|
|
市場調査レポート
商品コード
1584544
潜在性結核検査の世界市場:検査タイプ別、用途別、エンドユーザー別、地域別 - 予測(~2029年)Latent TB Testing Market by Test Type (Tuberculin Skin Test/TST, IGRA Test), Application (Household Contacts (HHC) of Tuberculosis (TB) Patients, People Living with HIV), End User (Diagnostic Labs, Hospitals), Region - Global Forecast to 2029 |
||||||
カスタマイズ可能
|
|||||||
| 潜在性結核検査の世界市場:検査タイプ別、用途別、エンドユーザー別、地域別 - 予測(~2029年) |
|
出版日: 2024年11月01日
発行: MarketsandMarkets
ページ情報: 英文 261 Pages
納期: 即納可能
|
全表示
- 概要
- 図表
- 目次
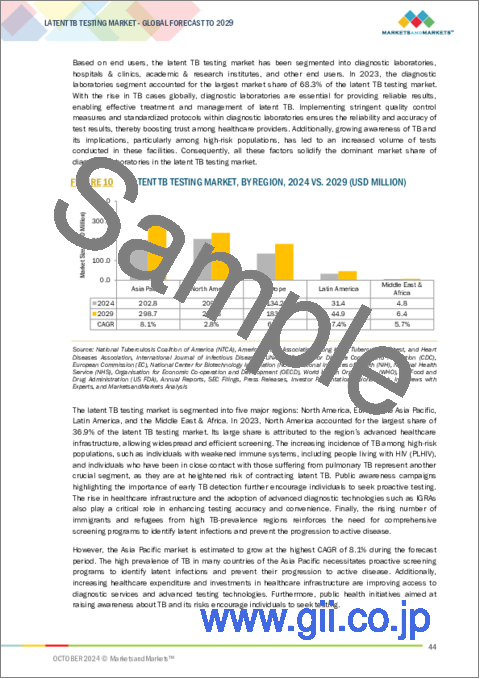
世界の潜在性結核検査の市場規模は、2024年の推定5億8,250万米ドルから2029年に7億7,340万米ドルに達すると予測され、予測期間にCAGRで5.8%の成長が見込まれます。
世界中の結核対策プログラムに対する資金や助成金の増加が、市場の重要な成長要因として浮上しています。現在、各国政府やWHOやGlobal Fundを含む国際機関によって、特に早期発見と予防の面で、増加する結核の負担に対処するために多額の資金が投入されています。これらの資金は、特に医療インフラが一般的に脆弱な高負担地域における診断施設へのアクセスの向上に充てられています。また、潜在性結核の正確な診断と適切な管理を行うために、患者を担当する医療従事者のトレーニングも奨励されています。それとは別に、研究助成金や国際的な協力関係も、結核検査を日常的な医療サービスに組み込むことを可能にしています。このように、拡大する資金によって潜在性結核検査が利用可能になり、世界の結核制圧への取り組みが強化されています。このことが、世界の潜在性結核検査市場の成長を促進しています。
| 調査範囲 | |
|---|---|
| 調査対象年 | 2022年~2029年 |
| 基準年 | 2023年 |
| 予測期間 | 2024年~2029年 |
| 単位 | 米ドル |
| セグメント | 検査タイプ、用途、エンドユーザー |
| 対象地域 | 北米、欧州、アジア太平洋、ラテンアメリカ、中東・アフリカ |
「検査タイプ別では、インターフェロンγ遊離試験(IGRA)セグメントが予測期間に市場でもっとも速い成長率を示す見込みです。」
インターフェロンγ遊離試験(IGRA)検査は、予測期間にもっとも高いCAGRを占めると予測されます。主な促進要因の1つは、従来のツベルクリン皮膚検査(TST)よりも精度が高いことです。IGRA検査は、TSTの偽陽性の原因となり得る事前のBCGワクチン接種による干渉を受けません。したがって、IGRAはBCGワクチン接種率が高い集団で好まれます。さらに、IGRAはより迅速で安定した結果が得られる傾向があるため、患者の来院回数が減り、検査全体の効率が向上します。免疫不全の患者や医療従事者など、リスクの高い集団にこれらの検査が浸透しつつあることが、需要の増加を支え続けています。WHOをはじめとするさまざまな国際保健機関による好ましい規制承認や推奨も、検査の普及を後押ししています。このような要因とともに、結核の研究資金の増加や診断法の進歩も、潜在性結核検査市場におけるIGRA検査セグメントの高成長を後押ししています。
「用途別では、肺結核家庭内接触者セグメントが予測期間に市場でもっとも高い成長率を占めました。」
結核患者と密接に接触している人は、潜在性結核に感染する確率が非常に高く、このことが、定期的なスクリーニングが疾患の早期診断と予防措置による介入を可能にする上で極めて重要である理由です。潜在性結核検査がハイリスクグループに果たす重要な役割が医療提供者や公衆衛生機関によって認識されつつあることも、正確な潜在性結核診断へのニーズを高めています。さらに、潜在性結核が活動性結核になるのを抑制するために、政府や結核対策プログラムが家庭内接触者をスクリーニングすることが、市場セグメントの成長をさらに促進しています。これらすべての要因が、潜在性結核検査市場におけるこの用途の急成長に寄与しています。
「アジア太平洋:潜在性結核検査市場でもっとも急成長している地域」
アジア太平洋は、高い罹患率を記録しているインドや中国など、結核の負担が大きく、その結果、必要不可欠な潜在性結核のスクリーニングと管理へのニーズが高まっています。政府や国際機関の資金援助や結核撲滅キャンペーンが活発化したことで、アジア太平洋では診断薬や医療インフラへのアクセスが向上しています。地域社会や医療従事者の間で、潜在性結核の早期発見の必要性に対する認識が高まっていることも、さらなる検査需要を大きく高めています。潜在性結核検査市場における同セグメントのもう1つの主な成長要因は、支援的な規制枠組みと医療費の増加です。これらすべての要因が、アジア太平洋における潜在性結核検査市場の成長を促進しています。
当レポートでは、世界の潜在性結核検査市場について調査分析し、主な促進要因と抑制要因、競合情勢、将来の動向などの情報を提供しています。
目次
第1章 イントロダクション
第2章 調査手法
第3章 エグゼクティブサマリー
第4章 重要考察
- 潜在性結核検査市場の概要
- 潜在性結核検査市場:検査タイプ別(2024年・2029年)
- 潜在性結核検査市場:用途別(2024年・2029年)
- 潜在性結核検査市場:エンドユーザー別(2024年・2029年)
- 潜在性結核検査市場:地理的成長機会
第5章 市場の概要
- イントロダクション
- 市場力学
- 促進要因
- 抑制要因
- 機会
- 課題
- 価格分析
- 平均販売価格:製品別
- IGRA検査の参考価格:地域別
- 特許分析
- バリューチェーン分析
- サプライチェーン分析
- 貿易分析
- 輸入データ
- 輸出データ
- エコシステム分析
- ポーターのファイブフォース分析
- 主なステークホルダーと購入基準
- 規制分析
- 規制情勢
- 規制機関、政府機関、その他の組織
- 技術分析
- 主要技術
- 補完技術
- 主な会議とイベント(2024年~2025年)
- 顧客ビジネスに影響を与える動向/混乱
- 投資と資金調達のシナリオ
- ケーススタディ分析
- 主要国における潜在性結核対策戦略
- 北米
- 欧州
- アジア太平洋
- 潜在性結核検査市場に対するAIの影響
第6章 潜在性結核検査市場:検査タイプ別
- イントロダクション
- IGRA
- TST
第7章 潜在性結核検査市場:用途別
- イントロダクション
- HIV感染者
- 肺結核(TB)の家庭内接触者(HHC)/結核(TB)患者の家庭内接触者(HHC)
- その他の用途
第8章 潜在性結核検査市場:エンドユーザー別
- イントロダクション
- 診断検査室
- 病院・診療所
- 学術・研究機関
- その他のエンドユーザー
第9章 潜在性結核検査市場:地域別
- イントロダクション
- 北米
- 北米のマクロ経済の見通し
- 米国
- カナダ
- アジア太平洋
- アジア太平洋のマクロ経済の見通し
- 中国
- 日本
- インド
- 韓国
- オーストラリア
- シンガポール
- その他のアジア太平洋
- 欧州
- 欧州のマクロ経済の見通し
- 英国
- フランス
- ドイツ
- スペイン
- イタリア
- ベルギー
- スウェーデン
- デンマーク
- その他の欧州
- ラテンアメリカ
- ラテンアメリカのマクロ経済の見通し
- ブラジル
- メキシコ
- その他のラテンアメリカ
- 中東・アフリカ
- 中東・アフリカのマクロ経済の見通し
- 南アフリカ
- サウジアラビア
- アラブ首長国連邦
- クウェート
- その他の中東・アフリカ
第10章 競合情勢
- イントロダクション
- 主要企業戦略/有力企業
- 収益分配分析
- 市場シェア分析
- 企業の評価マトリクス:主要企業(2023年)
- 企業の評価マトリクス:スタートアップ/中小企業(2023年)
- 企業の評価マトリクス:スタートアップ/中小企業(2023年)
- 評価と財務指標
- ブランド/製品の比較分析
- QIAGEN
- REVVITY
- BIOMERIEUX
- 競合シナリオ
第11章 企業プロファイル
- 主要企業
- QIAGEN
- REVVITY (OXFORD IMMUNOTEC)
- BEIJING WANTAI BIOPHARMACEUTICAL CO., LTD.
- SANOFI
- ENDO, INC.
- BIOMERIEUX
- SD BIOSENSOR, INC.
- LIONEX GMBH
- SERUM INSTITUTE OF INDIA PVT. LTD.
- ARKRAY, INC.
- その他の企業
- ZHI FEI BIOLOGICAL
- AID AUTOIMMUN DIAGNOSTIKA GMBH
- BODITECH MED INC.
- BIONEOVAN CO., LTD.
- BIOPANDA REAGENTS LTD.
第12章 付録
List of Tables
- TABLE 1 LATENT TB TESTING MARKET: RISK ASSESSMENT
- TABLE 2 COMMERCIALLY AVAILABLE IGRA TESTS, BY KEY PLAYER
- TABLE 3 AVERAGE SELLING PRICE OF LATENT TB TESTS, 2022-2024
- TABLE 4 INDICATIVE PRICE OF IGRA TESTS, BY REGION, 2022-2024
- TABLE 5 LATENT TB TESTING MARKET: LIST OF MAJOR PATENTS, 2022-2023
- TABLE 6 IMPORT DATA FOR DIAGNOSTIC AND LABORATORY REAGENTS (HS CODE 3822), BY COUNTRY, 2019-2023 (USD MILLION)
- TABLE 7 EXPORT DATA FOR DIAGNOSTIC AND LABORATORY REAGENTS (HS CODE 3822), BY COUNTRY, 2019-2023 (USD MILLION)
- TABLE 8 LATENT TB TESTING MARKET: ROLE IN ECOSYSTEM
- TABLE 9 LATENT TB TESTING MARKET: PORTER'S FIVE FORCES ANALYSIS
- TABLE 10 INFLUENCE OF STAKEHOLDERS ON BUYING PROCESS OF LATENT TB TESTS (%)
- TABLE 11 KEY BUYING CRITERIA FOR LATENT TB TESTS, BY TYPE
- TABLE 12 CLASSIFICATION OF DEVICES IN EUROPE
- TABLE 13 JAPAN: TIME, COST, AND COMPLEXITY OF REGISTRATION PROCESS
- TABLE 14 AUSTRALIA: CLASSIFICATION OF IVD MEDICAL DEVICES
- TABLE 15 NORTH AMERICA: KEY REGULATORY BODIES, GOVERNMENT AGENCIES, AND OTHER ORGANIZATIONS
- TABLE 16 EUROPE: KEY REGULATORY BODIES, GOVERNMENT AGENCIES, AND OTHER ORGANIZATIONS
- TABLE 17 ASIA PACIFIC: KEY REGULATORY BODIES, GOVERNMENT AGENCIES, AND OTHER ORGANIZATIONS
- TABLE 18 LATIN AMERICA: KEY REGULATORY BODIES, GOVERNMENT AGENCIES, AND OTHER ORGANIZATIONS
- TABLE 19 REST OF THE WORLD: KEY REGULATORY BODIES, GOVERNMENT AGENCIES, AND OTHER ORGANIZATIONS
- TABLE 20 LATENT TB TESTING MARKET: DETAILED LIST OF KEY CONFERENCES & EVENTS
- TABLE 21 LATENT TB TESTING MARKET, BY TEST TYPE, 2022-2029 (USD MILLION)
- TABLE 22 LATENT TB TESTING MARKET, BY TEST TYPE, 2022-2029 (IN MILLIONS)
- TABLE 23 BCG VACCINATION COVERAGE, BY REGION, 2021 VS 2022 VS 2023 (%)
- TABLE 24 COMMERCIALLY AVAILABLE IGRA PRODUCTS, BY COMPANY
- TABLE 25 LATENT TB TESTING MARKET FOR IGRA, BY REGION, 2022-2029 (USD MILLION)
- TABLE 26 NORTH AMERICA: LATENT TB TESTING MARKET FOR IGRA, BY COUNTRY, 2022-2029 (USD MILLION)
- TABLE 27 EUROPE: LATENT TB TESTING MARKET FOR IGRA, BY COUNTRY, 2022-2029 (USD MILLION)
- TABLE 28 ASIA PACIFIC: LATENT TB TESTING MARKET FOR IGRA, BY COUNTRY, 2022-2029 (USD MILLION)
- TABLE 29 LATIN AMERICA: LATENT TB TESTING MARKET FOR IGRA, BY COUNTRY, 2022-2029 (USD MILLION)
- TABLE 30 MIDDLE EAST & AFRICA: LATENT TB TESTING MARKET FOR IGRA, BY COUNTRY, 2022-2029 (USD MILLION)
- TABLE 31 COMMERCIALLY AVAILABLE TST PRODUCTS IN MARKET, BY COMPANY
- TABLE 32 LATENT TB TESTING MARKET FOR TST, BY REGION, 2022-2029 (USD MILLION)
- TABLE 33 NORTH AMERICA: LATENT TB TESTING MARKET FOR TST, BY COUNTRY, 2022-2029 (USD MILLION)
- TABLE 34 EUROPE: LATENT TB TESTING MARKET FOR TST, BY COUNTRY, 2022-2029 (USD MILLION)
- TABLE 35 ASIA PACIFIC: LATENT TB TESTING MARKET FOR TST, BY COUNTRY, 2022-2029 (USD MILLION)
- TABLE 36 LATIN AMERICA: LATENT TB TESTING MARKET FOR TST, BY COUNTRY, 2022-2029 (USD MILLION)
- TABLE 37 MIDDLE EAST & AFRICA: LATENT TB TESTING MARKET FOR TST, BY COUNTRY, 2022-2029 (USD MILLION)
- TABLE 38 LATENT TB TESTING MARKET, BY APPLICATION, 2022-2029 (USD MILLION)
- TABLE 39 LATENT TB TESTING MARKET FOR PEOPLE LIVING WITH HIV, BY REGION, 2022-2029 (USD MILLION)
- TABLE 40 NORTH AMERICA: LATENT TB TESTING MARKET FOR PEOPLE LIVING WITH HIV, BY COUNTRY, 2022-2029 (USD MILLION)
- TABLE 41 EUROPE: LATENT TB TESTING MARKET FOR PEOPLE LIVING WITH HIV, BY COUNTRY, 2022-2029 (USD MILLION)
- TABLE 42 ASIA PACIFIC: LATENT TB TESTING MARKET FOR PEOPLE LIVING WITH HIV, BY COUNTRY, 2022-2029 (USD MILLION)
- TABLE 43 LATIN AMERICA: LATENT TB TESTING MARKET FOR PEOPLE LIVING WITH HIV, BY COUNTRY, 2022-2029 (USD MILLION)
- TABLE 44 MIDDLE EAST & AFRICA: LATENT TB TESTING MARKET FOR PEOPLE LIVING WITH HIV, BY COUNTRY, 2022-2029 (USD MILLION)
- TABLE 45 LATENT TB TESTING MARKET FOR HOUSEHOLD CONTACTS WITH PULMONARY TB, BY REGION, 2022-2029 (USD MILLION)
- TABLE 46 NORTH AMERICA: LATENT TB TESTING MARKET FOR HOUSEHOLD CONTACTS WITH PULMONARY TB, BY COUNTRY, 2022-2029 (USD MILLION)
- TABLE 47 EUROPE: LATENT TB TESTING MARKET FOR HOUSEHOLD CONTACTS WITH PULMONARY TB, BY COUNTRY, 2022-2029 (USD MILLION)
- TABLE 48 ASIA PACIFIC: LATENT TB TESTING MARKET FOR HOUSEHOLD CONTACTS WITH PULMONARY TB, BY COUNTRY, 2022-2029 (USD MILLION)
- TABLE 49 LATIN AMERICA: LATENT TB TESTING MARKET FOR HOUSEHOLD CONTACTS WITH PULMONARY TB, BY COUNTRY, 2022-2029 (USD MILLION)
- TABLE 50 MIDDLE EAST & AFRICA: LATENT TB TESTING MARKET FOR HOUSEHOLD CONTACTS WITH PULMONARY TB, BY COUNTRY, 2022-2029 (USD MILLION)
- TABLE 51 LATENT TB TESTING MARKET FOR OTHER APPLICATIONS, BY REGION, 2022-2029 (USD MILLION)
- TABLE 52 NORTH AMERICA: LATENT TB TESTING MARKET FOR OTHER APPLICATIONS, BY COUNTRY, 2022-2029 (USD MILLION)
- TABLE 53 EUROPE: LATENT TB TESTING MARKET FOR OTHER APPLICATIONS, BY COUNTRY, 2022-2029 (USD MILLION)
- TABLE 54 ASIA PACIFIC: LATENT TB TESTING MARKET FOR OTHER APPLICATIONS, BY COUNTRY, 2022-2029 (USD MILLION)
- TABLE 55 LATIN AMERICA: LATENT TB TESTING MARKET FOR OTHER APPLICATIONS, BY COUNTRY, 2022-2029 (USD MILLION)
- TABLE 56 MIDDLE EAST & AFRICA: LATENT TB TESTING MARKET FOR OTHER APPLICATIONS, BY COUNTRY, 2022-2029 (USD MILLION)
- TABLE 57 LATENT TB TESTING MARKET, BY END USER, 2022-2029 (USD MILLION)
- TABLE 58 LATENT TB TESTING MARKET FOR DIAGNOSTIC LABORATORIES, BY REGION, 2022-2029 (USD MILLION)
- TABLE 59 NORTH AMERICA: LATENT TB TESTING MARKET FOR DIAGNOSTIC LABORATORIES, BY COUNTRY, 2022-2029 (USD MILLION)
- TABLE 60 EUROPE: LATENT TB TESTING MARKET FOR DIAGNOSTIC LABORATORIES, BY COUNTRY, 2022-2029 (USD MILLION)
- TABLE 61 ASIA PACIFIC: LATENT TB TESTING MARKET FOR DIAGNOSTIC LABORATORIES, BY COUNTRY, 2022-2029 (USD MILLION)
- TABLE 62 LATIN AMERICA: LATENT TB TESTING MARKET FOR DIAGNOSTIC LABORATORIES, BY COUNTRY, 2022-2029 (USD MILLION)
- TABLE 63 MIDDLE EAST & AFRICA: LATENT TB TESTING MARKET FOR DIAGNOSTIC LABORATORIES, BY COUNTRY, 2022-2029 (USD MILLION)
- TABLE 64 US: NUMBER OF COMMUNITY HOSPITALS, 2020 -VS 2021
- TABLE 65 LATENT TB TESTING MARKET FOR HOSPITALS & CLINICS, BY REGION, 2022-2029 (USD MILLION)
- TABLE 66 NORTH AMERICA: LATENT TB TESTING MARKET FOR HOSPITALS & CLINICS, BY COUNTRY, 2022-2029 (USD MILLION)
- TABLE 67 EUROPE: LATENT TB TESTING MARKET FOR HOSPITALS & CLINICS, BY COUNTRY, 2022-2029 (USD MILLION)
- TABLE 68 ASIA PACIFIC: LATENT TB TESTING MARKET FOR HOSPITALS & CLINICS, BY COUNTRY, 2022-2029 (USD MILLION)
- TABLE 69 LATIN AMERICA: LATENT TB TESTING MARKET FOR HOSPITALS & CLINICS, BY COUNTRY, 2022-2029 (USD MILLION)
- TABLE 70 MIDDLE EAST & AFRICA: LATENT TB TESTING MARKET FOR HOSPITALS & CLINICS, BY COUNTRY, 2022-2029 (USD MILLION)
- TABLE 71 LATENT TB TESTING MARKET FOR ACADEMIC & RESEARCH INSTITUTES, BY REGION, 2022-2029 (USD MILLION)
- TABLE 72 NORTH AMERICA: LATENT TB TESTING MARKET FOR ACADEMIC & RESEARCH INSTITUTES, BY COUNTRY, 2022-2029 (USD MILLION)
- TABLE 73 EUROPE: LATENT TB TESTING MARKET FOR ACADEMIC & RESEARCH INSTITUTES, BY COUNTRY, 2022-2029 (USD MILLION)
- TABLE 74 ASIA PACIFIC: LATENT TB TESTING MARKET FOR ACADEMIC & RESEARCH INSTITUTES, BY COUNTRY, 2022-2029 (USD MILLION)
- TABLE 75 LATIN AMERICA: LATENT TB TESTING MARKET FOR ACADEMIC & RESEARCH INSTITUTES, BY COUNTRY, 2022-2029 (USD MILLION)
- TABLE 76 MIDDLE EAST & AFRICA: LATENT TB TESTING MARKET FOR ACADEMIC & RESEARCH INSTITUTES, BY COUNTRY, 2022-2029 (USD MILLION)
- TABLE 77 LATENT TB TESTING MARKET FOR OTHER END USERS, BY REGION, 2022-2029 (USD MILLION)
- TABLE 78 NORTH AMERICA: LATENT TB TESTING MARKET FOR OTHER END USERS, BY COUNTRY, 2022-2029 (USD MILLION)
- TABLE 79 EUROPE: LATENT TB TESTING MARKET FOR OTHER END USERS, BY COUNTRY, 2022-2029 (USD MILLION)
- TABLE 80 ASIA PACIFIC: LATENT TB TESTING MARKET FOR OTHER END USERS, BY COUNTRY, 2022-2029 (USD MILLION)
- TABLE 81 LATIN AMERICA: LATENT TB TESTING MARKET FOR OTHER END USERS, BY COUNTRY, 2022-2029 (USD MILLION)
- TABLE 82 MIDDLE EAST & AFRICA: LATENT TB TESTING MARKET FOR OTHER END USERS, BY COUNTRY, 2022-2029 (USD MILLION)
- TABLE 83 LATENT TB TESTING MARKET, BY REGION, 2022-2029 (USD MILLION)
- TABLE 84 NORTH AMERICA: MACROINDICATORS FOR LATENT TB TESTING MARKET
- TABLE 85 NORTH AMERICA: LATENT TB TESTING MARKET, BY COUNTRY, 2022-2029 (USD MILLION)
- TABLE 86 NORTH AMERICA: LATENT TB TESTING MARKET, BY TEST TYPE, 2022-2029 (USD MILLION)
- TABLE 87 NORTH AMERICA: LATENT TB TESTING MARKET, BY TEST TYPE, 2022-2029 (IN MILLIONS)
- TABLE 88 NORTH AMERICA: LATENT TB TESTING MARKET, BY APPLICATION, 2022-2029 (USD MILLION)
- TABLE 89 NORTH AMERICA: LATENT TB TESTING MARKET, BY END USER, 2022-2029 (USD MILLION)
- TABLE 90 US: MAIN TB/LTBI INSTITUTIONS
- TABLE 91 US: LATENT TB TESTING MARKET, BY TEST TYPE, 2022-2029 (USD MILLION)
- TABLE 92 US: LATENT TB TESTING MARKET, BY TEST TYPE, 2022-2029 (IN MILLIONS)
- TABLE 93 US: LATENT TB TESTING MARKET, BY APPLICATION, 2022-2029 (USD MILLION)
- TABLE 94 US: LATENT TB TESTING MARKET, BY END USER, 2022-2029 (USD MILLION)
- TABLE 95 CANADA: MAIN TB/LTBI INSTITUTIONS
- TABLE 96 CANADA: LATENT TB TESTING MARKET, BY TEST TYPE, 2022-2029 (USD MILLION)
- TABLE 97 CANADA: LATENT TB TESTING MARKET, BY TEST TYPE, 2022-2029 (IN MILLIONS)
- TABLE 98 CANADA: LATENT TB TESTING MARKET, BY APPLICATION, 2022-2029 (USD MILLION)
- TABLE 99 CANADA: LATENT TB TESTING MARKET, BY END USER, 2022-2029 (USD MILLION)
- TABLE 100 ASIA PACIFIC: MACROECONOMIC INDICATORS FOR LATENT TB TESTING MARKET
- TABLE 101 ASIA PACIFIC: LATENT TB TESTING MARKET, BY COUNTRY, 2022-2029 (USD MILLION)
- TABLE 102 ASIA PACIFIC: LATENT TB TESTING MARKET, BY TEST TYPE, 2022-2029 (USD MILLION)
- TABLE 103 ASIA PACIFIC: LATENT TB TESTING MARKET, BY TEST TYPE, 2022-2029 (IN MILLIONS)
- TABLE 104 ASIA PACIFIC: LATENT TB TESTING MARKET, BY APPLICATION, 2022-2029 (USD MILLION)
- TABLE 105 ASIA PACIFIC: LATENT TB TESTING MARKET, BY END USER, 2022-2029 (USD MILLION)
- TABLE 106 CHINA: MAIN TB/LTBI INSTITUTIONS
- TABLE 107 CHINA: LATENT TB TESTING MARKET, BY TEST TYPE, 2022-2029 (USD MILLION)
- TABLE 108 CHINA: LATENT TB TESTING MARKET, BY TEST TYPE, 2022-2029 (IN MILLIONS)
- TABLE 109 CHINA: LATENT TB TESTING MARKET, BY APPLICATION, 2022-2029 (USD MILLION)
- TABLE 110 CHINA: LATENT TB TESTING MARKET, BY END USER, 2022-2029 (USD MILLION)
- TABLE 111 JAPAN: MAIN TB/LTBI INSTITUTIONS
- TABLE 112 JAPAN: LATENT TB TESTING MARKET, BY TEST TYPE, 2022-2029 (USD MILLION)
- TABLE 113 JAPAN: LATENT TB TESTING MARKET, BY TEST TYPE, 2022-2029 (IN MILLIONS)
- TABLE 114 JAPAN: LATENT TB TESTING MARKET, BY APPLICATION, 2022-2029 (USD MILLION)
- TABLE 115 JAPAN: LATENT TB TESTING MARKET, BY END USER, 2022-2029 (USD MILLION)
- TABLE 116 INDIA: MAIN TB/LTBI INSTITUTIONS
- TABLE 117 INDIA: LATENT TB TESTING MARKET, BY TEST TYPE, 2022-2029 (USD MILLION)
- TABLE 118 INDIA: LATENT TB TESTING MARKET, BY TEST TYPE, 2022-2029 (IN MILLIONS)
- TABLE 119 INDIA: LATENT TB TESTING MARKET, BY APPLICATION, 2022-2029 (USD MILLION)
- TABLE 120 INDIA: LATENT TB TESTING MARKET, BY END USER, 2022-2029 (USD MILLION)
- TABLE 121 SOUTH KOREA: MAIN TB/LTBI INSTITUTIONS
- TABLE 122 SOUTH KOREA: LATENT TB TESTING MARKET, BY TEST TYPE, 2022-2029 (USD MILLION)
- TABLE 123 SOUTH KOREA: LATENT TB TESTING MARKET, BY TEST TYPE, 2022-2029 (IN MILLIONS)
- TABLE 124 SOUTH KOREA: LATENT TB TESTING MARKET, BY APPLICATION, 2022-2029 (USD MILLION)
- TABLE 125 SOUTH KOREA: LATENT TB TESTING MARKET, BY END USER, 2022-2029 (USD MILLION)
- TABLE 126 AUSTRALIA: MAIN TB/LTBI INSTITUTIONS
- TABLE 127 AUSTRALIA: LATENT TB TESTING MARKET, BY TEST TYPE, 2022-2029 (USD MILLION)
- TABLE 128 AUSTRALIA: LATENT TB TESTING MARKET, BY TEST TYPE, 2022-2029 (IN MILLIONS)
- TABLE 129 AUSTRALIA: LATENT TB TESTING MARKET, BY APPLICATION, 2022-2029 (USD MILLION)
- TABLE 130 AUSTRALIA: LATENT TB TESTING MARKET, BY END USER, 2022-2029 (USD MILLION)
- TABLE 131 SINGAPORE: MAIN TB/LTBI INSTITUTIONS
- TABLE 132 SINGAPORE: LATENT TB TESTING MARKET, BY TEST TYPE, 2022-2029 (USD MILLION)
- TABLE 133 SINGAPORE: LATENT TB TESTING MARKET, BY TEST TYPE, 2022-2029 (IN MILLIONS)
- TABLE 134 SINGAPORE: LATENT TB TESTING MARKET, BY APPLICATION, 2022-2029 (USD MILLION)
- TABLE 135 SINGAPORE: LATENT TB TESTING MARKET, BY END USER, 2022-2029 (USD MILLION)
- TABLE 136 REST OF ASIA PACIFIC: LATENT TB TESTING MARKET, BY TEST TYPE, 2022-2029 (USD MILLION)
- TABLE 137 REST OF ASIA PACIFIC: LATENT TB TESTING MARKET, BY TEST TYPE, 2022-2029 (IN MILLIONS)
- TABLE 138 REST OF ASIA PACIFIC: LATENT TB TESTING MARKET, BY APPLICATION, 2022-2029 (USD MILLION)
- TABLE 139 REST OF ASIA PACIFIC: LATENT TB TESTING MARKET, BY END USER, 2022-2029 (USD MILLION)
- TABLE 140 EUROPE: MACROINDICATORS FOR LATENT TB TESTING MARKET
- TABLE 141 EUROPE: LATENT TB TESTING MARKET, BY COUNTRY, 2022-2029 (USD MILLION)
- TABLE 142 EUROPE: LATENT TB TESTING MARKET, BY TEST TYPE, 2022-2029 (USD MILLION)
- TABLE 143 EUROPE: LATENT TB TESTING MARKET, BY TEST TYPE, 2022-2029 (IN MILLIONS)
- TABLE 144 EUROPE: LATENT TB TESTING MARKET, BY APPLICATION, 2022-2029 (USD MILLION)
- TABLE 145 EUROPE: LATENT TB TESTING MARKET, BY END USER, 2022-2029 (USD MILLION)
- TABLE 146 UK: MAIN TB/LTBI INSTITUTIONS
- TABLE 147 UK: LATENT TB TESTING MARKET, BY TEST TYPE, 2022-2029 (USD MILLION)
- TABLE 148 UK: LATENT TB TESTING MARKET, BY TEST TYPE, 2022-2029 (IN MILLIONS)
- TABLE 149 UK: LATENT TB TESTING MARKET, BY APPLICATION, 2022-2029 (USD MILLION)
- TABLE 150 UK: LATENT TB TESTING MARKET, BY END USER, 2022-2029 (USD MILLION)
- TABLE 151 FRANCE: MAIN TB/LTBI INSTITUTIONS
- TABLE 152 FRANCE: LATENT TB TESTING MARKET, BY TEST TYPE, 2022-2029 (USD MILLION)
- TABLE 153 FRANCE: LATENT TB TESTING MARKET, BY TEST TYPE, 2022-2029 (IN MILLIONS)
- TABLE 154 FRANCE: LATENT TB TESTING MARKET, BY APPLICATION, 2022-2029 (USD MILLION)
- TABLE 155 FRANCE: LATENT TB TESTING MARKET, BY END USER, 2022-2029 (USD MILLION)
- TABLE 156 GERMANY: MAIN TB/LTBI INSTITUTIONS
- TABLE 157 GERMANY: LATENT TB TESTING MARKET, BY TEST TYPE, 2022-2029 (USD MILLION)
- TABLE 158 GERMANY: LATENT TB TESTING MARKET, BY TEST TYPE, 2022-2029 (IN MILLIONS)
- TABLE 159 GERMANY: LATENT TB TESTING MARKET, BY APPLICATION, 2022-2029 (USD MILLION)
- TABLE 160 GERMANY: LATENT TB TESTING MARKET, BY END USER, 2022-2029 (USD MILLION)
- TABLE 161 SPAIN: MAIN TB/LTBI INSTITUTIONS
- TABLE 162 SPAIN: LATENT TB TESTING MARKET, BY TEST TYPE, 2022-2029 (USD MILLION)
- TABLE 163 SPAIN: LATENT TB TESTING MARKET, BY TEST TYPE, 2022-2029 (IN MILLIONS)
- TABLE 164 SPAIN: LATENT TB TESTING MARKET, BY APPLICATION, 2022-2029 (USD MILLION)
- TABLE 165 SPAIN: LATENT TB TESTING MARKET, BY END USER, 2022-2029 (USD MILLION)
- TABLE 166 ITALY: MAIN TB/LTBI INSTITUTIONS
- TABLE 167 ITALY: LATENT TB TESTING MARKET, BY TEST TYPE, 2022-2029 (USD MILLION)
- TABLE 168 ITALY: LATENT TB TESTING MARKET, BY TEST TYPE, 2022-2029 (IN MILLIONS)
- TABLE 169 ITALY: LATENT TB TESTING MARKET, BY APPLICATION, 2022-2029 (USD MILLION)
- TABLE 170 ITALY: LATENT TB TESTING MARKET, BY END USER, 2022-2029 (USD MILLION)
- TABLE 171 BELGIUM: MAIN TB/LTBI INSTITUTIONS
- TABLE 172 BELGIUM: LATENT TB TESTING MARKET, BY TEST TYPE, 2022-2029 (USD MILLION)
- TABLE 173 BELGIUM: LATENT TB TESTING MARKET, BY TEST TYPE, 2022-2029 (IN MILLIONS)
- TABLE 174 BELGIUM: LATENT TB TESTING MARKET, BY APPLICATION, 2022-2029 (USD MILLION)
- TABLE 175 BELGIUM: LATENT TB TESTING MARKET, BY END USER, 2022-2029 (USD MILLION)
- TABLE 176 SWEDEN: LATENT TB TESTING MARKET, BY TEST TYPE, 2022-2029 (USD MILLION)
- TABLE 177 SWEDEN: LATENT TB TESTING MARKET, BY TEST TYPE, 2022-2029 (IN MILLIONS)
- TABLE 178 SWEDEN: LATENT TB TESTING MARKET, BY APPLICATION, 2022-2029 (USD MILLION)
- TABLE 179 SWEDEN: LATENT TB TESTING MARKET, BY END USER, 2022-2029 (USD MILLION)
- TABLE 180 DENMARK: MAIN TB/LTBI INSTITUTIONS
- TABLE 181 DENMARK: LATENT TB TESTING MARKET, BY TEST TYPE, 2022-2029 (USD MILLION)
- TABLE 182 DENMARK: LATENT TB TESTING MARKET, BY TEST TYPE, 2022-2029 (IN MILLIONS)
- TABLE 183 DENMARK: LATENT TB TESTING MARKET, BY APPLICATION, 2022-2029 (USD MILLION)
- TABLE 184 DENMARK: LATENT TB TESTING MARKET, BY END USER, 2022-2029 (USD MILLION)
- TABLE 185 REST OF EUROPE: LATENT TB TESTING MARKET, BY TEST TYPE, 2022-2029 (USD MILLION)
- TABLE 186 REST OF EUROPE: LATENT TB TESTING MARKET, BY TEST TYPE, 2022-2029 (IN MILLIONS)
- TABLE 187 REST OF EUROPE: LATENT TB TESTING MARKET, BY APPLICATION, 2022-2029 (USD MILLION)
- TABLE 188 REST OF EUROPE: LATENT TB TESTING MARKET, BY END USER, 2022-2029 (USD MILLION)
- TABLE 189 LATIN AMERICA: MACROINDICATORS FOR LATENT TB TESTING MARKET
- TABLE 190 LATIN AMERICA: LATENT TB TESTING MARKET, BY COUNTRY, 2022-2029 (USD MILLION)
- TABLE 191 LATIN AMERICA: LATENT TB TESTING MARKET, BY TEST TYPE, 2022-2029 (USD MILLION)
- TABLE 192 LATIN AMERICA: LATENT TB TESTING MARKET, BY TEST TYPE, 2022-2029 (IN MILLIONS)
- TABLE 193 LATIN AMERICA: LATENT TB TESTING MARKET, BY APPLICATION, 2022-2029 (USD MILLION)
- TABLE 194 LATIN AMERICA: LATENT TB TESTING MARKET, BY END USER, 2022-2029 (USD MILLION)
- TABLE 195 BRAZIL: MAIN TB/LTBI INSTITUTIONS
- TABLE 196 BRAZIL: LATENT TB TESTING MARKET, BY TEST TYPE, 2022-2029 (USD MILLION)
- TABLE 197 BRAZIL: LATENT TB TESTING MARKET, BY TEST TYPE, 2022-2029 (IN MILLIONS)
- TABLE 198 BRAZIL: LATENT TB TESTING MARKET, BY APPLICATION, 2022-2029 (USD MILLION)
- TABLE 199 BRAZIL: LATENT TB TESTING MARKET, BY END USER, 2022-2029 (USD MILLION)
- TABLE 200 MEXICO: MAIN TB/LTBI INSTITUTIONS
- TABLE 201 MEXICO: LATENT TB TESTING MARKET, BY TEST TYPE, 2022-2029 (USD MILLION)
- TABLE 202 MEXICO: LATENT TB TESTING MARKET, BY TEST TYPE, 2022-2029 (IN MILLIONS)
- TABLE 203 MEXICO: LATENT TB TESTING MARKET, BY APPLICATION, 2022-2029 (USD MILLION)
- TABLE 204 MEXICO: LATENT TB TESTING MARKET, BY END USER, 2022-2029 (USD MILLION)
- TABLE 205 REST OF LATIN AMERICA: LATENT TB TESTING MARKET, BY TEST TYPE, 2022-2029 (USD MILLION)
- TABLE 206 REST OF LATIN AMERICA: LATENT TB TESTING MARKET, BY TEST TYPE, 2022-2029 (IN MILLIONS)
- TABLE 207 REST OF LATIN AMERICA: LATENT TB TESTING MARKET, BY APPLICATION, 2022-2029 (USD MILLION)
- TABLE 208 REST OF LATIN AMERICA: LATENT TB TESTING MARKET, BY END USER, 2022-2029 (USD MILLION)
- TABLE 209 MIDDLE EAST & AFRICA: LATENT TB TESTING MARKET, BY COUNTRY, 2022-2029 (USD MILLION)
- TABLE 210 MIDDLE EAST & AFRICA: LATENT TB TESTING MARKET, BY TEST TYPE, 2022-2029 (USD MILLION)
- TABLE 211 MIDDLE EAST & AFRICA: LATENT TB TESTING MARKET, BY TEST TYPE, 2022-2029 (IN MILLIONS)
- TABLE 212 MIDDLE EAST & AFRICA: LATENT TB TESTING MARKET, BY APPLICATION, 2022-2029 (USD MILLION)
- TABLE 213 MIDDLE EAST & AFRICA: LATENT TB TESTING MARKET, BY END USER, 2022-2029 (USD MILLION)
- TABLE 214 SOUTH AFRICA: MAIN TB/LTBI INSTITUTIONS
- TABLE 215 SOUTH AFRICA: LATENT TB TESTING MARKET, BY TEST TYPE, 2022-2029 (USD MILLION)
- TABLE 216 SOUTH AFRICA: LATENT TB TESTING MARKET, BY TEST TYPE, 2022-2029 (IN MILLIONS)
- TABLE 217 SOUTH AFRICA: LATENT TB TESTING MARKET, BY APPLICATION, 2022-2029 (USD MILLION)
- TABLE 218 SOUTH AFRICA: LATENT TB TESTING MARKET, BY END USER, 2022-2029 (USD MILLION)
- TABLE 219 SAUDI ARABIA: LATENT TB TESTING MARKET, BY TEST TYPE, 2022-2029 (USD MILLION)
- TABLE 220 SAUDI ARABIA: LATENT TB TESTING MARKET, BY TEST TYPE, 2022-2029 (IN MILLIONS)
- TABLE 221 SAUDI ARABIA: LATENT TB TESTING MARKET, BY APPLICATION, 2022-2029 (USD MILLION)
- TABLE 222 SAUDI ARABIA: LATENT TB TESTING MARKET, BY END USER, 2022-2029 (USD MILLION)
- TABLE 223 UAE: LATENT TB TESTING MARKET, BY TEST TYPE, 2022-2029 (USD MILLION)
- TABLE 224 UAE: LATENT TB TESTING MARKET, BY TEST TYPE, 2022-2029 (IN MILLIONS)
- TABLE 225 UAE: LATENT TB TESTING MARKET, BY APPLICATION, 2022-2029 (USD MILLION)
- TABLE 226 UAE: LATENT TB TESTING MARKET, BY END USER, 2022-2029 (USD MILLION)
- TABLE 227 KUWAIT: LATENT TB TESTING MARKET, BY TEST TYPE, 2022-2029 (USD MILLION)
- TABLE 228 KUWAIT: LATENT TB TESTING MARKET, BY TEST TYPE, 2022-2029 (IN MILLIONS)
- TABLE 229 KUWAIT: LATENT TB TESTING MARKET, BY APPLICATION, 2022-2029 (USD MILLION)
- TABLE 230 KUWAIT: LATENT TB TESTING MARKET, BY END USER, 2022-2029 (USD MILLION)
- TABLE 231 REST OF MIDDLE EAST & AFRICA: LATENT TB TESTING MARKET, BY TEST TYPE, 2022-2029 (USD MILLION)
- TABLE 232 REST OF MIDDLE EAST & AFRICA: LATENT TB TESTING MARKET, BY TEST TYPE, 2022-2029 (IN MILLIONS)
- TABLE 233 REST OF MIDDLE EAST & AFRICA: LATENT TB TESTING MARKET, BY APPLICATION, 2022-2029 (USD MILLION)
- TABLE 234 REST OF MIDDLE EAST & AFRICA: LATENT TB TESTING MARKET, BY END USER, 2022-2029 (USD MILLION)
- TABLE 235 OVERVIEW OF STRATEGIES DEPLOYED BY KEY MANUFACTURING COMPANIES
- TABLE 236 LATENT TB TESTING MARKET: DEGREE OF COMPETITION
- TABLE 237 LATENT TB TESTING MARKET: TEST TYPE FOOTPRINT
- TABLE 238 LATENT TB TESTING MARKET: APPLICATION FOOTPRINT
- TABLE 239 LATENT TB TESTING MARKET: REGION FOOTPRINT
- TABLE 240 LATENT TB TESTING MARKET: DETAILED LIST OF KEY STARTUPS/SME PLAYERS
- TABLE 241 LATENT TB TESTING MARKET: COMPETITIVE BENCHMARKING OF STARTUP/SME PLAYERS
- TABLE 242 LATENT TB TESTING MARKET: PRODUCT LAUNCHES, JANUARY 2021-SEPTEMBER 2024
- TABLE 243 LATENT TB TESTING MARKET: DEALS, JANUARY 2021-SEPTEMBER 2024
- TABLE 244 QIAGEN: COMPANY OVERVIEW
- TABLE 245 QIAGEN: PRODUCTS OFFERED
- TABLE 246 QIAGEN: PRODUCT LAUNCHES & APPROVALS, JANUARY 2021-SEPTEMBER 2024
- TABLE 247 QIAGEN: DEALS, JANUARY 2021-SEPTEMBER 2024
- TABLE 248 QIAGEN: EXPANSIONS, JANUARY 2021-SEPTEMBER 2024
- TABLE 249 REVVITY: COMPANY OVERVIEW
- TABLE 250 REVVITY: PRODUCTS OFFERED
- TABLE 251 REVVITY: PRODUCT LAUNCHES & APPROVALS, JANUARY 2021-SEPTEMBER 2024
- TABLE 252 BEIJING WANTAI BIOPHARMACEUTICAL: COMPANY OVERVIEW
- TABLE 253 BEIJING WANTAI BIOPHARMACEUTICAL: PRODUCTS OFFERED
- TABLE 254 SANOFI: COMPANY OVERVIEW
- TABLE 255 SANOFI: PRODUCTS OFFERED
- TABLE 256 ENDO: COMPANY OVERVIEW
- TABLE 257 ENDO: PRODUCTS OFFERED
- TABLE 258 BIOMERIEUX: COMPANY OVERVIEW
- TABLE 259 BIOMERIEUX: PRODUCTS OFFERED
- TABLE 260 BIOMERIEUX: PRODUCT APPROVALS, JANUARY 2021-SEPTEMBER 2024
- TABLE 261 SD BIOSENSOR: COMPANY OVERVIEW
- TABLE 262 SD BIOSENSOR: PRODUCTS OFFERED
- TABLE 263 SD BIOSENSOR: DEALS, JANUARY 2021-SEPTEMBER 2024
- TABLE 264 LIONEX GMBH: COMPANY OVERVIEW
- TABLE 265 LIONEX GMBH: PRODUCTS OFFERED
- TABLE 266 SERUM INSTITUTE OF INDIA: COMPANY OVERVIEW
- TABLE 267 SERUM INSTITUTE OF INDIA: PRODUCTS OFFERED
- TABLE 268 ARKRAY: COMPANY OVERVIEW
- TABLE 269 ARKRAY: PRODUCTS OFFERED
- TABLE 270 ARKRAY: EXPANSIONS, JANUARY 2021-SEPTEMBER 2024
List of Figures
- FIGURE 1 LATENT TB TESTING MARKET: RESEARCH DESIGN
- FIGURE 2 BOTTOM-UP APPROACH: COMPANY REVENUE ESTIMATION APPROACH
- FIGURE 3 CAGR PROJECTIONS: SUPPLY-SIDE ANALYSIS
- FIGURE 4 LATENT TB TESTING MARKET: TOP-DOWN APPROACH
- FIGURE 5 LATENT TB TESTING MARKET: DATA TRIANGULATION
- FIGURE 6 LATENT TB TESTING MARKET: STUDY ASSUMPTIONS
- FIGURE 7 LATENT TB TESTING MARKET, BY TEST TYPE, 2024 VS. 2029 (USD MILLION)
- FIGURE 8 LATENT TB TESTING MARKET, BY APPLICATION, 2024 VS. 2029 (USD MILLION)
- FIGURE 9 LATENT TB TESTING MARKET, BY END USER, 2024 VS. 2029 (USD MILLION)
- FIGURE 10 LATENT TB TESTING MARKET, BY REGION, 2024 VS. 2029 (USD MILLION)
- FIGURE 11 INCREASING PREVALENCE OF LATENT TB AND GROWING FINANCIAL SUPPORT FOR TB CONTROL TO DRIVE MARKET
- FIGURE 12 IGRA WILL CONTINUE TO DOMINATE MARKET TILL 2029
- FIGURE 13 PEOPLE LIVING WITH HIV SEGMENT TO HOLD LARGEST MARKET SHARE
- FIGURE 14 DIAGNOSTIC LABORATORIES SEGMENT TO RETAIN DOMINANT MARKET SHARE TILL 2029
- FIGURE 15 ASIA PACIFIC TO REGISTER HIGHEST GROWTH
- FIGURE 16 LATENT TB TESTING MARKET: DRIVERS, RESTRAINTS, OPPORTUNITIES, AND CHALLENGES
- FIGURE 17 PATENT ANALYSIS FOR LATENT TB TESTS (JANUARY 2014-DECEMBER 2023)
- FIGURE 18 VALUE CHAIN ANALYSIS: MAJOR VALUE ADDED DURING MANUFACTURING AND ASSEMBLY PHASES
- FIGURE 19 LATENT TB TESTING MARKET: SUPPLY CHAIN ANALYSIS
- FIGURE 20 LATENT TB TESTING MARKET: ECOSYSTEM ANALYSIS
- FIGURE 21 LATENT TB TESTING MARKET: PORTER'S FIVE FORCES ANALYSIS
- FIGURE 22 INFLUENCE OF STAKEHOLDERS ON BUYING PROCESS OF LATENT TB TESTS
- FIGURE 23 KEY BUYING CRITERIA FOR LATENT TB TESTS
- FIGURE 24 TRENDS/DISRUPTIONS IMPACTING CUSTOMER BUSINESS
- FIGURE 25 DEALS AND FUNDING IN LATENT TB TESTING MARKET
- FIGURE 26 MARKET POTENTIAL OF AI TO ENHANCE R&D IN LATENT TB TESTING MARKET
- FIGURE 27 NORTH AMERICA: LATENT TB TESTING MARKET SNAPSHOT
- FIGURE 28 ASIA PACIFIC: LATENT TB TESTING MARKET SNAPSHOT
- FIGURE 29 REVENUE ANALYSIS OF KEY PLAYERS IN LATENT TB TESTING MARKET (2021-2023)
- FIGURE 30 MARKET SHARE ANALYSIS OF KEY PLAYERS IN LATENT TB TESTING MARKET (2023)
- FIGURE 31 NORTH AMERICA: MARKET SHARE ANALYSIS OF KEY PLAYERS (2023)
- FIGURE 32 EUROPE: MARKET SHARE ANALYSIS OF KEY PLAYERS (2023)
- FIGURE 33 ASIA PACIFIC: MARKET SHARE ANALYSIS OF KEY PLAYERS (2023)
- FIGURE 34 LATENT TB TESTING MARKET: COMPANY EVALUATION MATRIX (KEY PLAYERS), 2023
- FIGURE 35 LATENT TB TESTING MARKET: COMPANY FOOTPRINT
- FIGURE 36 LATENT TB TESTING MARKET: COMPANY EVALUATION MATRIX (STARTUPS/SMES), 2023
- FIGURE 37 EV/EBITDA OF KEY VENDORS
- FIGURE 38 YEAR-TO-DATE (YTD) PRICE TOTAL RETURN AND 5-YEAR STOCK BETA OF KEY VENDORS
- FIGURE 39 BRAND COMPARISON FOR IGRA
- FIGURE 40 QIAGEN: COMPANY SNAPSHOT (2023)
- FIGURE 41 REVVITY: COMPANY SNAPSHOT (2023)
- FIGURE 42 BEIJING WANTAI BIOPHARMACEUTICAL: COMPANY SNAPSHOT (2023)
- FIGURE 43 SANOFI: COMPANY SNAPSHOT (2023)
- FIGURE 44 ENDO: COMPANY SNAPSHOT (2023)
- FIGURE 45 BIOMERIEUX: COMPANY SNAPSHOT (2023)
- FIGURE 46 SD BIOSENSOR: COMPANY SNAPSHOT (2023)
The latent TB testing market will rise in value from estimated USD 582.5 million in 2024 to USD 773.4 million in 2029, at a CAGR of 5.8% over the forecast period. Increased funding and grants for TB control programs across the globe have emerged as an important growth factor in the latent TB testing market. Substantial funding is now being committed by governments and international bodies, including WHO and the Global Fund, to address the increasing burden of TB, especially in terms of early detection and prevention. These funds are being channeled into increasing access to diagnostic facilities, especially in high-burden regions where the healthcare infrastructure is generally weak. The availability of funds also encourages the training of healthcare professionals who work with a patient to accurately diagnose and properly manage cases of latent TB. Apart from that, research grants and international collaborations are also enabling TB testing integration into routine healthcare services. Thus, this growing funding is rendering latent TB tests available and enhancing the efforts to control TB globally. This, in turn, is fuelling the growth of the global latent TB testing market.
| Scope of the Report | |
|---|---|
| Years Considered for the Study | 2022-2029 |
| Base Year | 2023 |
| Forecast Period | 2024-2029 |
| Units Considered | Value (USD) |
| Segments | Test Type, Application, and End User |
| Regions covered | North America, Europe, Asia Pacific, Latin America, and the Middle East & Africa |
"Interferon gamma release assay (IGRA) segment is expected to have the fastest growth rate in the latent TB testing market, by test type, during the forecast period."
The latent TB testing market is segmented into tuberculin skin test (TST) and interferon gamma release assay (IGRA), based on test type. Interferon gamma release assay (IGRA) tests are projected to account for the highest CAGR during the forecast period. One major driving factor is its higher accuracy than conventional tuberculin skin tests (TST); IGRA tests do not suffer from interference by prior BCG vaccination, which can cause false positives for TST. Thus, IGRA is preferred in populations that have high BCG vaccination coverage. Furthermore, IGRA tends to offer faster and more consistent results, meaning that patients require fewer visits, enhancing overall efficiency in testing. Growing penetration of these tests in high-risk populations, such as patients who are immunocompromised and health care workers, continue to support increased demand. Favourable regulatory approvals and recommendations by various international health bodies, including the WHO, have also helped increase their adoption. Along with such factors, rising funding for research in TB and advancements in diagnostics are also driving the high growth of the IGRA test segment in the latent TB testing market.
"Household contacts with pulmonary TB segment accounted for the highest growth rate in the latent TB testing market, by application, during the forecast period."
Based on application, the latent TB testing market is bifurcated into household contacts (HHC) with pulmonary tuberculosis (TB)/household contacts (HHC) of tuberculosis (TB) patients, people living with HIV, and other applications. The household contacts with pulmonary TB application segment is expected to have the highest CAGR during the forecast period. Individuals in close contact with a TB patient have a much higher probability of becoming infected with latent TB, which is the reason why regular screenings are crucial in being able to diagnose the illness early and intervene by taking preventive measures. Increasing recognition of the critical role that latent TB testing plays among high-risk groups by healthcare providers and public health agencies also drives the need for accurate latent TB diagnostics. In addition, screening of contacts across the households by the governments and TB control programs in efforts to curb latent infections from becoming active TB is further driving growth in the market segment. Collectively, all these factors are contributing to the fast growth of this application within the latent TB testing market.
"Asia Pacific: The fastest-growing region in latent TB testing market."
The worldwide market for latent TB testing is categorized into North America, Europe, Asia Pacific, Latin America, and the Middle East & Africa. Notably, the Asia Pacific region is anticipated to experience the most substantial growth in the forecast period. The Asia Pacific region bears a high burden of tuberculosis, such as in India and China, which record a high incidence rate and thereby drive the need for essential latent TB screening and management. Growth in government and international organization funding and campaigns against TB has also led to better access to diagnostics and healthcare infrastructure in Asia Pacific. Awareness among the communities and among healthcare workers regarding the need for early detection of latent TB has also been significantly increasing the demand for further testing. Another major growth driver of this segment in the latent TB testing market is the increasing healthcare expenditure, along with supportive regulatory frameworks. All these factors propel increased growth of the latent TB testing market in the Asia Pacific region.
The break-up of the profile of primary participants in the latent TB testing market:
- By Company Type: Tier 1 - 40%, Tier 2 - 30%, and Tier 3 - 30%
- By Designation: C-level - 27%, D-level - 18%, and Others - 55%
- By Region: North America - 51%, Europe - 21%, Asia Pacific - 18%, Latin America - 6%, and Middle East & Africa- 4%
The key players in this market are QIAGEN (Netherlands), Revvity (US), Beijing Wantai Biopharmaceutical Co., Ltd. (China), Sanofi (France), Endo, Inc. (US), bioMerieux (France), SD Biosensor, INC. (South Korea), Lionex GmbH (Germany), Sanofi (France), Serum Institute of India Pvt. Ltd. (India), ARKRAY, Inc. (Japan), Zhi Fei Biological (China), AID Autoimmun Diagnostika GmbH (Germany), Boditech Med Inc. (South Korea), Bioneovan Co., Ltd (China), and Biopanda Reagents Ltd (UK).
Research Coverage:
This research report categorizes the latent TB testing market by test type (tuberculin skin test (TST) and interferon gamma release assay (IGRA)), by application (household contacts with pulmonary TB, people living with HIV, and other applications), by end user (diagnostic laboratories, hospitals & clinics, academic & research institutes, and other end users), and region (North America, Europe, Asia Pacific, Latin America, and the Middle East & Africa). The scope of the report covers detailed information regarding the major factors, such as drivers, restraints, opportunities, and challenges influencing the growth of the latent TB testing market. A detailed analysis of the key industry players has been done to provide insights into their business overview, solutions, key strategies, acquisitions, and agreements. New product & service launches, and recent developments associated with the latent TB testing market. Competitive analysis of upcoming startups in the latent TB testing market ecosystem is covered in this report.
Reasons to buy this report:
The report will help the market leaders/new entrants in this market with information on the closest approximations of the revenue numbers for the overall latent TB testing market and the subsegments. This report will help stakeholders understand the competitive landscape and gain more insights to position their businesses better and plan suitable go-to-market strategies. The report also helps stakeholders understand the pulse of the market and provides them with information on key market drivers, restraints, opportunities, and challenges.
The report provides insights on the following pointers:
- Analysis of key drivers (rising incidence of latent tuberculosis, widespread use of BCG vaccine, and increased funding and grants for TB control programs), opportunities (Growth opportunities in emerging economies), restraints (Unfavorable reimbursement scenario), and challenges (changing regulatory landscape and operational barriers and labor shortage) influencing the growth of the latent TB testing market.
- Product Development/Innovation: Detailed insights on upcoming technologies, research & development activities, and new product launches in the latent TB testing market.
- Market Development: Comprehensive information about lucrative markets - the report analyses the latent TB testing market across varied regions.
- Market Diversification: Exhaustive information about new products, untapped geographies, recent developments, and investments in the latent TB testing market.
- Competitive Assessment: In-depth assessment of market shares, growth strategies, product offerings of leading players like QIAGEN (Netherlands), Revvity (US), Beijing Wantai Biopharmaceutical Co., Ltd. (China), Sanofi (France), and Endo, Inc. (US).
TABLE OF CONTENTS
1 INTRODUCTION
- 1.1 STUDY OBJECTIVES
- 1.2 MARKET DEFINITION
- 1.3 STUDY SCOPE
- 1.3.1 MARKETS COVERED
- 1.3.2 INCLUSIONS & EXCLUSIONS
- 1.3.3 YEARS CONSIDERED
- 1.3.4 CURRENCY CONSIDERED
- 1.4 STAKEHOLDERS
2 RESEARCH METHODOLOGY
- 2.1 RESEARCH DATA
- 2.1.1 SECONDARY DATA
- 2.1.1.1 Key secondary sources
- 2.1.1.2 Key data from secondary sources
- 2.1.1.3 Objectives of secondary research
- 2.1.2 PRIMARY DATA
- 2.1.2.1 Key primary sources
- 2.1.2.2 Key data from primary sources
- 2.1.2.3 Key industry insights
- 2.1.2.4 Breakdown of primaries
- 2.1.2.4.1 Breakdown of primary interviews: supply & demand-side
- 2.1.2.4.2 Breakdown of primary interviews: by company type, designation, and region
- 2.1.1 SECONDARY DATA
- 2.2 MARKET SIZE ESTIMATION
- 2.2.1 BOTTOM-UP APPROACH
- 2.2.1.1 Company revenue estimation approach
- 2.2.1.2 Presentations of companies and primary interviews
- 2.2.1.3 Growth forecast
- 2.2.1.4 CAGR projections
- 2.2.2 TOP-DOWN APPROACH
- 2.2.1 BOTTOM-UP APPROACH
- 2.3 MARKET BREAKDOWN AND DATA TRIANGULATION
- 2.4 MARKET SHARE ANALYSIS
- 2.5 ASSUMPTIONS
- 2.5.1 STUDY ASSUMPTIONS
- 2.5.2 GROWTH RATE ASSUMPTIONS
- 2.6 LIMITATIONS
- 2.7 RISK ASSESSMENT
3 EXECUTIVE SUMMARY
4 PREMIUM INSIGHTS
- 4.1 LATENT TB TESTING MARKET OVERVIEW
- 4.2 LATENT TB TESTING MARKET, BY TEST TYPE, 2024 VS. 2029
- 4.3 LATENT TB TESTING MARKET, BY APPLICATION, 2024 VS. 2029
- 4.4 LATENT TB TESTING MARKET, BY END USER, 2024 VS. 2029
- 4.5 LATENT TB TESTING MARKET: GEOGRAPHIC GROWTH OPPORTUNITIES
5 MARKET OVERVIEW
- 5.1 INTRODUCTION
- 5.2 MARKET DYNAMICS
- 5.2.1 DRIVERS
- 5.2.1.1 Rising incidence of latent tuberculosis
- 5.2.1.2 Widespread use of BCG vaccine
- 5.2.1.3 Increased funding and grants for TB control programs
- 5.2.2 RESTRAINTS
- 5.2.2.1 Unfavorable reimbursement scenario
- 5.2.3 OPPORTUNITIES
- 5.2.3.1 Growth opportunities in emerging economies
- 5.2.4 CHALLENGES
- 5.2.4.1 Changing regulatory landscape
- 5.2.4.2 Operational barriers and labor shortage
- 5.2.1 DRIVERS
- 5.3 PRICING ANALYSIS
- 5.3.1 AVERAGE SELLING PRICE, BY PRODUCT
- 5.3.2 INDICATIVE PRICE OF IGRA TESTS, BY REGION
- 5.4 PATENT ANALYSIS
- 5.4.1 LIST OF MAJOR PATENTS
- 5.5 VALUE CHAIN ANALYSIS
- 5.6 SUPPLY CHAIN ANALYSIS
- 5.7 TRADE ANALYSIS
- 5.7.1 IMPORT DATA
- 5.7.2 EXPORT DATA
- 5.8 ECOSYSTEM ANALYSIS
- 5.8.1 ROLE IN ECOSYSTEM
- 5.9 PORTER'S FIVE FORCES ANALYSIS
- 5.9.1 THREAT OF NEW ENTRANTS
- 5.9.2 THREAT OF SUBSTITUTES
- 5.9.3 BARGAINING POWER OF BUYERS
- 5.9.4 BARGAINING POWER OF SUPPLIERS
- 5.9.5 INTENSITY OF COMPETITIVE RIVALRY
- 5.10 KEY STAKEHOLDERS AND BUYING CRITERIA
- 5.10.1 KEY STAKEHOLDERS IN BUYING PROCESS
- 5.10.2 KEY BUYING CRITERIA
- 5.11 REGULATORY ANALYSIS
- 5.11.1 REGULATORY LANDSCAPE
- 5.11.1.1 North America
- 5.11.1.1.1 US
- 5.11.1.1.2 Canada
- 5.11.1.2 Europe
- 5.11.1.2.1 Germany
- 5.11.1.2.2 UK
- 5.11.1.2.3 France
- 5.11.1.2.4 Italy
- 5.11.1.2.5 Spain
- 5.11.1.3 Asia Pacific
- 5.11.1.3.1 China
- 5.11.1.3.2 Japan
- 5.11.1.3.3 India
- 5.11.1.3.4 Australia
- 5.11.1.3.5 South Korea
- 5.11.1.3.6 Singapore
- 5.11.1.4 Latin America
- 5.11.1.4.1 Brazil
- 5.11.1.4.2 Mexico
- 5.11.1.5 Middle East
- 5.11.1.5.1 Africa
- 5.11.1.1 North America
- 5.11.2 REGULATORY BODIES, GOVERNMENT AGENCIES, AND OTHER ORGANIZATIONS
- 5.11.2.1 North America
- 5.11.2.2 Europe
- 5.11.2.3 Asia Pacific
- 5.11.2.4 Latin America
- 5.11.2.5 Rest of the world
- 5.11.1 REGULATORY LANDSCAPE
- 5.12 TECHNOLOGY ANALYSIS
- 5.12.1 KEY TECHNOLOGIES
- 5.12.1.1 Tuberculin skin test
- 5.12.2 COMPLEMENTARY TECHNOLOGIES
- 5.12.2.1 Interferon gamma released assay
- 5.12.1 KEY TECHNOLOGIES
- 5.13 KEY CONFERENCES AND EVENTS, 2024-2025
- 5.14 TRENDS/DISRUPTIONS IMPACTING CUSTOMER BUSINESS
- 5.15 INVESTMENT & FUNDING SCENARIO
- 5.16 CASE STUDY ANALYSIS
- 5.16.1 CASE STUDY 1: EXPLORING CYTOKINE USAGE IN MONITORING LTBI TREATMENT
- 5.16.2 CASE STUDY 2: ASSESSING DIAGNOSTIC ACCURACY OF ESAT6-CFP10 (EC) SKIN TEST FOR LTBI DETECTION
- 5.16.3 CASE STUDY 3: EVALUATING MTB-SPECIFIC CYTOKINE RESPONSES FOR ENHANCED LTBI DETECTION IN HEU INFANTS
- 5.17 LATENT TB CONTROL STRATEGIES IN MAJOR COUNTRIES
- 5.17.1 NORTH AMERICA
- 5.17.1.1 US
- 5.17.1.2 Canada
- 5.17.2 EUROPE
- 5.17.2.1 Germany
- 5.17.2.2 UK
- 5.17.3 ASIA PACIFIC
- 5.17.3.1 Japan
- 5.17.3.2 China
- 5.17.3.3 India
- 5.17.3.4 Singapore
- 5.17.1 NORTH AMERICA
- 5.18 IMPACT OF AI ON LATENT TB TESTING MARKET
- 5.18.1 KEY USE CASES
6 LATENT TB TESTING MARKET, BY TEST TYPE
- 6.1 INTRODUCTION
- 6.2 IGRA
- 6.2.1 HIGHER SPECIFICITY AND ACCURACY TO BOOST MARKET SEGMENT
- 6.3 TST
- 6.3.1 COST-EFFECTIVENESS TO PROPEL MARKET GROWTH
7 LATENT TB TESTING MARKET, BY APPLICATION
- 7.1 INTRODUCTION
- 7.2 PEOPLE LIVING WITH HIV
- 7.2.1 RISING RATES OF TB-HIV COINFECTION TO PROPEL GROWTH
- 7.3 HOUSEHOLD CONTACTS (HHC) WITH PULMONARY TUBERCULOSIS (TB)/ HOUSEHOLD CONTACTS (HHC) OF TUBERCULOSIS (TB) PATIENTS
- 7.3.1 NEED FOR ROUTINE SCREENING FOR HOUSEHOLD CONTACTS TO BOOST SEGMENT
- 7.4 OTHER APPLICATIONS
8 LATENT TB TESTING MARKET, BY END USER
- 8.1 INTRODUCTION
- 8.2 DIAGNOSTIC LABORATORIES
- 8.2.1 WELL-DEVELOPED INFRASTRUCTURE AND CAPABILITIES TO SUPPORT DEMAND FOR TESTING METHODS
- 8.3 HOSPITALS & CLINICS
- 8.3.1 GROWING NUMBER OF HOSPITALS AND GOVERNMENT INVESTMENTS IN HEALTHCARE TO BOOST SEGMENT
- 8.4 ACADEMIC & RESEARCH INSTITUTES
- 8.4.1 RISING INDUSTRY-ACADEMIA COLLABORATIONS TO SUPPORT MARKET GROWTH
- 8.5 OTHER END USERS
9 LATENT TB TESTING MARKET, BY REGION
- 9.1 INTRODUCTION
- 9.2 NORTH AMERICA
- 9.2.1 NORTH AMERICA: MACROECONOMIC OUTLOOK
- 9.2.2 US
- 9.2.2.1 US to dominate North American latent TB testing market
- 9.2.3 CANADA
- 9.2.3.1 Improved screening and treatment for latent TB to drive market
- 9.3 ASIA PACIFIC
- 9.3.1 ASIA PACIFIC: MACROECONOMIC OUTLOOK
- 9.3.2 CHINA
- 9.3.2.1 Growing public access to modern healthcare to fuel market growth
- 9.3.3 JAPAN
- 9.3.3.1 Presence of universal healthcare reimbursement policy to drive market
- 9.3.4 INDIA
- 9.3.4.1 High TB burden and growing government initiatives to drive demand for latent TB screening
- 9.3.5 SOUTH KOREA
- 9.3.5.1 Rising healthcare spending in innovative diagnostic technologies to support market growth
- 9.3.6 AUSTRALIA
- 9.3.6.1 Diverse immigrant population to fuel demand for latent TB screening
- 9.3.7 SINGAPORE
- 9.3.7.1 Enhanced healthcare infrastructure to propel market growth
- 9.3.8 REST OF ASIA PACIFIC
- 9.4 EUROPE
- 9.4.1 EUROPE: MACROECONOMIC OUTLOOK
- 9.4.2 UK
- 9.4.2.1 Growing number of diagnostic centers to fuel market
- 9.4.3 FRANCE
- 9.4.3.1 Presence of robust healthcare system to augment market growth
- 9.4.4 GERMANY
- 9.4.4.1 Higher healthcare spending and favorable government policies to favor market growth
- 9.4.5 SPAIN
- 9.4.5.1 Improving healthcare infrastructure to drive demand
- 9.4.6 ITALY
- 9.4.6.1 Improved quality of medical care to spur market growth
- 9.4.7 BELGIUM
- 9.4.7.1 Targeted screening for high-risk groups to fuel demand
- 9.4.8 SWEDEN
- 9.4.8.1 Growing immigrant population from high-prevalence regions to propel demand
- 9.4.9 DENMARK
- 9.4.9.1 Importance of latent TB testing in public health strategy to support market growth
- 9.4.10 REST OF EUROPE
- 9.5 LATIN AMERICA
- 9.5.1 LATIN AMERICA: MACROECONOMIC OUTLOOK
- 9.5.2 BRAZIL
- 9.5.2.1 Developed public health systems and improving healthcare infrastructure to favor market growth
- 9.5.3 MEXICO
- 9.5.3.1 Modernization of healthcare infrastructure to augment market growth
- 9.5.4 REST OF LATIN AMERICA
- 9.6 MIDDLE EAST & AFRICA
- 9.6.1 MIDDLE EAST & AFRICA: MACROECONOMIC OUTLOOK
- 9.6.2 SOUTH AFRICA
- 9.6.2.1 Rising government initiatives for TB screening to augment market growth
- 9.6.3 SAUDI ARABIA
- 9.6.3.1 Enhancements in healthcare infrastructure to support market growth
- 9.6.4 UAE
- 9.6.4.1 UAE ranks among the world's leading countries with low TB rates
- 9.6.5 KUWAIT
- 9.6.5.1 Expansion of healthcare infrastructure to augment market growth
- 9.6.6 REST OF MIDDLE EAST & AFRICA
10 COMPETITIVE LANDSCAPE
- 10.1 INTRODUCTION
- 10.2 KEY PLAYER STRATEGIES/RIGHT TO WIN
- 10.2.1 OVERVIEW OF STRATEGIES ADOPTED BY PLAYERS IN LATENT TB TESTING MARKET
- 10.3 REVENUE SHARE ANALYSIS
- 10.4 MARKET SHARE ANALYSIS
- 10.4.1 MARKET SHARE ANALYSIS, BY REGION
- 10.4.1.1 North America
- 10.4.1.2 Europe
- 10.4.1.3 Asia Pacific
- 10.4.1 MARKET SHARE ANALYSIS, BY REGION
- 10.5 COMPANY EVALUATION MATRIX: KEY PLAYERS, 2023
- 10.5.1 STARS
- 10.5.2 EMERGING LEADERS
- 10.5.3 PERVASIVE PLAYERS
- 10.5.4 PARTICIPANTS
- 10.5.5 COMPANY FOOTPRINT: KEY PLAYERS, 2023
- 10.5.5.1 Company footprint
- 10.5.5.2 Test type footprint
- 10.5.5.3 Application footprint
- 10.5.5.4 Region footprint
- 10.6 COMPANY EVALUATION MATRIX: STARTUPS/SMES, 2023
- 10.6.1 PROGRESSIVE COMPANIES
- 10.6.2 RESPONSIVE COMPANIES
- 10.6.3 DYNAMIC COMPANIES
- 10.6.4 STARTING BLOCKS
- 10.7 COMPANY EVALUATION MATRIX: STARTUPS/SMES, 2023
- 10.7.1 COMPETITIVE BENCHMARKING OF STARTUP/SME PLAYERS, 2023
- 10.8 VALUATION & FINANCIAL METRICS
- 10.9 BRAND/PRODUCT COMPARATIVE ANALYSIS
- 10.9.1 QIAGEN
- 10.9.2 REVVITY
- 10.9.3 BIOMERIEUX
- 10.10 COMPETITIVE SCENARIO
- 10.10.1 PRODUCT LAUNCHES
- 10.10.2 DEALS
11 COMPANY PROFILES
- 11.1 KEY PLAYERS
- 11.1.1 QIAGEN
- 11.1.1.1 Business overview
- 11.1.1.2 Products offered
- 11.1.1.3 Recent developments
- 11.1.1.3.1 Product launches & approvals
- 11.1.1.3.2 Deals
- 11.1.1.3.3 Expansions
- 11.1.1.4 MnM view
- 11.1.1.4.1 Right to win
- 11.1.1.4.2 Strategic choices
- 11.1.1.4.3 Weaknesses & competitive threats
- 11.1.2 REVVITY (OXFORD IMMUNOTEC)
- 11.1.2.1 Business overview
- 11.1.2.2 Products offered
- 11.1.2.3 Recent developments
- 11.1.2.3.1 Product launches & approvals
- 11.1.2.4 MnM view
- 11.1.2.4.1 Right to win
- 11.1.2.4.2 Strategic choices
- 11.1.2.4.3 Weaknesses & competitive threats
- 11.1.3 BEIJING WANTAI BIOPHARMACEUTICAL CO., LTD.
- 11.1.3.1 Business overview
- 11.1.3.2 Products offered
- 11.1.3.3 MnM view
- 11.1.3.3.1 Right to win
- 11.1.3.3.2 Strategic choices
- 11.1.3.3.3 Weaknesses & competitive threats
- 11.1.4 SANOFI
- 11.1.4.1 Business overview
- 11.1.4.2 MnM view
- 11.1.4.2.1 Right to win
- 11.1.4.2.2 Strategic choices
- 11.1.4.2.3 Weaknesses & competitive threats
- 11.1.5 ENDO, INC.
- 11.1.5.1 Business overview
- 11.1.5.2 Products offered
- 11.1.5.3 MnM view
- 11.1.5.3.1 Key strengths
- 11.1.5.3.2 Strategic choices
- 11.1.5.3.3 Weaknesses and competitive threats
- 11.1.6 BIOMERIEUX
- 11.1.6.1 Business overview
- 11.1.6.2 Products offered
- 11.1.6.3 Recent developments
- 11.1.6.3.1 Product approvals
- 11.1.7 SD BIOSENSOR, INC.
- 11.1.7.1 Business overview
- 11.1.7.2 Products offered
- 11.1.7.3 Recent developments
- 11.1.7.3.1 Deals
- 11.1.8 LIONEX GMBH
- 11.1.8.1 Business overview
- 11.1.8.2 Products offered
- 11.1.9 SERUM INSTITUTE OF INDIA PVT. LTD.
- 11.1.9.1 Business overview
- 11.1.9.2 Products offered
- 11.1.10 ARKRAY, INC.
- 11.1.10.1 Business overview
- 11.1.10.2 Products offered
- 11.1.10.3 Recent developments
- 11.1.10.3.1 Expansions
- 11.1.1 QIAGEN
- 11.2 OTHER PLAYERS
- 11.2.1 ZHI FEI BIOLOGICAL
- 11.2.2 AID AUTOIMMUN DIAGNOSTIKA GMBH
- 11.2.3 BODITECH MED INC.
- 11.2.4 BIONEOVAN CO., LTD.
- 11.2.5 BIOPANDA REAGENTS LTD.
12 APPENDIX
- 12.1 DISCUSSION GUIDE
- 12.2 KNOWLEDGESTORE: MARKETSANDMARKETS' SUBSCRIPTION PORTAL
- 12.3 CUSTOMIZATION OPTIONS
- 12.4 RELATED REPORTS
- 12.5 AUTHOR DETAILS