)
- TABLE 90 GASTROINTESTINAL TUMOR MODEL: LATIN AMERICA PATIENT-DERIVED XENOGRAFT (PDX) MODEL MARKET, BY COUNTRY, 2021-2028 (USD MILLION)
- TABLE 91 GASTROINTESTINAL TUMOR MODEL: MIDDLE EAST & AFRICA PATIENT-DERIVED XENOGRAFT (PDX) MODEL MARKET, BY COUNTRY, 2021-2028 (USD MILLION)
- 10.3 GYNECOLOGICAL TUMOR MODEL
- 10.3.1 HIGH INCIDENCE OF TARGET CANCERS
- TABLE 92 GYNECOLOGICAL TUMOR MODEL: PATIENT-DERIVED XENOGRAFT (PDX) MODEL MARKET, BY REGION, 2021-2028 (USD MILLION)
- TABLE 93 GYNECOLOGICAL TUMOR MODEL: NORTH AMERICA PATIENT-DERIVED XENOGRAFT (PDX) MODEL MARKET, BY COUNTRY, 2021-2028 (USD MILLION)
- TABLE 94 GYNECOLOGICAL TUMOR MODEL: EUROPE PATIENT-DERIVED XENOGRAFT (PDX) MODEL MARKET, BY COUNTRY, 2021-2028 (USD MILLION)
- TABLE 95 GYNECOLOGICAL TUMOR MODEL: ASIA PACIFIC PATIENT-DERIVED XENOGRAFT (PDX) MODEL MARKET, BY COUNTRY, 2021-2028 (USD MILLION)
- TABLE 96 GYNECOLOGICAL TUMOR MODEL: LATIN AMERICA PATIENT-DERIVED XENOGRAFT (PDX) MODEL MARKET, BY COUNTRY, 2021-2028 (USD MILLION)
- TABLE 97 GYNECOLOGICAL TUMOR MODEL: MIDDLE EAST & AFRICA PATIENT-DERIVED XENOGRAFT (PDX) MODEL MARKET, BY COUNTRY, 2021-2028 (USD MILLION)
- 10.4 RESPIRATORY TUMOR MODEL
- 10.4.1 INCREASING PREVALENCE OF LUNG CANCER
- TABLE 98 RESPIRATORY TUMOR MODEL: PATIENT-DERIVED XENOGRAFT (PDX) MODEL MARKET, BY REGION, 2021-2028 (USD MILLION)
- TABLE 99 RESPIRATORY TUMOR MODEL: NORTH AMERICA PATIENT-DERIVED XENOGRAFT (PDX) MODEL MARKET, BY COUNTRY, 2021-2028 (USD MILLION)
- TABLE 100 RESPIRATORY TUMOR MODEL: EUROPE PATIENT-DERIVED XENOGRAFT (PDX) MODEL MARKET, BY COUNTRY, 2021-2028 (USD MILLION)
- TABLE 101 RESPIRATORY TUMOR MODEL: ASIA PACIFIC PATIENT-DERIVED XENOGRAFT (PDX) MODEL MARKET, BY COUNTRY, 2021-2028 (USD MILLION)
- TABLE 102 RESPIRATORY TUMOR MODEL: LATIN AMERICA PATIENT-DERIVED XENOGRAFT (PDX) MODEL MARKET, BY COUNTRY, 2021-2028 (USD MILLION)
- TABLE 103 RESPIRATORY TUMOR MODEL: MIDDLE EAST & AFRICA PATIENT-DERIVED XENOGRAFT (PDX) MODEL MARKET, BY COUNTRY, 2021-2028 (USD MILLION)
- 10.5 UROLOGICAL TUMOR MODEL
- 10.5.1 INCREASING FOCUS OF COMPANIES ON EXPANDING PORTFOLIO OF UROLOGICAL TUMOR MODELS
- TABLE 104 UROLOGICAL TUMOR MODEL: PATIENT-DERIVED XENOGRAFT (PDX) MODEL MARKET, BY REGION, 2021-2028 (USD MILLION)
- TABLE 105 UROLOGICAL TUMOR MODEL: NORTH AMERICA PATIENT-DERIVED XENOGRAFT (PDX) MODEL MARKET, BY COUNTRY, 2021-2028 (USD MILLION)
- TABLE 106 UROLOGICAL TUMOR MODEL: EUROPE PATIENT-DERIVED XENOGRAFT (PDX) MODEL MARKET, BY COUNTRY, 2021-2028 (USD MILLION)
- TABLE 107 UROLOGICAL TUMOR MODEL: ASIA PACIFIC PATIENT-DERIVED XENOGRAFT (PDX) MODEL MARKET, BY COUNTRY, 2021-2028 (USD MILLION)
- TABLE 108 UROLOGICAL TUMOR MODEL: LATIN AMERICA PATIENT-DERIVED XENOGRAFT (PDX) MODEL MARKET, BY COUNTRY, 2021-2028 (USD MILLION)
- TABLE 109 UROLOGICAL TUMOR MODEL: MIDDLE EAST & AFRICA PATIENT-DERIVED XENOGRAFT (PDX) MODEL MARKET, BY COUNTRY, 2021-2028 (USD MILLION)
- 10.6 HEMATOLOGICAL TUMOR MODEL
- 10.6.1 ADVANCEMENTS IN TRANSLATIONAL RESEARCH AND INCREASING DEMAND FOR PERSONALIZED MEDICINE
- TABLE 110 HEMATOLOGICAL TUMOR MODEL: PATIENT-DERIVED XENOGRAFT (PDX) MODEL MARKET, BY REGION, 2021-2028 (USD MILLION)
- TABLE 111 HEMATOLOGICAL TUMOR MODEL: NORTH AMERICA PATIENT-DERIVED XENOGRAFT (PDX) MODEL MARKET, BY COUNTRY, 2021-2028 (USD MILLION)
- TABLE 112 HEMATOLOGICAL TUMOR MODEL: EUROPE PATIENT-DERIVED XENOGRAFT (PDX) MODEL MARKET, BY COUNTRY, 2021-2028 (USD MILLION)
- TABLE 113 HEMATOLOGICAL TUMOR MODEL: ASIA PACIFIC PATIENT-DERIVED XENOGRAFT (PDX) MODEL MARKET, BY COUNTRY, 2021-2028 (USD MILLION)
- TABLE 114 HEMATOLOGICAL TUMOR MODEL: LATIN AMERICA PATIENT-DERIVED XENOGRAFT (PDX) MODEL MARKET, BY COUNTRY, 2021-2028 (USD MILLION)
- TABLE 115 HEMATOLOGICAL TUMOR MODEL: MIDDLE EAST & AFRICA PATIENT-DERIVED XENOGRAFT (PDX) MODEL MARKET, BY COUNTRY, 2021-2028 (USD MILLION)
- 10.7 OTHERS
- TABLE 116 OTHERS: PATIENT-DERIVED XENOGRAFT (PDX) MODEL MARKET, BY REGION, 2021-2028 (USD MILLION)
- TABLE 117 OTHERS: NORTH AMERICA PATIENT-DERIVED XENOGRAFT (PDX) MODEL MARKET, BY COUNTRY, 2021-2028 (USD MILLION)
- TABLE 118 OTHERS: EUROPE PATIENT-DERIVED XENOGRAFT (PDX) MODEL MARKET, BY COUNTRY, 2021-2028 (USD MILLION)
- TABLE 119 OTHERS: ASIA PACIFIC PATIENT-DERIVED XENOGRAFT (PDX) MODEL MARKET, BY COUNTRY, 2021-2028 (USD MILLION)
- TABLE 120 OTHERS: LATIN AMERICA PATIENT-DERIVED XENOGRAFT (PDX) MODEL MARKET, BY COUNTRY, 2021-2028 (USD MILLION)
- TABLE 121 OTHERS: MIDDLE EAST & AFRICA PATIENT-DERIVED XENOGRAFT (PDX) MODEL MARKET, BY COUNTRY, 2021-2028 (USD MILLION)
11 PATIENT-DERIVED XENOGRAFT (PDX) MODEL MARKET, BY REGION
- 11.1 INTRODUCTION
- TABLE 122 PATIENT-DERIVED XENOGRAFT MODEL MARKET, BY REGION, 2021-2028 (USD MILLION)
- 11.2 NORTH AMERICA
- FIGURE 23 NORTH AMERICA: PATIENT-DERIVED XENOGRAFT (PDX) MODEL MARKET SNAPSHOT
- TABLE 123 NORTH AMERICA: PATIENT-DERIVED XENOGRAFT (PDX) MODEL MARKET, BY COUNTRY, 2021-2028 (USD MILLION)
- TABLE 124 NORTH AMERICA: PATIENT-DERIVED XENOGRAFT MODEL MARKET, BY TYPE, 2021-2028 (USD MILLION)
- TABLE 125 NORTH AMERICA: PATIENT-DERIVED XENOGRAFT MODEL MARKET, BY IMPLANTATION METHOD, 2021-2028 (USD MILLION)
- TABLE 126 NORTH AMERICA: PATIENT-DERIVED XENOGRAFT (PDX) MODEL MARKET, BY TUMOR TYPE, 2021-2028 (USD MILLION)
- TABLE 127 NORTH AMERICA: PATIENT-DERIVED XENOGRAFT (PDX) MODEL MARKET, BY APPLICATION, 2021-2028 (USD MILLION)
- TABLE 128 NORTH AMERICA: PATIENT-DERIVED XENOGRAFT (PDX) MODEL MARKET, BY END USER, 2021-2028 (USD MILLION)
- 11.2.1 US
- 11.2.1.1 Growing federal research and development efforts to drive market
- TABLE 129 US: PATIENT-DERIVED XENOGRAFT (PDX) MODEL MARKET, BY TYPE, 2021-2028 (USD MILLION)
- TABLE 130 US: PATIENT-DERIVED XENOGRAFT (PDX) MODEL MARKET, BY IMPLANTATION METHOD, 2021-2028 (USD MILLION)
- TABLE 131 US: PATIENT-DERIVED XENOGRAFT (PDX) MODEL MARKET, BY TUMOR TYPE, 2021-2028 (USD MILLION)
- TABLE 132 US: PATIENT-DERIVED XENOGRAFT (PDX) MODEL MARKET, BY APPLICATION, 2021-2028 (USD MILLION)
- TABLE 133 US: PATIENT-DERIVED XENOGRAFT (PDX) MODEL MARKET, BY END USER, 2021-2028 (USD MILLION)
- 11.2.2 CANADA
- 11.2.2.1 Government-led investments in stem cell research to drive market
- TABLE 134 CANADA: PATIENT-DERIVED XENOGRAFT (PDX) MODEL MARKET, BY TYPE, 2021-2028 (USD MILLION)
- TABLE 135 CANADA: PATIENT-DERIVED XENOGRAFT (PDX) MODEL MARKET, BY IMPLANTATION METHOD, 2021-2028 (USD MILLION)
- TABLE 136 CANADA: PATIENT-DERIVED XENOGRAFT (PDX) MODEL MARKET, BY TUMOR TYPE, 2021-2028 (USD MILLION)
- TABLE 137 CANADA: PATIENT-DERIVED XENOGRAFT (PDX) MODEL MARKET, BY APPLICATION, 2021-2028 (USD MILLION)
- TABLE 138 CANADA: PATIENT-DERIVED XENOGRAFT (PDX) MODEL MARKET, BY END USER, 2021-2028 (USD MILLION)
- 11.2.3 NORTH AMERICA: IMPACT OF RECESSION
- 11.3 EUROPE
- TABLE 139 EUROPE: PATIENT-DERIVED XENOGRAFT (PDX) MODEL MARKET, BY COUNTRY, 2021-2028 (USD MILLION)
- TABLE 140 EUROPE: PATIENT-DERIVED XENOGRAFT (PDX) MODEL MARKET, BY TYPE, 2021-2028 (USD MILLION)
- TABLE 141 EUROPE: PATIENT-DERIVED XENOGRAFT (PDX) MODEL MARKET, BY IMPLANTATION METHOD, 2021-2028 (USD MILLION)
- TABLE 142 EUROPE: PATIENT-DERIVED XENOGRAFT (PDX) MODEL MARKET, BY TUMOR TYPE, 2021-2028 (USD MILLION)
- TABLE 143 EUROPE: PATIENT-DERIVED XENOGRAFT (PDX) MODEL MARKET, BY APPLICATION, 2021-2028 (USD MILLION)
- TABLE 144 EUROPE: PATIENT-DERIVED XENOGRAFT (PDX) MODEL MARKET, BY END USER, 2021-2028 (USD MILLION)
- 11.3.1 GERMANY
- 11.3.1.1 Increasing investments in biotechnology industry to boost market growth
- TABLE 145 GERMANY: PATIENT-DERIVED XENOGRAFT (PDX) MODEL MARKET, BY TYPE, 2021-2028 (USD MILLION)
- TABLE 146 GERMANY: PATIENT-DERIVED XENOGRAFT (PDX) MODEL MARKET, BY IMPLANTATION METHOD, 2021-2028 (USD MILLION)
- TABLE 147 GERMANY: PATIENT-DERIVED XENOGRAFT (PDX) MODEL MARKET, BY TUMOR TYPE, 2021-2028 (USD MILLION)
- TABLE 148 GERMANY: PATIENT-DERIVED XENOGRAFT (PDX) MODEL MARKET, BY APPLICATION, 2021-2028 (USD MILLION)
- TABLE 149 GERMANY: PATIENT-DERIVED XENOGRAFT (PDX) MODEL MARKET, BY END USER, 2021-2028 (USD MILLION)
- 11.3.2 FRANCE
- 11.3.2.1 Increasing government funding for cancer research to support market growth
- TABLE 150 FRANCE: PATIENT-DERIVED XENOGRAFT (PDX) MODEL MARKET, BY TYPE, 2021-2028 (USD MILLION)
- TABLE 151 FRANCE: PATIENT-DERIVED XENOGRAFT (PDX) MODEL MARKET, BY IMPLANTATION METHOD, 2021-2028 (USD MILLION)
- TABLE 152 FRANCE: PATIENT-DERIVED XENOGRAFT (PDX) MODEL MARKET, BY TUMOR TYPE, 2021-2028 (USD MILLION)
- TABLE 153 FRANCE: PATIENT-DERIVED XENOGRAFT (PDX) MODEL MARKET, BY APPLICATION, 2021-2028 (USD MILLION)
- TABLE 154 FRANCE: PATIENT-DERIVED XENOGRAFT (PDX) MODEL MARKET, BY END USER, 2021-2028 (USD MILLION)
- 11.3.3 UK
- 11.3.3.1 Rising focus on innovating oncology therapeutics to drive market
- TABLE 155 UK: PATIENT-DERIVED XENOGRAFT (PDX) MODEL MARKET, BY TYPE, 2021-2028 (USD MILLION)
- TABLE 156 UK: PATIENT-DERIVED XENOGRAFT (PDX) MODEL MARKET, BY IMPLANTATION METHOD, 2021-2028 (USD MILLION)
- TABLE 157 UK: PATIENT-DERIVED XENOGRAFT (PDX) MODEL MARKET, BY TUMOR TYPE, 2021-2028 (USD MILLION)
- TABLE 158 UK: PATIENT-DERIVED XENOGRAFT (PDX) MODEL MARKET, BY APPLICATION, 2021-2028 (USD MILLION)
- TABLE 159 UK: PATIENT-DERIVED XENOGRAFT (PDX) MODEL MARKET, BY END USER, 2021-2028 (USD MILLION)
- 11.3.4 ITALY
- 11.3.4.1 Growing biotechnology and pharmaceutical industries to drive market
- TABLE 160 ITALY: PATIENT-DERIVED XENOGRAFT (PDX) MODEL MARKET, BY TYPE, 2021-2028 (USD MILLION)
- TABLE 161 ITALY: PATIENT-DERIVED XENOGRAFT (PDX) MODEL MARKET, BY IMPLANTATION METHOD, 2021-2028 (USD MILLION)
- TABLE 162 ITALY: PATIENT-DERIVED XENOGRAFT (PDX) MODEL MARKET, BY TUMOR TYPE, 2021-2028 (USD MILLION)
- TABLE 163 ITALY: PATIENT-DERIVED XENOGRAFT (PDX) MODEL MARKET, BY APPLICATION, 2021-2028 (USD MILLION)
- TABLE 164 ITALY: PATIENT-DERIVED XENOGRAFT (PDX) MODEL MARKET, BY END USER, 2021-2028 (USD MILLION)
- 11.3.5 SPAIN
- 11.3.5.1 Increasing investments in clinical trials to promote market growth
- TABLE 165 SPAIN: PATIENT-DERIVED XENOGRAFT (PDX) MODEL MARKET, BY TYPE, 2021-2028 (USD MILLION)
- TABLE 166 SPAIN: PATIENT-DERIVED XENOGRAFT (PDX) MODEL MARKET, BY IMPLANTATION METHOD, 2021-2028 (USD MILLION)
- TABLE 167 SPAIN: PATIENT-DERIVED XENOGRAFT (PDX) MODEL MARKET, BY TUMOR TYPE, 2021-2028 (USD MILLION)
- TABLE 168 SPAIN: PATIENT-DERIVED XENOGRAFT (PDX) MODEL MARKET, BY APPLICATION, 2021-2028 (USD MILLION)
- TABLE 169 SPAIN: PATIENT-DERIVED XENOGRAFT (PDX) MODEL MARKET, BY END USER, 2021-2028 (USD MILLION)
- 11.3.6 REST OF EUROPE
- TABLE 170 REST OF EUROPE: PATIENT-DERIVED XENOGRAFT (PDX) MODEL MARKET, BY TYPE, 2021-2028 (USD MILLION)
- TABLE 171 REST OF EUROPE: PATIENT-DERIVED XENOGRAFT (PDX) MODEL MARKET, BY IMPLANTATION METHOD, 2021-2028 (USD MILLION)
- TABLE 172 REST OF EUROPE: PATIENT-DERIVED XENOGRAFT (PDX) MODEL MARKET, BY TUMOR TYPE, 2021-2028 (USD MILLION)
- TABLE 173 REST OF EUROPE: PATIENT-DERIVED XENOGRAFT (PDX) MODEL MARKET, BY APPLICATION, 2021-2028 (USD MILLION)
- TABLE 174 REST OF EUROPE: PATIENT-DERIVED XENOGRAFT (PDX) MODEL MARKET, BY END USER, 2021-2028 (USD MILLION)
- 11.3.7 EUROPE: IMPACT OF RECESSION
- 11.4 ASIA PACIFIC
- FIGURE 24 ASIA PACIFIC: PATIENT-DERIVED XENOGRAFT (PDX) MODEL MARKET SNAPSHOT
- TABLE 175 ASIA PACIFIC: PATIENT-DERIVED XENOGRAFT (PDX) MODEL MARKET, BY COUNTRY, 2021-2028 (USD MILLION)
- TABLE 176 ASIA PACIFIC: PATIENT-DERIVED XENOGRAFT (PDX) MODEL MARKET, BY TYPE, 2021-2028 (USD MILLION)
- TABLE 177 ASIA PACIFIC: PATIENT-DERIVED XENOGRAFT (PDX) MODEL MARKET, BY IMPLANTATION METHOD, 2021-2028 (USD MILLION)
- TABLE 178 ASIA PACIFIC: PATIENT-DERIVED XENOGRAFT (PDX) MODEL MARKET, BY TUMOR TYPE, 2021-2028 (USD MILLION)
- TABLE 179 ASIA PACIFIC: PATIENT-DERIVED XENOGRAFT (PDX) MODEL MARKET, BY APPLICATION, 2021-2028 (USD MILLION)
- TABLE 180 ASIA PACIFIC: PATIENT-DERIVED XENOGRAFT (PDX) MODEL MARKET, BY END USER, 2021-2028 (USD MILLION)
- 11.4.1 CHINA
- 11.4.1.1 Government-led fundings for clinical trials in oncology to drive market
- TABLE 181 CHINA: PATIENT-DERIVED XENOGRAFT (PDX) MODEL MARKET, BY TYPE, 2021-2028 (USD MILLION)
- TABLE 182 CHINA: PATIENT-DERIVED XENOGRAFT (PDX) MODEL MARKET, BY IMPLANTATION METHOD, 2021-2028 (USD MILLION)
- TABLE 183 CHINA: PATIENT-DERIVED XENOGRAFT (PDX) MODEL MARKET, BY TUMOR TYPE, 2021-2028 (USD MILLION)
- TABLE 184 CHINA: PATIENT-DERIVED XENOGRAFT (PDX) MODEL MARKET, BY APPLICATION, 2021-2028 (USD MILLION)
- TABLE 185 CHINA: PATIENT-DERIVED XENOGRAFT (PDX) MODEL MARKET, BY END USER, 2021-2028 (USD MILLION)
- 11.4.2 JAPAN
- 11.4.2.1 Advancements in mice model technologies to drive market
- TABLE 186 JAPAN: PATIENT-DERIVED XENOGRAFT (PDX) MODEL MARKET, BY TYPE, 2021-2028 (USD MILLION)
- TABLE 187 JAPAN: PATIENT-DERIVED XENOGRAFT (PDX) MODEL MARKET, BY IMPLANTATION METHOD, 2021-2028 (USD MILLION)
- TABLE 188 JAPAN: PATIENT-DERIVED XENOGRAFT (PDX) MODEL MARKET, BY TUMOR TYPE, 2021-2028 (USD MILLION)
- TABLE 189 JAPAN: PATIENT-DERIVED XENOGRAFT (PDX) MODEL MARKET, BY APPLICATION, 2021-2028 (USD MILLION)
- TABLE 190 JAPAN: PATIENT-DERIVED XENOGRAFT (PDX) MODEL MARKET, BY END USER, 2021-2028 (USD MILLION)
- 11.4.3 INDIA
- 11.4.3.1 Favorable government policies for drug development to fuel demand
- TABLE 191 INDIA: PATIENT-DERIVED XENOGRAFT (PDX) MODEL MARKET, BY TYPE, 2021-2028 (USD MILLION)
- TABLE 192 INDIA: PATIENT-DERIVED XENOGRAFT (PDX) MODEL MARKET, BY IMPLANTATION METHOD, 2021-2028 (USD MILLION)
- TABLE 193 INDIA: PATIENT-DERIVED XENOGRAFT (PDX) MODEL MARKET, BY TUMOR TYPE, 2021-2028 (USD MILLION)
- TABLE 194 INDIA: PATIENT-DERIVED XENOGRAFT (PDX) MODEL MARKET, BY APPLICATION, 2021-2028 (USD MILLION)
- TABLE 195 INDIA: PATIENT-DERIVED XENOGRAFT (PDX) MODEL MARKET, BY END USER, 2021-2028 (USD MILLION)
- 11.4.4 SOUTH KOREA
- 11.4.4.1 Rising investments in preclinical drug development to drive demand
- TABLE 196 SOUTH KOREA: PATIENT-DERIVED XENOGRAFT (PDX) MODEL MARKET, BY TYPE, 2021-2028 (USD MILLION)
- TABLE 197 SOUTH KOREA: PATIENT-DERIVED XENOGRAFT (PDX) MODEL MARKET, BY IMPLANTATION METHOD, 2021-2028 (USD MILLION)
- TABLE 198 SOUTH KOREA: PATIENT-DERIVED XENOGRAFT (PDX) MODEL MARKET, BY TUMOR TYPE, 2021-2028 (USD MILLION)
- TABLE 199 SOUTH KOREA: PATIENT-DERIVED XENOGRAFT (PDX) MODEL MARKET, BY APPLICATION, 2021-2028 (USD MILLION)
- TABLE 200 SOUTH KOREA: PATIENT-DERIVED XENOGRAFT (PDX) MODEL MARKET, BY END USER, 2021-2028 (USD MILLION)
- 11.4.5 AUSTRALIA
- 11.4.5.1 Increased funding in clinical trials from research institutions to drive market
- TABLE 201 AUSTRALIA: PATIENT-DERIVED XENOGRAFT (PDX) MODEL MARKET, BY TYPE, 2021-2028 (USD MILLION)
- TABLE 202 AUSTRALIA: PATIENT-DERIVED XENOGRAFT (PDX) MODEL MARKET, BY IMPLANTATION METHOD, 2021-2028 (USD MILLION)
- TABLE 203 AUSTRALIA: PATIENT-DERIVED XENOGRAFT (PDX) MODEL MARKET, BY TUMOR TYPE, 2021-2028 (USD MILLION)
- TABLE 204 AUSTRALIA: PATIENT-DERIVED XENOGRAFT (PDX) MODEL MARKET, BY APPLICATION, 2021-2028 (USD MILLION)
- TABLE 205 AUSTRALIA: PATIENT-DERIVED XENOGRAFT (PDX) MODEL MARKET, BY END USER, 2021-2028 (USD MILLION)
- 11.4.6 REST OF ASIA PACIFIC
- TABLE 206 REST OF ASIA PACIFIC: PATIENT-DERIVED XENOGRAFT (PDX) MODEL MARKET, BY TYPE, 2021-2028 (USD MILLION)
- TABLE 207 REST OF ASIA PACIFIC: PATIENT-DERIVED XENOGRAFT (PDX) MODEL MARKET, BY IMPLANTATION METHOD, 2021-2028 (USD MILLION)
- TABLE 208 REST OF ASIA PACIFIC: PATIENT-DERIVED XENOGRAFT (PDX) MODEL MARKET, BY TUMOR TYPE, 2021-2028 (USD MILLION)
- TABLE 209 REST OF ASIA PACIFIC: PATIENT-DERIVED XENOGRAFT (PDX) MODEL MARKET, BY APPLICATION, 2021-2028 (USD MILLION)
- TABLE 210 REST OF ASIA PACIFIC: PATIENT-DERIVED XENOGRAFT (PDX) MODEL MARKET, BY END USER, 2021-2028 (USD MILLION)
- 11.4.7 ASIA PACIFIC: IMPACT OF RECESSION
- 11.5 LATIN AMERICA
- TABLE 211 LATIN AMERICA: PATIENT-DERIVED XENOGRAFT (PDX) MODEL MARKET, BY COUNTRY, 2021-2028 (USD MILLION)
- TABLE 212 LATIN AMERICA: PATIENT-DERIVED XENOGRAFT (PDX) MODEL MARKET, BY TYPE, 2021-2028 (USD MILLION)
- TABLE 213 LATIN AMERICA: PATIENT-DERIVED XENOGRAFT (PDX) MODEL MARKET, BY IMPLANTATION METHOD, 2021-2028 (USD MILLION)
- TABLE 214 LATIN AMERICA: PATIENT-DERIVED XENOGRAFT (PDX) MODEL MARKET, BY TUMOR TYPE, 2021-2028 (USD MILLION)
- TABLE 215 LATIN AMERICA: PATIENT-DERIVED XENOGRAFT (PDX) MODEL MARKET, BY APPLICATION, 2021-2028 (USD MILLION)
- TABLE 216 LATIN AMERICA: PATIENT-DERIVED XENOGRAFT (PDX) MODEL MARKET, BY END USER, 2021-2028 (USD MILLION)
- 11.5.1 BRAZIL
- 11.5.1.1 Growing need for advanced cancer research and therapeutic development to drive market
- TABLE 217 BRAZIL: PATIENT-DERIVED XENOGRAFT (PDX) MODEL MARKET, BY TYPE, 2021-2028 (USD MILLION)
- TABLE 218 BRAZIL: PATIENT-DERIVED XENOGRAFT (PDX) MODEL MARKET, BY IMPLANTATION METHOD, 2021-2028 (USD MILLION)
- TABLE 219 BRAZIL: PATIENT-DERIVED XENOGRAFT (PDX) MODEL MARKET, BY TUMOR TYPE, 2021-2028 (USD MILLION)
- TABLE 220 BRAZIL: PATIENT-DERIVED XENOGRAFT (PDX) MODEL MARKET, BY APPLICATION, 2021-2028 (USD MILLION)
- TABLE 221 BRAZIL: PATIENT-DERIVED XENOGRAFT (PDX) MODEL MARKET, BY END USER, 2021-2028 (USD MILLION)
- 11.5.2 REST OF LATIN AMERICA
- TABLE 222 REST OF LATIN AMERICA: PATIENT-DERIVED XENOGRAFT (PDX) MODEL MARKET, BY TYPE, 2021-2028 (USD MILLION)
- TABLE 223 REST OF LATIN AMERICA: PATIENT-DERIVED XENOGRAFT (PDX) MODEL MARKET, BY IMPLANTATION METHOD, 2021-2028 (USD MILLION)
- TABLE 224 REST OF LATIN AMERICA: PATIENT-DERIVED XENOGRAFT (PDX) MODEL MARKET, BY TUMOR TYPE, 2021-2028 (USD MILLION)
- TABLE 225 REST OF LATIN AMERICA: PATIENT-DERIVED XENOGRAFT (PDX) MODEL MARKET, BY APPLICATION, 2021-2028 (USD MILLION)
- TABLE 226 REST OF LATIN AMERICA: PATIENT-DERIVED XENOGRAFT (PDX) MODEL MARKET, BY END USER, 2021-2028 (USD MILLION)
- 11.5.3 LATIN AMERICA: IMPACT OF RECESSION
- 11.6 MIDDLE EAST & AFRICA
- TABLE 227 MIDDLE EAST & AFRICA: PATIENT-DERIVED XENOGRAFT (PDX) MODEL MARKET, BY COUNTRY, 2021-2028 (USD MILLION)
- TABLE 228 MIDDLE EAST & AFRICA: PATIENT-DERIVED XENOGRAFT (PDX) MODEL MARKET, BY TYPE, 2021-2028 (USD MILLION)
- TABLE 229 MIDDLE EAST & AFRICA: PATIENT-DERIVED XENOGRAFT (PDX) MODEL MARKET, BY IMPLANTATION METHOD, 2021-2028 (USD MILLION)
- TABLE 230 MIDDLE EAST & AFRICA: PATIENT-DERIVED XENOGRAFT (PDX) MODEL MARKET, BY TUMOR TYPE, 2021-2028 (USD MILLION)
- TABLE 231 MIDDLE EAST & AFRICA: PATIENT-DERIVED XENOGRAFT (PDX) MODEL MARKET, BY APPLICATION, 2021-2028 (USD MILLION)
- TABLE 232 MIDDLE EAST & AFRICA: PATIENT-DERIVED XENOGRAFT (PDX) MODEL MARKET, BY END USER, 2021-2028 (USD MILLION)
- 11.6.1 MIDDLE EAST
- 11.6.1.1 Growing use to probe treatment strategies aligned with individual patient profiles to drive market
- TABLE 233 MIDDLE EAST: PATIENT-DERIVED XENOGRAFT (PDX) MODEL MARKET, BY TYPE, 2021-2028 (USD MILLION)
- TABLE 234 MIDDLE EAST: PATIENT-DERIVED XENOGRAFT (PDX) MODEL MARKET, BY IMPLANTATION METHOD, 2021-2028 (USD MILLION)
- TABLE 235 MIDDLE EAST: PATIENT-DERIVED XENOGRAFT (PDX) MODEL MARKET, BY TUMOR TYPE, 2021-2028 (USD MILLION)
- TABLE 236 MIDDLE EAST: PATIENT-DERIVED XENOGRAFT (PDX) MODEL MARKET, BY APPLICATION, 2021-2028 (USD MILLION)
- TABLE 237 MIDDLE EAST: PATIENT-DERIVED XENOGRAFT (PDX) MODEL MARKET, BY END USER, 2021-2028 (USD MILLION)
- 11.6.2 AFRICA
- 11.6.2.1 Rising incidence of cancer and expanding research infrastructure to drive market
- TABLE 238 AFRICA: PATIENT-DERIVED XENOGRAFT (PDX) MODEL MARKET, BY TYPE, 2021-2028 (USD MILLION)
- TABLE 239 AFRICA: PATIENT-DERIVED XENOGRAFT (PDX) MODEL MARKET, BY IMPLANTATION METHOD, 2021-2028 (USD MILLION)
- TABLE 240 AFRICA: PATIENT-DERIVED XENOGRAFT (PDX) MODEL MARKET, BY TUMOR TYPE, 2021-2028 (USD MILLION)
- TABLE 241 AFRICA: PATIENT-DERIVED XENOGRAFT (PDX) MODEL MARKET, BY APPLICATION, 2021-2028 (USD MILLION)
- TABLE 242 AFRICA: PATIENT-DERIVED XENOGRAFT (PDX) MODEL MARKET, BY END USER, 2021-2028 (USD MILLION)
- 11.6.3 MIDDLE EAST & AFRICA: IMPACT OF RECESSION
12 COMPETITIVE LANDSCAPE
- 12.1 OVERVIEW
- 12.2 KEY STRATEGIES ADOPTED BY MAJOR PLAYERS
- FIGURE 25 PATIENT-DERIVED XENOGRAFT (PDX) MODEL MARKET: KEY STRATEGIES ADOPTED BY MAJOR PLAYERS
- 12.3 REVENUE SHARE ANALYSIS OF TOP FOUR PLAYERS, 2022
- FIGURE 26 REVENUE SHARE ANALYSIS FOR TOP FOUR COMPANIES, 2022
- 12.4 MARKET SHARE ANALYSIS OF TOP FOUR PLAYERS, 2020-2022
- FIGURE 27 PATIENT-DERIVED XENOGRAFT (PDX) MODEL MARKET SHARE ANALYSIS OF TOP FOUR PLAYERS, 2020-2022
- 12.5 COMPANY EVALUATION MATRIX FOR KEY PLAYERS, 2022
- 12.5.1 STARS
- 12.5.2 EMERGING LEADERS
- 12.5.3 PERVASIVE PLAYERS
- 12.5.4 PARTICIPANTS
- FIGURE 28 PATIENT-DERIVED XENOGRAFT (PDX) MODEL MARKET: COMPANY EVALUATION MATRIX FOR KEY PLAYERS, 2022
- 12.6 COMPANY EVALUATION MATRIX FOR STARTUPS/SMALL AND MEDIUM-SIZED ENTERPRISES (SMES), 2022
- 12.6.1 PROGRESSIVE COMPANIES
- 12.6.2 STARTING BLOCKS
- 12.6.3 RESPONSIVE COMPANIES
- 12.6.4 DYNAMIC COMPANIES
- FIGURE 29 PATIENT-DERIVED XENOGRAFT (PDX) MODEL MARKET: COMPANY EVALUATION MATRIX FOR STARTUPS/SMES, 2022
- 12.7 COMPETITIVE BENCHMARKING
- 12.7.1 PATIENT-DERIVED XENOGRAFT (PDX) MODEL MARKET: LIST OF STARTUPS/SMES
- TABLE 243 PATIENT-DERIVED XENOGRAFT (PDX) MODEL MARKET: LIST OF STARTUPS/SMES
- 12.7.2 PATIENT-DERIVED XENOGRAFT (PDX) MODEL MARKET: COMPETITIVE BENCHMARKING OF STARTUPS/SMES
- TABLE 244 PATIENT-DERIVED XENOGRAFT (PDX) MODEL MARKET: COMPETITIVE BENCHMARKING OF STARTUPS/SMES
- 12.8 COMPETITIVE SCENARIOS AND TRENDS
- 12.8.1 DEALS
- TABLE 245 PATIENT-DERIVED XENOGRAFT (PDX) MODEL MARKET: DEALS, 2021-2023
- 12.8.2 OTHERS
- TABLE 246 PATIENT-DERIVED XENOGRAFT (PDX) MODEL MARKET: OTHERS, 2021-2023
13 COMPANY PROFILES
- 13.1 KEY PLAYERS
- (Business Overview, Products/Services/Solutions Offered, MnM View, Key Strengths and Right to Win, Strategic Choices Made, Weaknesses and Competitive Threats, Recent Developments)**
- 13.1.1 JSR CORPORATION
- TABLE 247 JSR CORPORATION: COMPANY OVERVIEW
- FIGURE 30 JSR CORPORATION: COMPANY SNAPSHOT
- 13.1.2 WUXI APPTEC
- TABLE 248 WUXI APPTEC: COMPANY OVERVIEW
- FIGURE 31 WUXI APPTEC: COMPANY SNAPSHOT
- 13.1.3 THE JACKSON LABORATORY
- TABLE 249 THE JACKSON LABORATORY: COMPANY OVERVIEW
- FIGURE 32 THE JACKSON LABORATORY: COMPANY SNAPSHOT
- 13.1.4 CHARLES RIVER LABORATORIES
- TABLE 250 CHARLES RIVER LABORATORIES: COMPANY OVERVIEW
- FIGURE 33 CHARLES RIVER LABORATORIES: COMPANY SNAPSHOT
- 13.1.5 TACONIC BIOSCIENCES, INC.
- TABLE 251 TACONIC BIOSCIENCES, INC.: COMPANY OVERVIEW
- 13.1.6 ONCODESIGN PRECISION MEDICINE
- TABLE 252 ONCODESIGN PRECISION MEDICINE: COMPANY OVERVIEW
- FIGURE 34 ONCODESIGN PRECISION MEDICINE: COMPANY SNAPSHOT
- 13.1.7 INOTIV, INC.
- TABLE 253 INOTIV, INC.: COMPANY OVERVIEW
- FIGURE 35 INOTIV, INC.: COMPANY SNAPSHOT
- 13.1.8 PHARMATEST SERVICES
- TABLE 254 PHARMATEST SERVICES: COMPANY OVERVIEW
- 13.1.9 HERA BIOLABS
- TABLE 255 HERA BIOLABS: COMPANY OVERVIEW
- 13.1.10 EPO BERLIN-BUCH GMBH
- TABLE 256 EPO BERLIN-BUCH GMBH: COMPANY OVERVIEW
- 13.1.11 XENTECH
- TABLE 257 XENTECH: COMPANY OVERVIEW
- 13.1.12 UROSPHERE
- TABLE 258 UROSPHERE: COMPANY OVERVIEW
- 13.1.13 ALTOGEN LABS
- TABLE 259 ALTOGEN LABS: COMPANY OVERVIEW
- 13.1.14 ABNOVA CORPORATION
- TABLE 260 ABNOVA CORPORATION: COMPANY OVERVIEW
- 13.1.15 GENESIS BIOTECHNOLOGY GROUP
- TABLE 261 GENESIS BIOTECHNOLOGY GROUP: COMPANY OVERVIEW
- *Business Overview, Products/Services/Solutions Offered, MnM View, Key Strengths and Right to Win, Strategic Choices Made, Weaknesses and Competitive Threats, Recent Developments might not be captured in case of unlisted companies.
- 13.2 OTHER PLAYERS
- 13.2.1 BIOCYTOGEN PHARMACEUTICALS CO., LTD.
- 13.2.2 CREATIVE ANIMODEL
- 13.2.3 BIODURO-SUNDIA INC.
- 13.2.4 ARAGEN LIFE SCIENCES
- 13.2.5 SHANGHAI LIDE BIOTECH CO., LTD.
- 13.2.6 CERTIS ONCOLOGY SOLUTIONS
- 13.2.7 INNOSER
- 13.2.8 IVRS AB
- 13.2.9 BEIJING IDMO CO. LTD.
- 13.2.10 SHANGHAI CHEM PARTNER CO. LTD.
14 APPENDIX
- 14.1 KNOWLEDGESTORE: MARKETSANDMARKETS' SUBSCRIPTION PORTAL
- 14.2 CUSTOMIZATION OPTIONS
- 14.3 RELATED REPORTS
- 14.4 AUTHOR DETAILS