|
|
市場調査レポート
商品コード
1419620
脊椎インプラントの世界市場:製品別(デバイス、生物製剤、刺激装置)、用途別、手術タイプ別、地域別、顧客のアンメットニーズ-2028年までの予測Spinal Implants Market by Product (Devices, Biologics, Stimulators), Application, Surgery, Region & Customer Unmet Need Forecast to 2028 |
||||||
カスタマイズ可能
|
|||||||
| 脊椎インプラントの世界市場:製品別(デバイス、生物製剤、刺激装置)、用途別、手術タイプ別、地域別、顧客のアンメットニーズ-2028年までの予測 |
|
出版日: 2024年01月25日
発行: MarketsandMarkets
ページ情報: 英文 322 Pages
納期: 即納可能
|
全表示
- 概要
- 目次
| 調査範囲 | |
|---|---|
| 調査対象年 | 2020年~2028年 |
| 基準年 | 2022年 |
| 予測期間 | 2023年~2028年 |
| 単位 | 金額(10億米ドル) |
| セグメント | 製品別、手術タイプ別、用途別、エンドユーザー別、地域別 |
| 対象地域 | 北米、欧州、アジア太平洋、ラテンアメリカ、中東・アフリカ |
世界の脊椎インプラントの市場規模は、2023年の112億米ドルから2028年には143億米ドルに達し、予測期間中にCAGR 5.0%で成長すると予測されています。
脊椎インプラント市場の急成長が予測される背景には、魅力的な利点が数多くあります。特に、脊椎インプラントは、人口の高齢化、脊椎疾患の調査件数の増加、脊椎手術技術の進歩のための公的/民間組織による研究・資金提供イニシアチブの増加によって牽引されています。さらに、早期診断と早期治療の有効性が向上したことで、脊椎疾患の早期発見が増加しており、脊椎インプラントが最前線のソリューションとして位置づけられる先進的な治療方法に対する需要が高まっています。これらの要因によって、市場は堅調な拡大局面を迎えることになり、その結果、今後予測される数年間における脊椎インプラントの採用状況が形成されることになります。
手術タイプ別では、2022年~2028年の予測期間では、開腹手術分野が最大の市場シェアを記録しました。このセグメントの成長は、重度の脊柱側弯症や進行した後弯症のような複雑な脊柱変形を伴う症例によるもので、開腹手術が頻繁に好まれます。開腹手術特有の直接的な可視化により、外科医は複雑な解剖学的異常に効果的に対処する能力を高めることができます。さらに、外科医が開腹手技に慣れ親しんでいることや専門知識が、手技の選択に影響することもあります。場合によっては、特に伝統的な手法の訓練を受けた外科医にとっては、開腹手術が好まれることもあり、このセグメントの進歩にさらに拍車をかけています。
エンドユーザー別では、2022年には、病院セグメントが世界の脊椎インプラント市場で最大のシェアを占めました。このセグメントの成長は、日常的な処置から複雑な介入に至るまで脊椎手術の大部分を占めること、高度な脊椎インプラント技術の早期導入、臨床実践による動向の形成などの要因によって推進されています。
アジア太平洋市場は、ヘルスケアインフラの強化を目的とした新興国の政府機関による投資の増加により成長を遂げています。その他の特典として、独立系整形外科センターの増加、患者数の増加、アジア太平洋における脊椎インプラント製品の提供を拡大する業界各社の戦略的イニシアチブがあります。
当レポートでは、世界の脊椎インプラント市場について調査し、製品別、手術タイプ別、用途別、エンドユーザー別、地域別動向、および市場に参入する企業のプロファイルなどをまとめています。
目次
第1章 イントロダクション
第2章 調査手法
第3章 エグゼクティブサマリー
第4章 重要考察
第5章 市場概要
- イントロダクション
- ポーターのファイブフォース分析
- 規制分析
- 償還シナリオ
- 生態系/市場マップ
- バリューチェーン分析
- サプライチェーン分析
- 価格分析
- 特許分析
- 貿易分析
- 2023年~2024年の主要な会議とイベント
- 顧客のビジネスに影響を与える動向/混乱
- 主要な利害関係者と購入基準
- 脊椎インプラント市場:不況の影響
- アンメットニーズと主要な問題点
第6章 脊椎インプラント市場、製品別
- イントロダクション
- 胸部および腰部固定装置
- 頸椎固定装置
- 脊椎用生物製剤
- VCF治療装置
- 脊椎減圧装置
- 非融合デバイス
- 脊椎骨刺激装置
第7章 脊椎インプラント市場、手術タイプ別
- イントロダクション
- 開腹手術
- 低侵襲手術
第8章 脊椎インプラント市場、用途別
- イントロダクション
- 脊椎の固定
- VCF手技
- 可動性温存/非融合
- 脊椎減圧症
第9章 脊椎インプラント市場、エンドユーザー別
- イントロダクション
- 病院
- 整形外科センター
第10章 脊椎インプラント市場、地域別
- イントロダクション
- 北米
- 欧州
- アジア太平洋
- ラテンアメリカ
- 中東・アフリカ
第11章 競合情勢
- 概要
- 主要参入企業の戦略/強み
- 収益シェア分析
- 市場シェア分析
- 企業評価マトリックス
- スタートアップ/中小企業の評価マトリックス
- 競合シナリオと動向
第12章 企業プロファイル
- 主要参入企業
- MEDTRONIC PLC
- JOHNSON & JOHNSON MEDTECH
- NUVASIVE, INC.
- STRYKER CORPORATION
- GLOBUS MEDICAL, INC.
- ZIMMER BIOMET HOLDINGS, INC.
- ORTHOFIX INTERNATIONAL N.V.
- ULRICH GMBH & CO. KG
- B. BRAUN MELSUNGEN AG
- INTEGRA LIFESCIENCES HOLDINGS CORPORATION
- BOSTON SCIENTIFIC CORPORATION
- ABBOTT LABORATORIES
- SPINEART
- CENTINEL SPINE LLC
- PREMIA SPINE
- EXACTECH, INC.
- LIFE SPINE, INC.
- ATEC SPINE
- その他の企業
- KUROS BIOSCIENCES LTD.
- BIOVENTUS LLC
- COLFAX CORPORATION
- AESCULAP INC.
- IMPLANET
- CAMBER SPINE TECHNOLOGIES
- CAPTIVA SPINE, INC.
- MEDACTA INTERNATIONAL
- AURORA SPINE CORPORATION
第13章 付録
Report Description
| Scope of the Report | |
|---|---|
| Years Considered for the Study | 2020-2028 |
| Base Year | 2022 |
| Forecast Period | 2023-2028 |
| Units Considered | Value (USD) Billion |
| Segments | Product, type of surgery, Application, End User, and Region |
| Regions covered | North America, Europe, Asia Pacific, Latin America and Middle East & Africa |
The global spinal implants market is projected to reach USD 14.3 billion by 2028 from USD 11.2 billion in 2023, growing at a CAGR of 5.0% during the forecast period. The projected surge in market growth for spinal implants is underpinned by a confluence of compelling advantages. Notably, spinal implants are driven by an aging population, rising cases of spinal conditions, and an increase in research & funding initiatives from public/private organizations for the advancement in spine surgical technologies. Furthermore, the escalating early detection of spinal disorders with improved efficacy for early diagnosis and treatment amplifies the demand for advanced treatment modalities, with spinal implants positioned as a forefront solution. These factors are poised to usher in a phase of robust expansion within the market, consequently shaping the landscape of spinal implant adoption in the forthcoming forecasting years.
"Open surgeries segment to register largest market share in 2022-2028."
Based on the type of surgery, the spinal implant market is segmented into Open surgeries and minimally invasive surgeries. The open surgeries segment registered the largest market share over the forecast period of 2022-2028. The growth of this segment is due to the cases involving intricate spinal deformities like severe scoliosis or advanced kyphosis; open surgeries are frequently the preferred approach. The direct visualization inherent in open procedures enhances surgeons' ability to effectively address complex anatomical abnormalities. Moreover, surgeons' familiarity and expertise with open techniques can influence the choice of procedure. In some cases, particularly for surgeons trained in traditional methods, open surgeries may be preferred., further fueling the segment's advancement.
"Hospitals segment held the largest share of spinal implants market in 2022, by End-user."
Based on the end-user, the spinal implants market is segmented into Hospitals and orthopedic centers. In 2022, the hospitals segment accounted for the largest share of the global spinal implants market. The growth of this segment is being propelled by factors including a significant portion of spinal surgeries, ranging from routine procedures to complex interventions, early adoption of advanced spinal implant technologies, and shaping trends through their clinical practices.
"Asia Pacific to register significant growth rate in the market during the forecast period."
For the forecasting period 2023-2028, the APAC region is expected to register a significant growth rate in the market during the forecast period. Asia Pacific comprises India, China, Japan, Australia, South Korea, and RoAPAC. The Asia-Pacific (APAC) region has witnessed a significant increase in the market growth rate for spinal implants. There are several drivers that contributed to this growth:
The Asia Pacific market is experiencing growth due to increased investments by government agencies in emerging countries aimed at enhancing healthcare infrastructure. Additionally, the market is benefiting from the rise in independent orthopedic centers, a substantial patient population, and strategic initiatives by industry players to expand their offerings of spinal implant products in the Asia Pacific region.
A breakdown of the primary participants referred to for this report is provided below:
- By Company Type: Tier 1-45%, Tier 2-34%, and Tier 3- 21%
- By Designation: C-level-47%, Director-level-33%, and Others-20%
- By Region: North America-35%, Europe-32%, Asia Pacific-25%, Latin America-6%, and the Middle East & Africa-2%
Prominent players in this market are Medtronic (Ireland), Johnson & Johnson- Depuy Synthes (US), Stryker Corporation (US), NuVasive (US), among others.
Research Coverage
- The report studies the spinal implants market based on products, type of surgery, application, end user, and region.
- The report analyzes factors (such as drivers, restraints, opportunities, and challenges) affecting the market growth.
- The report evaluates the opportunities and challenges in the market for stakeholders and provides details of the competitive landscape for market leaders.
- The report studies micro markets with respect to their growth trends, prospects, and contributions to the global spinal implants market
- The report forecasts the revenue of market segments with respect to five major regions.
Key Benefits of Buying the Report:
The report will help the new entrants/ market leaders/smaller firms in this market with investment evaluation viability within the spinal implants market through a thorough analysis of comprehensive data, thereby facilitating robust risk assessment and enabling well-informed investment determinations. Benefit from meticulous market segmentation encompassing application, end-user, and regional dimensions, affording tailored insights for precise segment targeting. The report also provides an all-encompassing evaluation of encapsulating pivotal trends, challenges, growth catalysts and prospects, thereby empowering strategic decision-making with astute discernment.
The report provides insights on the following pointers:
- Analysis of key drivers (increasing prevalence of spinal disorder diseases, Increasing geriatric population worldwide, Ongoing advancements in spine surgery technologies), restraints (high cost of treatment procedures and uncertainty in reimbursement structure), opportunities (Growth in the number of hospitals and surgical centers, Emerging markets offers high-growth opportunities), and challenges (lack of adequate physicians and limited awareness) influencing the growth of the spinal implants market
- Product Development/Innovation: Detailed insights on upcoming technologies, research & development activities, and new product & service launches in the spinal implants market.
- Market Development: Comprehensive information about lucrative markets - the report analyses the spinal implants market across varied regions.
- Market Diversification: Exhaustive information about new products, untapped geographies, recent developments, and investments in the spinal implants market
- Competitive Assessment: In-depth assessment of market shares, growth strategies, and service offerings of leading players like Medtronic (Ireland), Johnson & Johnson- Depuy Synthes (US), Stryker Corporation (US), NuVasive (US), among others.
TABLE OF CONTENTS
1 INTRODUCTION
- 1.1 STUDY OBJECTIVES
- 1.2 MARKET DEFINITION
- 1.2.1 INCLUSIONS AND EXCLUSIONS
- 1.3 MARKET SCOPE
- 1.3.1 MARKETS COVERED
- 1.3.2 REGIONS COVERED
- 1.3.3 YEARS CONSIDERED
- 1.4 CURRENCY CONSIDERED
- 1.5 MARKET STAKEHOLDERS
- 1.6 SUMMARY OF CHANGES
- 1.6.1 RECESSION IMPACT
2 RESEARCH METHODOLOGY
- 2.1 RESEARCH DATA
- 2.2 RESEARCH DESIGN
- 2.2.1 SECONDARY RESEARCH
- 2.2.1.1 Key data from secondary sources
- 2.2.2 PRIMARY RESEARCH
- 2.2.2.1 Primary sources
- 2.2.2.2 Key industry insights
- 2.2.2.3 Breakdown of primaries
- FIGURE 1 BREAKDOWN OF PRIMARY INTERVIEWS: SUPPLY AND DEMAND-SIDE PARTICIPANTS
- FIGURE 2 BREAKDOWN OF PRIMARY INTERVIEWS: BY COMPANY TYPE, DESIGNATION, AND REGION
- 2.2.1 SECONDARY RESEARCH
- 2.3 MARKET SIZE ESTIMATION
- FIGURE 3 RESEARCH METHODOLOGY: HYPOTHESIS BUILDING
- 2.3.1 BOTTOM-UP APPROACH
- 2.3.1.1 Approach 1: Company revenue estimation approach
- FIGURE 4 SPINAL IMPLANTS MARKET SIZE ESTIMATION: APPROACH 1 (COMPANY REVENUE ESTIMATION)
- 2.3.1.2 Approach 2: Customer-based market estimation
- FIGURE 5 SPINAL IMPLANTS MARKET SIZE ESTIMATION: BOTTOM-UP APPROACH
- 2.3.1.3 Approach 3: Top-down approach
- 2.3.1.4 Approach 4: Primary interviews
- FIGURE 6 TOP-DOWN APPROACH
- 2.4 DATA TRIANGULATION AND MARKET BREAKDOWN
- FIGURE 7 DATA TRIANGULATION METHODOLOGY
- 2.5 MARKET SHARE ASSESSMENT
- 2.6 STUDY ASSUMPTIONS
- 2.7 RISK ASSESSMENT
- TABLE 1 ASSOCIATED RISKS
- 2.8 RESEARCH LIMITATIONS
- 2.9 GROWTH RATE ASSUMPTIONS
- 2.10 RECESSION IMPACT
3 EXECUTIVE SUMMARY
- FIGURE 8 SPINAL IMPLANTS MARKET, BY TYPE OF SURGERY, 2023 VS. 2028 (USD MILLION)
- FIGURE 9 SPINAL IMPLANTS MARKET, BY PRODUCT, 2023 VS. 2028 (USD MILLION)
- FIGURE 10 SPINAL IMPLANTS MARKET, BY APPLICATION, 2023 VS. 2028 (USD MILLION)
- FIGURE 11 SPINAL IMPLANTS MARKET, BY END USER, 2023 VS. 2028 (USD MILLION)
- FIGURE 12 SPINAL IMPLANTS MARKET: GEOGRAPHICAL SNAPSHOT
4 PREMIUM INSIGHTS
- 4.1 SPINAL IMPLANTS MARKET OVERVIEW
- FIGURE 13 INCREASING INCIDENCE OF SPINAL DISORDERS TO DRIVE MARKET
- 4.2 SPINAL IMPLANTS MARKET: REGIONAL LANDSCAPE, 2023 VS. 2028 (USD MILLION)
- FIGURE 14 NORTH AMERICA TO RETAIN LARGEST MARKET SHARE
- 4.3 NORTH AMERICA: SPINAL IMPLANTS MARKET, BY COUNTRY AND END USER, 2022 (USD MILLION)
- FIGURE 15 US AND HOSPITALS HELD LARGEST MARKET SHARES IN 2022
- 4.4 SPINAL IMPLANTS MARKET: GEOGRAPHIC GROWTH OPPORTUNITIES
- FIGURE 16 CHINA TO REGISTER HIGHEST CAGR OVER FORECAST PERIOD
5 MARKET OVERVIEW
- 5.1 INTRODUCTION
- FIGURE 17 SPINAL IMPLANTS MARKET: DRIVERS, RESTRAINTS, OPPORTUNITIES, AND CHALLENGES
- 5.1.1 DRIVERS
- 5.1.1.1 Innovation in spine surgery technologies
- 5.1.1.2 Increasing incidence of spinal disorders
- 5.1.1.3 Launch of advanced bone graft products
- 5.1.1.4 Increasing demand for MIS procedures
- 5.1.1.5 Growing funding and support for research
- TABLE 2 RECENT FUNDING FOR R&D IN SPINAL IMPLANTS
- 5.1.2 RESTRAINTS
- 5.1.2.1 High procedural costs
- 5.1.2.2 Unfavorable regulatory processes and limited reimbursement
- 5.1.3 OPPORTUNITIES
- 5.1.3.1 Rising number of hospitals and surgical centers
- 5.1.3.2 Emerging markets
- 5.1.4 CHALLENGES
- 5.1.4.1 Lack of personnel and limited awareness & education for MIS
- 5.2 PORTER'S FIVE FORCES ANALYSIS
- TABLE 3 SPINAL IMPLANTS MARKET: PORTER'S FIVE FORCES ANALYSIS
- 5.2.1 THREAT OF NEW ENTRANTS
- 5.2.2 THREAT OF SUBSTITUTES
- 5.2.3 BARGAINING POWER OF SUPPLIERS
- 5.2.4 BARGAINING POWER OF BUYERS
- 5.2.5 INTENSITY OF COMPETITIVE RIVALRY
- 5.3 REGULATORY ANALYSIS
- 5.3.1 REGULATORY BODIES, GOVERNMENT AGENCIES, AND OTHER ORGANIZATIONS
- TABLE 4 NORTH AMERICA: REGULATORY BODIES, GOVERNMENT AGENCIES, AND OTHER ORGANIZATIONS
- TABLE 5 EUROPE: REGULATORY BODIES, GOVERNMENT AGENCIES, AND OTHER ORGANIZATIONS
- TABLE 6 ASIA PACIFIC: REGULATORY BODIES, GOVERNMENT AGENCIES, AND OTHER ORGANIZATIONS
- TABLE 7 LATIN AMERICA: REGULATORY BODIES, GOVERNMENT AGENCIES, AND OTHER ORGANIZATIONS
- TABLE 8 MIDDLE EAST & AFRICA: REGULATORY BODIES, GOVERNMENT AGENCIES, AND OTHER ORGANIZATIONS
- 5.3.1.1 Key regulatory guidelines
- 5.3.1.1.1 US
- 5.3.1.1 Key regulatory guidelines
- TABLE 9 US FDA: MEDICAL DEVICE REGULATORY APPROVAL PROCESS
- 5.3.1.1.2 Europe
- 5.3.1.1.3 Japan
- 5.4 REIMBURSEMENT SCENARIO
- TABLE 10 REIMBURSEMENT CODES FOR VARIOUS PROCEDURES
- 5.5 ECOSYSTEM/MARKET MAP
- 5.6 VALUE CHAIN ANALYSIS
- 5.6.1 RESEARCH & DEVELOPMENT
- 5.6.2 PROCUREMENT AND PRODUCT DEVELOPMENT
- 5.6.3 MARKETING, SALES AND DISTRIBUTION, AND POST-SALES SERVICES
- FIGURE 18 SPINAL IMPLANTS MARKET: VALUE CHAIN ANALYSIS
- 5.7 SUPPLY CHAIN ANALYSIS
- 5.7.1 PROMINENT COMPANIES
- 5.7.2 SMALL & MEDIUM-SIZED COMPANIES
- 5.7.3 END USERS
- FIGURE 19 SPINAL IMPLANTS MARKET: SUPPLY CHAIN ANALYSIS
- 5.8 PRICING ANALYSIS
- FIGURE 20 AVERAGE SELLING PRICE OF SPINAL IMPLANTS, BY PRODUCT (FY 2021-2023)
- FIGURE 21 AVERAGE SELLING PRICE TREND OF SPINAL IMPLANTS (2021-2023), BY REGION (USD)
- 5.9 PATENT ANALYSIS
- FIGURE 22 PATENT DETAILS FOR SPINAL IMPLANTS (JANUARY 2013-DECEMBER 2023)
- 5.10 TRADE ANALYSIS
- TABLE 11 IMPORT DATA FOR SPINAL IMPLANTS (HS CODE 9021), BY COUNTRY, 2018-2022 (USD THOUSAND)
- TABLE 12 EXPORT DATA FOR SPINAL IMPLANTS (HS CODE 3822), BY COUNTRY, 2018-2022 (USD THOUSAND)
- 5.11 KEY CONFERENCES AND EVENTS, 2023-2024
- TABLE 13 SPINAL IMPLANTS MARKET: DETAILED LIST OF KEY CONFERENCES AND EVENTS IN 2023-2024
- 5.12 TRENDS/DISRUPTIONS IMPACTING CUSTOMERS' BUSINESSES
- FIGURE 23 EMERGING TRENDS AND OPPORTUNITIES AFFECTING FUTURE REVENUE MIX
- 5.13 KEY STAKEHOLDERS AND BUYING CRITERIA
- 5.13.1 KEY STAKEHOLDERS IN BUYING PROCESS
- TABLE 14 KEY REQUIREMENTS OF STAKEHOLDERS WHILE BUYING SPINAL IMPLANTS (%)
- 5.13.2 KEY STAKEHOLDERS BUYING CRITERIA
- FIGURE 24 INFLUENCE OF STAKEHOLDERS ON BUYING PROCESS OF SPINAL IMPLANTS
- TABLE 15 INFLUENCE OF STAKEHOLDERS ON BUYING PROCESS FOR KEY PRODUCT SEGMENTS (%)
- 5.14 SPINAL IMPLANTS MARKET: RECESSION IMPACT
- 5.15 UNMET NEEDS AND KEY PAIN POINTS
- TABLE 16 SPINAL IMPLANTS MARKET: UNMET CUSTOMER NEEDS AND KEY PAIN POINTS
6 SPINAL IMPLANTS MARKET, BY PRODUCT
- 6.1 INTRODUCTION
- TABLE 17 SPINAL IMPLANTS MARKET, BY PRODUCT, 2021-2028 (USD MILLION)
- 6.2 THORACIC & LUMBAR FUSION DEVICES
- TABLE 18 THORACIC & LUMBAR FUSION DEVICES MARKET, BY TYPE, 2021-2028 (USD MILLION)
- TABLE 19 THORACIC & LUMBAR FUSION DEVICES MARKET, BY REGION, 2021-2028 (USD MILLION)
- 6.2.1 THORACIC & LUMBAR FUSION DEVICES MARKET, BY APPLICATION
- TABLE 20 THORACIC & LUMBAR FUSION DEVICES MARKET, BY APPLICATION, 2021-2028 (USD MILLION)
- 6.2.2 THORACIC & LUMBAR FUSION DEVICES MARKET, BY TYPE OF SURGERY
- TABLE 21 THORACIC & LUMBAR FUSION DEVICES MARKET, BY TYPE OF SURGERY, 2021-2028 (USD MILLION)
- 6.2.3 POSTERIOR THORACIC & LUMBAR FUSION DEVICES
- 6.2.3.1 Posterior fusion devices to account for largest market share
- TABLE 22 POSTERIOR THORACIC & LUMBAR FUSION DEVICES MARKET, BY REGION, 2021-2028 (USD MILLION)
- 6.2.4 INTERBODY THORACIC & LUMBAR FUSION DEVICES
- TABLE 23 INTERBODY THORACIC & LUMBAR FUSION DEVICES MARKET, BY REGION, 2021-2028 (USD MILLION)
- 6.2.4.1 Interbody thoracic & lumbar fusion devices market, by approach
- TABLE 24 INTERBODY THORACIC & LUMBAR FUSION DEVICES MARKET, BY APPROACH, 2021-2028 (USD MILLION)
- 6.2.4.1.1 Anterior lumbar interbody fusion devices
- TABLE 25 ANTERIOR LUMBAR INTERBODY FUSION DEVICES MARKET, BY REGION, 2021-2028 (USD MILLION)
- 6.2.4.1.2 Posterior lumbar interbody fusion devices
- TABLE 26 POSTERIOR LUMBAR INTERBODY FUSION DEVICES MARKET, BY REGION, 2021-2028 (USD MILLION)
- 6.2.4.1.3 Transforaminal lumbar interbody fusion devices
- TABLE 27 TRANSFORAMINAL LUMBAR INTERBODY FUSION DEVICES MARKET, BY REGION, 2021-2028 (USD MILLION)
- 6.2.4.1.4 Axial lumbar interbody fusion devices
- TABLE 28 AXIAL LUMBAR INTERBODY FUSION DEVICES MARKET, BY REGION, 2021-2028 (USD MILLION)
- 6.2.4.2 Interbody thoracic & lumbar fusion devices market, by material
- TABLE 29 INTERBODY THORACIC & LUMBAR FUSION DEVICES MARKET, BY MATERIAL, 2021-2028 (USD MILLION)
- 6.2.4.2.1 Non-bone interbody fusion devices
- TABLE 30 NON-BONE INTERBODY FUSION DEVICES MARKET, BY REGION, 2021-2028 (USD MILLION)
- 6.2.4.2.2 Bone interbody fusion devices
- TABLE 31 BONE INTERBODY FUSION DEVICES MARKET, BY REGION, 2021-2028 (USD MILLION)
- 6.2.5 ANTERIOR THORACIC & LUMBAR FUSION DEVICES
- 6.2.5.1 Minimized impact, low chance of blood loss and infection to support demand
- TABLE 32 ANTERIOR THORACIC & LUMBAR FUSION DEVICES MARKET, BY REGION, 2021-2028 (USD MILLION)
- 6.3 CERVICAL FUSION DEVICES
- TABLE 33 CERVICAL FUSION DEVICES MARKET, BY TYPE, 2021-2028 (USD MILLION)
- TABLE 34 CERVICAL FUSION DEVICES MARKET, BY REGION, 2021-2028 (USD MILLION)
- 6.3.1 CERVICAL FUSION DEVICES MARKET, BY APPLICATION
- TABLE 35 CERVICAL FUSION DEVICES MARKET, BY APPLICATION, 2021-2028 (USD MILLION)
- 6.3.2 CERVICAL FUSION DEVICES MARKET, BY TYPE OF SURGERY
- TABLE 36 CERVICAL FUSION DEVICES MARKET, BY TYPE OF SURGERY, 2021-2028 (USD MILLION)
- 6.3.3 ANTERIOR CERVICAL FUSION DEVICES
- TABLE 37 ANTERIOR CERVICAL FUSION DEVICES MARKET, BY TYPE, 2021-2028 (USD MILLION)
- TABLE 38 ANTERIOR CERVICAL FUSION DEVICES MARKET, BY REGION, 2021-2028 (USD MILLION)
- 6.3.3.1 Anterior cervical plates
- 6.3.3.1.1 Anterior cervical plates to hold largest share of cervical fusion devices market
- 6.3.3.1 Anterior cervical plates
- TABLE 39 ANTERIOR CERVICAL PLATES MARKET, BY REGION, 2021-2028 (USD MILLION)
- 6.3.3.2 Anterior cervical screw systems
- 6.3.3.2.1 Potential risk of injury to limit acceptance
- 6.3.3.2 Anterior cervical screw systems
- TABLE 40 ANTERIOR CERVICAL SCREW SYSTEMS MARKET, BY REGION, 2021-2028 (USD MILLION)
- 6.3.3.3 Cervical interbody fusion devices
- 6.3.3.3.1 Growing use of PEEK over titanium-based devices to support market growth
- 6.3.3.3 Cervical interbody fusion devices
- TABLE 41 CERVICAL INTERBODY FUSION DEVICES MARKET, BY REGION, 2021-2028 (USD MILLION)
- 6.3.4 POSTERIOR CERVICAL FUSION DEVICES
- TABLE 42 POSTERIOR CERVICAL FUSION DEVICES MARKET, BY TYPE, 2021-2028 (USD MILLION)
- TABLE 43 POSTERIOR CERVICAL FUSION DEVICES MARKET, BY REGION, 2021-2028 (USD MILLION)
- 6.3.4.1 Posterior cervical plates
- 6.3.4.1.1 Plates to hold largest share of posterior cervical fusion devices
- 6.3.4.1 Posterior cervical plates
- TABLE 44 POSTERIOR CERVICAL PLATES MARKET, BY REGION, 2021-2028 (USD MILLION)
- 6.3.4.2 Posterior cervical screws
- 6.3.4.2.1 Growing preference for screws over plates in some procedures to favor market growth
- 6.3.4.2 Posterior cervical screws
- TABLE 45 POSTERIOR CERVICAL SCREWS MARKET, BY REGION, 2021-2028 (USD MILLION)
- 6.3.4.3 Posterior cervical rods
- 6.3.4.3.1 Advantages of newer screw-rod constructs to drive adoption
- 6.3.4.3 Posterior cervical rods
- TABLE 46 POSTERIOR CERVICAL RODS MARKET, BY REGION, 2021-2028 (USD MILLION)
- 6.4 SPINE BIOLOGICS
- TABLE 47 SPINE BIOLOGICS MARKET, BY TYPE, 2021-2028 (USD MILLION)
- TABLE 48 SPINE BIOLOGICS MARKET, BY REGION, 2021-2028 (USD MILLION)
- 6.4.1 SPINE BIOLOGICS MARKET, BY APPLICATION
- TABLE 49 SPINE BIOLOGICS MARKET, BY APPLICATION, 2021-2028 (USD MILLION)
- 6.4.2 SPINE BIOLOGICS MARKET, BY TYPE OF SURGERY
- TABLE 50 SPINE BIOLOGICS MARKET, BY TYPE OF SURGERY, 2021-2028 (USD MILLION)
- 6.4.3 DEMINERALIZED BONE MATRIX
- 6.4.3.1 DBM to hold largest share of spine biologics market
- TABLE 51 DEMINERALIZED BONE MATRIX MARKET, BY REGION, 2021-2028 (USD MILLION)
- 6.4.4 BONE MORPHOGENETIC PROTEINS
- 6.4.4.1 Safety and effectiveness to drive adoption
- TABLE 52 BONE MORPHOGENETIC PROTEINS MARKET, BY REGION, 2021-2028 (USD MILLION)
- 6.4.5 BONE SUBSTITUTES
- 6.4.5.1 Easy availability, low cost, and other advantages to support adoption
- TABLE 53 BONE SUBSTITUTES MARKET, BY REGION, 2021-2028 (USD MILLION)
- 6.4.6 MACHINED BONES
- 6.4.6.1 Rising popularity of PEEK and titanium spaces to restrain usage
- TABLE 54 MACHINED BONES MARKET, BY REGION, 2021-2028 (USD MILLION)
- 6.4.7 CELL-BASED MATRICES
- 6.4.7.1 Complications such as risk of infection and sensory loss to affect demand
- TABLE 55 CELL-BASED MATRICES MARKET, BY REGION, 2021-2028 (USD MILLION)
- 6.4.8 ALLOGRAFT BONE
- 6.4.8.1 Lower chance of successful fusion to limit adoption
- TABLE 56 ALLOGRAFT BONE MARKET, BY REGION, 2021-2028 (USD MILLION)
- 6.5 VCF TREATMENT DEVICES
- TABLE 57 VCF TREATMENT DEVICES MARKET, BY TYPE, 2021-2028 (USD MILLION)
- TABLE 58 VCF TREATMENT DEVICES MARKET, BY REGION, 2021-2028 (USD MILLION)
- 6.5.1 VCF TREATMENT DEVICES MARKET, BY APPLICATION
- TABLE 59 VCF TREATMENT DEVICES MARKET, BY APPLICATION, 2021-2028 (USD MILLION)
- 6.5.2 VCF TREATMENT DEVICES MARKET, BY TYPE OF SURGERY
- TABLE 60 VCF TREATMENT DEVICES MARKET, BY TYPE OF SURGERY, 2021-2028 (USD MILLION)
- 6.5.3 BALLOON KYPHOPLASTY DEVICES
- 6.5.3.1 Better surgical outcomes to ensure sustained demand
- TABLE 61 BALLOON KYPHOPLASTY DEVICES MARKET, BY REGION, 2021-2028 (USD MILLION)
- 6.5.4 VERTEBROPLASTY DEVICES
- 6.5.4.1 Chances of cement leakage to limit adoption
- TABLE 62 VERTEBROPLASTY DEVICES MARKET, BY REGION, 2021-2028 (USD MILLION)
- 6.6 SPINAL DECOMPRESSION DEVICES
- TABLE 63 SPINAL DECOMPRESSION DEVICES MARKET, BY PROCEDURE, 2021-2028 (USD MILLION)
- TABLE 64 SPINAL DECOMPRESSION DEVICES MARKET, BY REGION, 2021-2028 (USD MILLION)
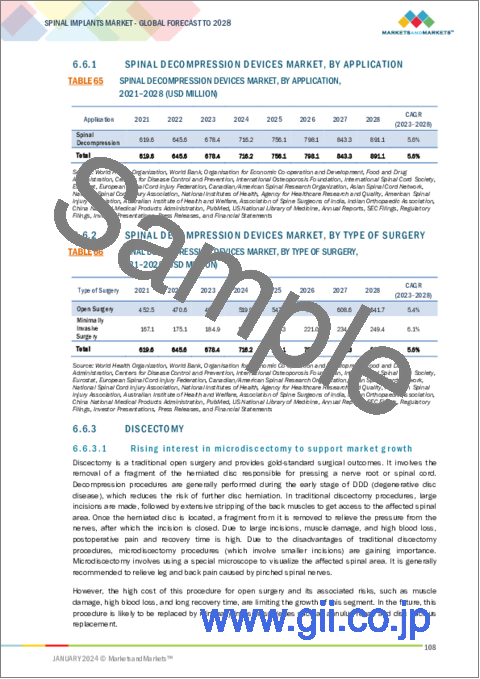
- 6.6.1 SPINAL DECOMPRESSION DEVICES MARKET, BY APPLICATION
- TABLE 65 SPINAL DECOMPRESSION DEVICES MARKET, BY APPLICATION, 2021-2028 (USD MILLION)
- 6.6.2 SPINAL DECOMPRESSION DEVICES MARKET, BY TYPE OF SURGERY
- TABLE 66 SPINAL DECOMPRESSION DEVICES MARKET, BY TYPE OF SURGERY, 2021-2028 (USD MILLION)
- 6.6.3 DISCECTOMY
- 6.6.3.1 Rising interest in microdiscectomy to support market growth
- TABLE 67 SPINAL DECOMPRESSION DEVICES MARKET FOR DISCECTOMY, BY REGION, 2021-2028 (USD MILLION)
- 6.6.4 LAMINOPLASTY, LAMINECTOMY, AND LAMINOTOMY
- 6.6.4.1 Rising incidence of spinal stenosis to drive market
- TABLE 68 SPINAL DECOMPRESSION DEVICES MARKET FOR LAMINOPLASTY, LAMINECTOMY, AND LAMINOTOMY, BY REGION, 2021-2028 (USD MILLION)
- 6.6.5 FORAMINOTOMY & FORAMINECTOMY
- 6.6.5.1 Growing preference for MIS treatment of cervical radiculopathy to support adoption
- TABLE 69 SPINAL DECOMPRESSION DEVICES MARKET FOR FORAMINOTOMY & FORAMINECTOMY, BY REGION, 2021-2028 (USD MILLION)
- 6.6.6 FACETECTOMY
- 6.6.6.1 Rising awareness of MIS and risks of facetectomy to restrain growth
- TABLE 70 SPINAL DECOMPRESSION DEVICES MARKET FOR FACETECTOMY, BY REGION, 2021-2028 (USD MILLION)
- 6.6.7 CORPECTOMY
- 6.6.7.1 Challenging procedures and inherent risks to affect demand
- TABLE 71 SPINAL DECOMPRESSION DEVICES MARKET FOR CORPECTOMY, BY REGION, 2021-2028 (USD MILLION)
- 6.7 NON-FUSION DEVICES
- TABLE 72 NON-FUSION DEVICES MARKET, BY TYPE, 2021-2028 (USD MILLION)
- TABLE 73 NON-FUSION DEVICES MARKET, BY REGION, 2021-2028 (USD MILLION)
- 6.7.1 NON-FUSION DEVICES MARKET, BY APPLICATION
- TABLE 74 NON-FUSION DEVICES MARKET, BY APPLICATION, 2021-2028 (USD MILLION)
- 6.7.2 NON-FUSION DEVICES MARKET, BY TYPE OF SURGERY
- TABLE 75 NON-FUSION DEVICES MARKET, BY TYPE OF SURGERY, 2021-2028 (USD MILLION)
- 6.7.3 DYNAMIC STABILIZATION DEVICES
- TABLE 76 DYNAMIC STABILIZATION DEVICES MARKET, BY TYPE, 2021-2028 (USD MILLION)
- TABLE 77 DYNAMIC STABILIZATION DEVICES MARKET, BY REGION, 2021-2028 (USD MILLION)
- 6.7.3.1 Interspinous process spacers
- 6.7.3.1.1 Positive outcomes and outpatient procedures to drive demand
- 6.7.3.1 Interspinous process spacers
- TABLE 78 INTERSPINOUS PROCESS SPACERS MARKET, BY REGION, 2021-2028 (USD MILLION)
- 6.7.3.2 Pedicle screw-based dynamic rod devices
- 6.7.3.2.1 Need for long-term safety and efficacy evidence to restrain adoption
- 6.7.3.2 Pedicle screw-based dynamic rod devices
- TABLE 79 PEDICLE SCREW-BASED DYNAMIC ROD DEVICES, BY REGION, 2021-2028 (USD MILLION)
- 6.7.3.3 Facet replacement products
- 6.7.3.3.1 Positive clinical outcomes and rising interest from players to support growth
- 6.7.3.3 Facet replacement products
- TABLE 80 FACET REPLACEMENT PRODUCTS MARKET, BY REGION, 2021-2028 (USD MILLION)
- 6.7.4 ARTIFICIAL DISCS
- TABLE 81 ARTIFICIAL DISCS MARKET, BY TYPE, 2021-2028 (USD MILLION)
- TABLE 82 ARTIFICIAL DISCS MARKET, BY REGION, 2021-2028 (USD MILLION)
- 6.7.4.1 Artificial cervical discs
- 6.7.4.1.1 Increasing adoption of artificial disc replacement to drive market
- 6.7.4.1 Artificial cervical discs
- TABLE 83 ARTIFICIAL CERVICAL DISCS MARKET, BY REGION, 2021-2028 (USD MILLION)
- 6.7.4.2 Artificial lumbar discs
- 6.7.4.2.1 Limited evidence for effectiveness and possibility of complications to hinder growth
- 6.7.4.2 Artificial lumbar discs
- TABLE 84 ARTIFICIAL LUMBAR DISCS MARKET, BY REGION, 2021-2028 (USD MILLION)
- 6.7.5 ANNULUS REPAIR DEVICES
- 6.7.5.1 Rising target patient population to drive usage
- TABLE 85 ANNULUS REPAIR DEVICES MARKET, BY REGION, 2021-2028 (USD MILLION)
- 6.7.6 NUCLEAR DISC PROSTHESES
- 6.7.6.1 Nuclear disc prostheses to register high growth
- TABLE 86 NUCLEAR DISC PROSTHESES MARKET, BY REGION, 2021-2028 (USD MILLION)
- 6.8 SPINE BONE STIMULATORS
- TABLE 87 SPINE BONE STIMULATORS MARKET, BY TYPE, 2021-2028 (USD MILLION)
- TABLE 88 SPINE BONE STIMULATORS MARKET, BY REGION, 2021-2028 (USD MILLION)
- 6.8.1 SPINE BONE STIMULATORS MARKET, BY APPLICATION
- TABLE 89 SPINE BONE STIMULATORS MARKET, BY APPLICATION, 2021-2028 (USD MILLION)
- 6.8.2 SPINE BONE STIMULATORS MARKET, BY TYPE OF SURGERY
- TABLE 90 SPINE BONE STIMULATORS MARKET, BY TYPE OF SURGERY, 2021-2028 (USD MILLION)
- 6.8.3 NONINVASIVE SPINE BONE STIMULATORS
- TABLE 91 NONINVASIVE SPINE BONE STIMULATORS MARKET, BY TYPE, 2021-2028 (USD MILLION)
- TABLE 92 NONINVASIVE SPINE BONE STIMULATORS MARKET, BY REGION, 2021-2028 (USD MILLION)
- 6.8.3.1 Pulsed electromagnetic field devices
- 6.8.3.2 CC & CMF devices
- 6.8.4 INVASIVE SPINE BONE STIMULATORS
- 6.8.4.1 Disadvantages and complications to affect pace of market growth
- TABLE 93 INVASIVE SPINE BONE STIMULATORS MARKET, BY REGION, 2021-2028 (USD MILLION)
7 SPINAL IMPLANTS MARKET, BY TYPE OF SURGERY
- 7.1 INTRODUCTION
- TABLE 94 SPINAL IMPLANTS MARKET, BY TYPE OF SURGERY, 2021-2028 (USD MILLION)
- 7.2 OPEN SURGERY
- 7.2.1 INCREASING PREFERENCE FOR OPEN SPINE SURGERIES TO DRIVE MARKET
- TABLE 95 SPINAL IMPLANTS MARKET FOR OPEN SURGERY, BY REGION, 2021-2028 (USD MILLION)
- 7.3 MINIMALLY INVASIVE SURGERY
- 7.3.1 MINIMALLY INVASIVE SURGERY SEGMENT TO REGISTER HIGHEST GROWTH
- TABLE 96 SPINAL IMPLANTS MARKET FOR MINIMALLY INVASIVE SURGERY, BY REGION, 2021-2028 (USD MILLION)
8 SPINAL IMPLANTS MARKET, BY APPLICATION
- 8.1 INTRODUCTION
- TABLE 97 SPINAL IMPLANTS MARKET, BY APPLICATION, 2021-2028 (USD MILLION)
- 8.2 SPINAL FUSION & FIXATION
- 8.2.1 SPINAL FUSION & FIXATION TECHNOLOGIES TO DOMINATE MARKET OVER FORECAST PERIOD
- TABLE 98 SPINAL IMPLANTS MARKET FOR SPINAL FUSION & FIXATION, BY REGION, 2021-2028 (USD MILLION)
- 8.3 VCF TREATMENT
- 8.3.1 INCREASING FRACTURE INCIDENCE TO SUPPORT MARKET GROWTH
- TABLE 99 SPINAL IMPLANTS MARKET FOR VCF TREATMENT, BY REGION, 2021-2028 (USD MILLION)
- 8.4 MOTION PRESERVATION/NON-FUSION
- 8.4.1 MOTION PRESERVATION/NON-FUSION TECHNOLOGIES TO WITNESS HIGHEST GROWTH
- TABLE 100 SPINAL IMPLANTS MARKET FOR MOTION PRESERVATION/NON-FUSION, BY REGION, 2021-2028 (USD MILLION)
- 8.5 SPINAL DECOMPRESSION
- 8.5.1 RISING INCIDENCE OF SPINAL TUMORS AND DEVELOPMENT OF MINIMALLY INVASIVE DEVICES TO DRIVE MARKET
- TABLE 101 SPINAL IMPLANTS MARKET FOR SPINAL DECOMPRESSION, BY REGION, 2021-2028 (USD MILLION)
9 SPINAL IMPLANTS MARKET, BY END USER
- 9.1 INTRODUCTION
- TABLE 102 SPINAL IMPLANTS MARKET, BY END USER, 2021-2028 (USD MILLION)
- 9.2 HOSPITALS
- 9.2.1 HOSPITALS TO HOLD LARGEST MARKET SHARE
- TABLE 103 SPINAL IMPLANTS MARKET FOR HOSPITALS, BY REGION, 2021-2028 (USD MILLION)
- 9.3 ORTHOPEDIC CENTERS
- 9.3.1 EARLY DETECTION OF SPINAL DISORDERS AND LOWER TURNAROUND TIME TO DRIVE MARKET
- TABLE 104 SPINAL IMPLANTS MARKET FOR ORTHOPEDIC CENTERS, BY REGION, 2021-2028 (USD MILLION)
10 SPINAL IMPLANTS MARKET, BY REGION
- 10.1 INTRODUCTION
- TABLE 105 SPINAL IMPLANTS MARKET, BY REGION, 2021-2028 (USD MILLION)
- 10.2 NORTH AMERICA
- 10.2.1 NORTH AMERICA: RECESSION IMPACT
- FIGURE 25 NORTH AMERICA: MARKET SNAPSHOT
- TABLE 106 NORTH AMERICA: SPINAL IMPLANTS MARKET, BY COUNTRY, 2021-2028 (USD MILLION)
- TABLE 107 NORTH AMERICA: SPINAL IMPLANTS MARKET, BY PRODUCT, 2021-2028 (USD MILLION)
- TABLE 108 NORTH AMERICA: THORACIC & LUMBAR FUSION DEVICES MARKET, BY TYPE, 2021-2028 (USD MILLION)
- TABLE 109 NORTH AMERICA: INTERBODY THORACIC & LUMBAR FUSION DEVICES MARKET, BY APPROACH, 2021-2028 (USD MILLION)
- TABLE 110 NORTH AMERICA: INTERBODY THORACIC & LUMBAR FUSION DEVICES MARKET, BY MATERIAL, 2021-2028 (USD MILLION)
- TABLE 111 NORTH AMERICA: CERVICAL FUSION DEVICES MARKET, BY TYPE, 2021-2028 (USD MILLION)
- TABLE 112 NORTH AMERICA: ANTERIOR CERVICAL FUSION DEVICES MARKET, BY TYPE, 2021-2028 (USD MILLION)
- TABLE 113 NORTH AMERICA: POSTERIOR CERVICAL FUSION DEVICES MARKET, BY TYPE, 2021-2028 (USD MILLION)
- TABLE 114 NORTH AMERICA: SPINE BIOLOGICS MARKET, BY TYPE, 2021-2028 (USD MILLION)
- TABLE 115 NORTH AMERICA: VCF TREATMENT DEVICES MARKET, BY TYPE, 2021-2028 (USD MILLION)
- TABLE 116 NORTH AMERICA: SPINAL DECOMPRESSION DEVICES MARKET, BY PROCEDURE, 2021-2028 (USD MILLION)
- TABLE 117 NORTH AMERICA: NON-FUSION DEVICES MARKET, BY TYPE, 2021-2028 (USD MILLION)
- TABLE 118 NORTH AMERICA: DYNAMIC STABILIZATION DEVICES MARKET, BY TYPE, 2021-2028 (USD MILLION)
- TABLE 119 NORTH AMERICA: ARTIFICIAL DISCS MARKET, BY TYPE, 2021-2028 (USD MILLION)
- TABLE 120 NORTH AMERICA: SPINE BONE STIMULATORS MARKET, BY TYPE, 2021-2028 (USD MILLION)
- TABLE 121 NORTH AMERICA: NONINVASIVE SPINE BONE STIMULATORS MARKET, BY TYPE, 2021-2028 (USD MILLION)
- TABLE 122 NORTH AMERICA: SPINAL IMPLANTS MARKET, BY TYPE OF SURGERY, 2021-2028 (USD MILLION)
- TABLE 123 NORTH AMERICA: SPINAL IMPLANTS MARKET, BY APPLICATION, 2021-2028 (USD MILLION)
- TABLE 124 NORTH AMERICA: SPINAL IMPLANTS MARKET, BY END USER, 2021-2028 (USD MILLION)
- 10.2.2 US
- 10.2.2.1 US to dominate North American market
- TABLE 125 US: SPINAL IMPLANTS MARKET, BY PRODUCT, 2021-2028 (USD MILLION)
- TABLE 126 US: THORACIC & LUMBAR FUSION DEVICES MARKET, BY TYPE, 2021-2028 (USD MILLION)
- TABLE 127 US: CERVICAL FUSION DEVICES MARKET, BY TYPE, 2021-2028 (USD MILLION)
- TABLE 128 US: SPINE BIOLOGICS MARKET, BY TYPE, 2021-2028 (USD MILLION)
- TABLE 129 US: VCF TREATMENT DEVICES MARKET, BY TYPE, 2021-2028 (USD MILLION)
- TABLE 130 US: SPINAL DECOMPRESSION DEVICES MARKET, BY PROCEDURE, 2021-2028 (USD MILLION)
- TABLE 131 US: NON-FUSION DEVICES MARKET, BY TYPE, 2021-2028 (USD MILLION)
- TABLE 132 US: SPINE BONE STIMULATORS MARKET, BY TYPE, 2021-2028 (USD MILLION)
- 10.2.3 CANADA
- 10.2.3.1 Inadequate reimbursement for spinal procedures to restrain market growth
- TABLE 133 CANADA: SPINAL IMPLANTS MARKET, BY PRODUCT, 2021-2028 (USD MILLION)
- TABLE 134 CANADA: THORACIC & LUMBAR FUSION DEVICES MARKET, BY TYPE, 2021-2028 (USD MILLION)
- TABLE 135 CANADA: CERVICAL FUSION DEVICES MARKET, BY TYPE, 2021-2028 (USD MILLION)
- TABLE 136 CANADA: SPINE BIOLOGICS MARKET, BY TYPE, 2021-2028 (USD MILLION)
- TABLE 137 CANADA: VCF TREATMENT DEVICES MARKET, BY TYPE, 2021-2028 (USD MILLION)
- TABLE 138 CANADA: SPINAL DECOMPRESSION DEVICES MARKET, BY PROCEDURE, 2021-2028 (USD MILLION)
- TABLE 139 CANADA: NON-FUSION DEVICES MARKET, BY TYPE, 2021-2028 (USD MILLION)
- TABLE 140 CANADA: SPINE BONE STIMULATORS MARKET, BY TYPE, 2021-2028 (USD MILLION)
- 10.3 EUROPE
- 10.3.1 EUROPE: RECESSION IMPACT
- TABLE 141 EUROPE: SPINAL IMPLANTS MARKET, BY COUNTRY, 2021-2028 (USD MILLION)
- TABLE 142 EUROPE: SPINAL IMPLANTS MARKET, BY PRODUCT, 2021-2028 (USD MILLION)
- TABLE 143 EUROPE: THORACIC & LUMBAR FUSION DEVICES MARKET, BY TYPE, 2021-2028 (USD MILLION)
- TABLE 144 EUROPE: INTERBODY THORACIC & LUMBAR FUSION DEVICES MARKET, BY APPROACH, 2021-2028 (USD MILLION)
- TABLE 145 EUROPE: INTERBODY THORACIC & LUMBAR FUSION DEVICES MARKET, BY MATERIAL, 2021-2028 (USD MILLION)
- TABLE 146 EUROPE: CERVICAL FUSION DEVICES MARKET, BY TYPE, 2021-2028 (USD MILLION)
- TABLE 147 EUROPE: ANTERIOR CERVICAL FUSION DEVICES MARKET, BY TYPE, 2021-2028 (USD MILLION)
- TABLE 148 EUROPE: POSTERIOR CERVICAL FUSION DEVICES MARKET, BY TYPE, 2021-2028 (USD MILLION)
- TABLE 149 EUROPE: SPINE BIOLOGICS MARKET, BY TYPE, 2021-2028 (USD MILLION)
- TABLE 150 EUROPE: VCF TREATMENT DEVICES MARKET, BY TYPE, 2021-2028 (USD MILLION)
- TABLE 151 EUROPE: SPINAL DECOMPRESSION DEVICES MARKET, BY PROCEDURE, 2021-2028 (USD MILLION)
- TABLE 152 EUROPE: NON-FUSION DEVICES MARKET, BY TYPE, 2021-2028 (USD MILLION)
- TABLE 153 EUROPE: DYNAMIC STABILIZATION DEVICES MARKET, BY TYPE, 2021-2028 (USD MILLION)
- TABLE 154 EUROPE: ARTIFICIAL DISCS MARKET, BY TYPE, 2021-2028 (USD MILLION)
- TABLE 155 EUROPE: SPINE BONE STIMULATORS MARKET, BY TYPE, 2021-2028 (USD MILLION)
- TABLE 156 EUROPE: NONINVASIVE SPINE BONE STIMULATORS MARKET, BY TYPE, 2021-2028 (USD MILLION)
- TABLE 157 EUROPE: SPINAL IMPLANTS MARKET, BY TYPE OF SURGERY, 2021-2028 (USD MILLION)
- TABLE 158 EUROPE: SPINAL IMPLANTS MARKET, BY APPLICATION, 2021-2028 (USD MILLION)
- TABLE 159 EUROPE: SPINAL IMPLANTS MARKET, BY END USER, 2021-2028 (USD MILLION)
- 10.3.2 GERMANY
- 10.3.2.1 Germany to hold largest market share in Europe
- TABLE 160 GERMANY: SPINAL IMPLANTS MARKET, BY PRODUCT, 2021-2028 (USD MILLION)
- TABLE 161 GERMANY: THORACIC & LUMBAR FUSION DEVICES MARKET, BY TYPE, 2021-2028 (USD MILLION)
- TABLE 162 GERMANY: CERVICAL FUSION DEVICES MARKET, BY TYPE, 2021-2028 (USD MILLION)
- TABLE 163 GERMANY: SPINE BIOLOGICS MARKET, BY TYPE, 2021-2028 (USD MILLION)
- TABLE 164 GERMANY: VCF TREATMENT DEVICES MARKET, BY TYPE, 2021-2028 (USD MILLION)
- TABLE 165 GERMANY: SPINAL DECOMPRESSION DEVICES MARKET, BY PROCEDURE, 2021-2028 (USD MILLION)
- TABLE 166 GERMANY: NON-FUSION DEVICES MARKET, BY TYPE, 2021-2028 (USD MILLION)
- TABLE 167 GERMANY: SPINE BONE STIMULATORS MARKET, BY TYPE, 2021-2028 (USD MILLION)
- 10.3.3 FRANCE
- 10.3.3.1 Rising healthcare expenditure to support market growth
- TABLE 168 FRANCE: SPINAL IMPLANTS MARKET, BY PRODUCT, 2021-2028 (USD MILLION)
- TABLE 169 FRANCE: THORACIC & LUMBAR FUSION DEVICES MARKET, BY TYPE, 2021-2028 (USD MILLION)
- TABLE 170 FRANCE: CERVICAL FUSION DEVICES MARKET, BY TYPE, 2021-2028 (USD MILLION)
- TABLE 171 FRANCE: SPINE BIOLOGICS MARKET, BY TYPE, 2021-2028 (USD MILLION)
- TABLE 172 FRANCE: VCF TREATMENT DEVICES MARKET, BY TYPE, 2021-2028 (USD MILLION)
- TABLE 173 FRANCE: SPINAL DECOMPRESSION DEVICES MARKET, BY PROCEDURE, 2021-2028 (USD MILLION)
- TABLE 174 FRANCE: NON-FUSION DEVICES MARKET, BY TYPE, 2021-2028 (USD MILLION)
- TABLE 175 FRANCE: SPINE BONE STIMULATORS MARKET, BY TYPE, 2021-2028 (USD MILLION)
- 10.3.4 UK
- 10.3.4.1 Rising cost of spinal surgeries to hinder market growth
- TABLE 176 UK: SPINAL IMPLANTS MARKET, BY PRODUCT, 2021-2028 (USD MILLION)
- TABLE 177 UK: THORACIC & LUMBAR FUSION DEVICES MARKET, BY TYPE, 2021-2028 (USD MILLION)
- TABLE 178 UK: CERVICAL FUSION DEVICES MARKET, BY TYPE, 2021-2028 (USD MILLION)
- TABLE 179 UK: SPINE BIOLOGICS MARKET, BY TYPE, 2021-2028 (USD MILLION)
- TABLE 180 UK: VCF TREATMENT DEVICES MARKET, BY TYPE, 2021-2028 (USD MILLION)
- TABLE 181 UK: SPINAL DECOMPRESSION DEVICES MARKET, BY PROCEDURE, 2021-2028 (USD MILLION)
- TABLE 182 UK: NON-FUSION DEVICES MARKET, BY TYPE, 2021-2028 (USD MILLION)
- TABLE 183 UK: SPINE BONE STIMULATORS MARKET, BY TYPE, 2021-2028 (USD MILLION)
- 10.3.5 ITALY
- 10.3.5.1 Demand for MIS to positively affect market growth
- TABLE 184 ITALY: SPINAL IMPLANTS MARKET, BY PRODUCT, 2021-2028 (USD MILLION)
- TABLE 185 ITALY: THORACIC & LUMBAR FUSION DEVICES MARKET, BY TYPE, 2021-2028 (USD MILLION)
- TABLE 186 ITALY: CERVICAL FUSION DEVICES MARKET, BY TYPE, 2021-2028 (USD MILLION)
- TABLE 187 ITALY: SPINE BIOLOGICS MARKET, BY TYPE, 2021-2028 (USD MILLION)
- TABLE 188 ITALY: VCF TREATMENT DEVICES MARKET, BY TYPE, 2021-2028 (USD MILLION)
- TABLE 189 ITALY: SPINAL DECOMPRESSION DEVICES MARKET, BY PROCEDURE, 2021-2028 (USD MILLION)
- TABLE 190 ITALY: NON-FUSION DEVICES MARKET, BY TYPE, 2021-2028 (USD MILLION)
- TABLE 191 ITALY: SPINE BONE STIMULATORS MARKET, BY TYPE, 2021-2028 (USD MILLION)
- 10.3.6 SPAIN
- 10.3.6.1 Growing preference for MIS to support market growth
- TABLE 192 SPAIN: SPINAL IMPLANTS MARKET, BY PRODUCT, 2021-2028 (USD MILLION)
- TABLE 193 SPAIN: THORACIC & LUMBAR FUSION DEVICES MARKET, BY TYPE, 2021-2028 (USD MILLION)
- TABLE 194 SPAIN: CERVICAL FUSION DEVICES MARKET, BY TYPE, 2021-2028 (USD MILLION)
- TABLE 195 SPAIN: SPINE BIOLOGICS MARKET, BY TYPE, 2021-2028 (USD MILLION)
- TABLE 196 SPAIN: VCF TREATMENT DEVICES MARKET, BY TYPE, 2021-2028 (USD MILLION)
- TABLE 197 SPAIN: SPINAL DECOMPRESSION DEVICES MARKET, BY PROCEDURE, 2021-2028 (USD MILLION)
- TABLE 198 SPAIN: NON-FUSION DEVICES MARKET, BY TYPE, 2021-2028 (USD MILLION)
- TABLE 199 SPAIN: SPINE BONE STIMULATORS MARKET, BY TYPE, 2021-2028 (USD MILLION)
- 10.3.7 REST OF EUROPE
- TABLE 200 REST OF EUROPE: SPINAL IMPLANTS MARKET, BY PRODUCT, 2021-2028 (USD MILLION)
- TABLE 201 REST OF EUROPE: THORACIC & LUMBAR FUSION DEVICES MARKET, BY TYPE, 2021-2028 (USD MILLION)
- TABLE 202 REST OF EUROPE: CERVICAL FUSION DEVICES MARKET, BY TYPE, 2021-2028 (USD MILLION)
- TABLE 203 V: SPINE BIOLOGICS MARKET, BY TYPE, 2021-2028 (USD MILLION)
- TABLE 204 REST OF EUROPE: VCF TREATMENT DEVICES MARKET, BY TYPE, 2021-2028 (USD MILLION)
- TABLE 205 REST OF EUROPE: SPINAL DECOMPRESSION DEVICES MARKET, BY PROCEDURE, 2021-2028 (USD MILLION)
- TABLE 206 REST OF EUROPE: NON-FUSION DEVICES MARKET, BY TYPE, 2021-2028 (USD MILLION)
- TABLE 207 REST OF EUROPE: SPINE BONE STIMULATORS MARKET, BY TYPE, 2021-2028 (USD MILLION)
- 10.4 ASIA PACIFIC
- 10.4.1 ASIA PACIFIC: RECESSION IMPACT
- FIGURE 26 ASIA PACIFIC: MARKET SNAPSHOT
- TABLE 208 ASIA PACIFIC: SPINAL IMPLANTS MARKET, BY COUNTRY, 2021-2028 (USD MILLION)
- TABLE 209 ASIA PACIFIC: SPINAL IMPLANTS MARKET, BY PRODUCT, 2021-2028 (USD MILLION)
- TABLE 210 ASIA PACIFIC: THORACIC & LUMBAR FUSION DEVICES MARKET, BY TYPE, 2021-2028 (USD MILLION)
- TABLE 211 ASIA PACIFIC: INTERBODY THORACIC & LUMBAR FUSION DEVICES MARKET, BY APPROACH, 2021-2028 (USD MILLION)
- TABLE 212 ASIA PACIFIC: INTERBODY THORACIC & LUMBAR FUSION DEVICES MARKET, BY MATERIAL, 2021-2028 (USD MILLION)
- TABLE 213 ASIA PACIFIC: CERVICAL FUSION DEVICES MARKET, BY TYPE, 2021-2028 (USD MILLION)
- TABLE 214 ASIA PACIFIC: ANTERIOR CERVICAL FUSION DEVICES MARKET, BY TYPE, 2021-2028 (USD MILLION)
- TABLE 215 ASIA PACIFIC: POSTERIOR CERVICAL FUSION DEVICES MARKET, BY TYPE, 2021-2028 (USD MILLION)
- TABLE 216 ASIA PACIFIC: SPINE BIOLOGICS MARKET, BY TYPE, 2021-2028 (USD MILLION)
- TABLE 217 ASIA PACIFIC: VCF TREATMENT DEVICES MARKET, BY TYPE, 2021-2028 (USD MILLION)
- TABLE 218 ASIA PACIFIC: SPINAL DECOMPRESSION DEVICES MARKET, BY PROCEDURE, 2021-2028 (USD MILLION)
- TABLE 219 ASIA PACIFIC: NON-FUSION DEVICES MARKET, BY TYPE, 2021-2028 (USD MILLION)
- TABLE 220 ASIA PACIFIC: DYNAMIC STABILIZATION DEVICES MARKET, BY TYPE, 2021-2028 (USD MILLION)
- TABLE 221 ASIA PACIFIC: ARTIFICIAL DISCS MARKET, BY TYPE, 2021-2028 (USD MILLION)
- TABLE 222 ASIA PACIFIC: SPINE BONE STIMULATORS MARKET, BY TYPE, 2021-2028 (USD MILLION)
- TABLE 223 ASIA PACIFIC: NONINVASIVE SPINE BONE STIMULATORS MARKET, BY TYPE, 2021-2028 (USD MILLION)
- TABLE 224 ASIA PACIFIC: SPINAL IMPLANTS MARKET, BY TYPE OF SURGERY, 2021-2028 (USD MILLION)
- TABLE 225 ASIA PACIFIC: SPINAL IMPLANTS MARKET, BY APPLICATION, 2021-2028 (USD MILLION)
- TABLE 226 ASIA PACIFIC: SPINAL IMPLANTS MARKET, BY END USER, 2021-2028 (USD MILLION)
- 10.4.2 JAPAN
- 10.4.2.1 Japan to dominate APAC market for spinal surgeries
- TABLE 227 JAPAN: SPINAL IMPLANTS MARKET, BY PRODUCT, 2021-2028 (USD MILLION)
- TABLE 228 JAPAN: THORACIC & LUMBAR FUSION DEVICES MARKET, BY TYPE, 2021-2028 (USD MILLION)
- TABLE 229 JAPAN: CERVICAL FUSION DEVICES MARKET, BY TYPE, 2021-2028 (USD MILLION)
- TABLE 230 JAPAN: SPINE BIOLOGICS MARKET, BY TYPE, 2021-2028 (USD MILLION)
- TABLE 231 JAPAN: VCF TREATMENT DEVICES MARKET, BY TYPE, 2021-2028 (USD MILLION)
- TABLE 232 JAPAN: SPINAL DECOMPRESSION DEVICES MARKET, BY PROCEDURE, 2021-2028 (USD MILLION)
- TABLE 233 JAPAN: NON-FUSION DEVICES MARKET, BY TYPE, 2021-2028 (USD MILLION)
- TABLE 234 JAPAN: SPINE BONE STIMULATORS MARKET, BY TYPE, 2021-2028 (USD MILLION)
- 10.4.3 CHINA
- 10.4.3.1 Limited reimbursement coverage for spinal surgeries to affect market growth
- TABLE 235 CHINA: SPINAL IMPLANTS MARKET, BY PRODUCT, 2021-2028 (USD MILLION)
- TABLE 236 CHINA: THORACIC & LUMBAR FUSION DEVICES MARKET, BY TYPE, 2021-2028 (USD MILLION)
- TABLE 237 CHINA: CERVICAL FUSION DEVICES MARKET, BY TYPE, 2021-2028 (USD MILLION)
- TABLE 238 CHINA: SPINE BIOLOGICS MARKET, BY TYPE, 2021-2028 (USD MILLION)
- TABLE 239 CHINA: VCF TREATMENT DEVICES MARKET, BY TYPE, 2021-2028 (USD MILLION)
- TABLE 240 CHINA: SPINAL DECOMPRESSION DEVICES MARKET, BY PROCEDURE, 2021-2028 (USD MILLION)
- TABLE 241 CHINA: NON-FUSION DEVICES MARKET, BY TYPE, 2021-2028 (USD MILLION)
- TABLE 242 CHINA: SPINE BONE STIMULATORS MARKET, BY TYPE, 2021-2028 (USD MILLION)
- 10.4.4 INDIA
- 10.4.4.1 Low surgical costs to support market growth
- TABLE 243 INDIA: SPINAL IMPLANTS MARKET, BY PRODUCT, 2021-2028 (USD MILLION)
- TABLE 244 INDIA: THORACIC & LUMBAR FUSION DEVICES MARKET, BY TYPE, 2021-2028 (USD MILLION)
- TABLE 245 INDIA: CERVICAL FUSION DEVICES MARKET, BY TYPE, 2021-2028 (USD MILLION)
- TABLE 246 INDIA: SPINE BIOLOGICS MARKET, BY TYPE, 2021-2028 (USD MILLION)
- TABLE 247 INDIA: VCF TREATMENT DEVICES MARKET, BY TYPE, 2021-2028 (USD MILLION)
- TABLE 248 INDIA: SPINAL DECOMPRESSION DEVICES MARKET, BY PROCEDURE, 2021-2028 (USD MILLION)
- TABLE 249 INDIA: NON-FUSION DEVICES MARKET, BY TYPE, 2021-2028 (USD MILLION)
- TABLE 250 INDIA: SPINE BONE STIMULATORS MARKET, BY TYPE, 2021-2028 (USD MILLION)
- 10.4.5 AUSTRALIA
- 10.4.5.1 Uneven distribution of specialists to hinder market growth
- TABLE 251 AUSTRALIA: SPINAL IMPLANTS MARKET, BY PRODUCT, 2021-2028 (USD MILLION)
- TABLE 252 AUSTRALIA: THORACIC & LUMBAR FUSION DEVICES MARKET, BY TYPE, 2021-2028 (USD MILLION)
- TABLE 253 AUSTRALIA: CERVICAL FUSION DEVICES MARKET, BY TYPE, 2021-2028 (USD MILLION)
- TABLE 254 AUSTRALIA: SPINE BIOLOGICS MARKET, BY TYPE, 2021-2028 (USD MILLION)
- TABLE 255 AUSTRALIA: VCF TREATMENT DEVICES MARKET, BY TYPE, 2021-2028 (USD MILLION)
- TABLE 256 AUSTRALIA: SPINAL DECOMPRESSION DEVICES MARKET, BY PROCEDURE, 2021-2028 (USD MILLION)
- TABLE 257 AUSTRALIA: NON-FUSION DEVICES MARKET, BY TYPE, 2021-2028 (USD MILLION)
- TABLE 258 AUSTRALIA: SPINE BONE STIMULATORS MARKET, BY TYPE, 2021-2028 (USD MILLION)
- 10.4.6 SOUTH KOREA
- 10.4.6.1 Growing medical tourism to favor demand for spinal implants
- TABLE 259 SOUTH KOREA: SPINAL IMPLANTS MARKET, BY PRODUCT, 2021-2028 (USD MILLION)
- TABLE 260 SOUTH KOREA: THORACIC & LUMBAR FUSION DEVICES MARKET, BY TYPE, 2021-2028 (USD MILLION)
- TABLE 261 SOUTH KOREA: CERVICAL FUSION DEVICES MARKET, BY TYPE, 2021-2028 (USD MILLION)
- TABLE 262 SOUTH KOREA: SPINE BIOLOGICS MARKET, BY TYPE, 2021-2028 (USD MILLION)
- TABLE 263 SOUTH KOREA: VCF TREATMENT DEVICES MARKET, BY TYPE, 2021-2028 (USD MILLION)
- TABLE 264 SOUTH KOREA: SPINAL DECOMPRESSION DEVICES MARKET, BY PROCEDURE, 2021-2028 (USD MILLION)
- TABLE 265 SOUTH KOREA: NON-FUSION DEVICES MARKET, BY TYPE, 2021-2028 (USD MILLION)
- TABLE 266 SOUTH KOREA: SPINE BONE STIMULATORS MARKET, BY TYPE, 2021-2028 (USD MILLION)
- 10.4.7 REST OF ASIA PACIFIC
- TABLE 267 REST OF ASIA PACIFIC: SPINAL IMPLANTS MARKET, BY PRODUCT, 2021-2028 (USD MILLION)
- TABLE 268 REST OF ASIA PACIFIC: THORACIC & LUMBAR FUSION DEVICES MARKET, BY TYPE, 2021-2028 (USD MILLION)
- TABLE 269 REST OF ASIA PACIFIC: CERVICAL FUSION DEVICES MARKET, BY TYPE, 2021-2028 (USD MILLION)
- TABLE 270 REST OF ASIA PACIFIC: SPINE BIOLOGICS MARKET, BY TYPE, 2021-2028 (USD MILLION)
- TABLE 271 REST OF ASIA PACIFIC: VCF TREATMENT DEVICES MARKET, BY TYPE, 2021-2028 (USD MILLION)
- TABLE 272 REST OF ASIA PACIFIC: SPINAL DECOMPRESSION DEVICES MARKET, BY PROCEDURE, 2021-2028 (USD MILLION)
- TABLE 273 REST OF ASIA PACIFIC: NON-FUSION DEVICES MARKET, BY TYPE, 2021-2028 (USD MILLION)
- TABLE 274 REST OF ASIA PACIFIC: SPINE BONE STIMULATORS MARKET, BY TYPE, 2021-2028 (USD MILLION)
- 10.5 LATIN AMERICA
- 10.5.1 LATIN AMERICA: RECESSION IMPACT
- TABLE 275 LATIN AMERICA: SPINAL IMPLANTS MARKET, BY COUNTRY, 2021-2028 (USD MILLION)
- TABLE 276 LATIN AMERICA: SPINAL IMPLANTS MARKET, BY PRODUCT, 2021-2028 (USD MILLION)
- TABLE 277 LATIN AMERICA: THORACIC & LUMBAR FUSION DEVICES MARKET, BY TYPE, 2021-2028 (USD MILLION)
- TABLE 278 LATIN AMERICA: INTERBODY THORACIC & LUMBAR FUSION DEVICES MARKET, BY APPROACH, 2021-2028 (USD MILLION)
- TABLE 279 LATIN AMERICA: INTERBODY THORACIC & LUMBAR FUSION DEVICES MARKET, BY MATERIAL, 2021-2028 (USD MILLION)
- TABLE 280 LATIN AMERICA: CERVICAL FUSION DEVICES MARKET, BY TYPE, 2021-2028 (USD MILLION)
- TABLE 281 LATIN AMERICA: ANTERIOR CERVICAL FUSION DEVICES MARKET, BY TYPE, 2021-2028 (USD MILLION)
- TABLE 282 LATIN AMERICA: POSTERIOR CERVICAL FUSION DEVICES MARKET, BY TYPE, 2021-2028 (USD MILLION)
- TABLE 283 LATIN AMERICA: SPINE BIOLOGICS MARKET, BY TYPE, 2021-2028 (USD MILLION)
- TABLE 284 LATIN AMERICA: VCF TREATMENT DEVICES MARKET, BY TYPE, 2021-2028 (USD MILLION)
- TABLE 285 LATIN AMERICA: SPINAL DECOMPRESSION DEVICES MARKET, BY PROCEDURE, 2021-2028 (USD MILLION)
- TABLE 286 LATIN AMERICA: NON-FUSION DEVICES MARKET, BY TYPE, 2021-2028 (USD MILLION)
- TABLE 287 LATIN AMERICA: DYNAMIC STABILIZATION DEVICES MARKET, BY TYPE, 2021-2028 (USD MILLION)
- TABLE 288 LATIN AMERICA: ARTIFICIAL DISCS MARKET, BY TYPE, 2021-2028 (USD MILLION)
- TABLE 289 LATIN AMERICA: SPINE BONE STIMULATORS MARKET, BY TYPE, 2021-2028 (USD MILLION)
- TABLE 290 LATIN AMERICA: NONINVASIVE SPINE BONE STIMULATORS MARKET, BY TYPE, 2021-2028 (USD MILLION)
- TABLE 291 LATIN AMERICA: SPINAL IMPLANTS MARKET, BY TYPE OF SURGERY, 2021-2028 (USD MILLION)
- TABLE 292 LATIN AMERICA: SPINAL IMPLANTS MARKET, BY APPLICATION, 2021-2028 (USD MILLION)
- TABLE 293 LATIN AMERICA: SPINAL IMPLANTS MARKET, BY END USER, 2021-2028 (USD MILLION)
- 10.5.2 BRAZIL
- 10.5.2.1 Brazil to dominate LATAM market
- TABLE 294 BRAZIL: SPINAL IMPLANTS MARKET, BY PRODUCT, 2021-2028 (USD MILLION)
- TABLE 295 BRAZIL: THORACIC & LUMBAR FUSION DEVICES MARKET, BY TYPE, 2021-2028 (USD MILLION)
- TABLE 296 BRAZIL: CERVICAL FUSION DEVICES MARKET, BY TYPE, 2021-2028 (USD MILLION)
- TABLE 297 BRAZIL: SPINE BIOLOGICS MARKET, BY TYPE, 2021-2028 (USD MILLION)
- TABLE 298 BRAZIL: VCF TREATMENT DEVICES MARKET, BY TYPE, 2021-2028 (USD MILLION)
- TABLE 299 BRAZIL: SPINAL DECOMPRESSION DEVICES MARKET, BY PROCEDURE, 2021-2028 (USD MILLION)
- TABLE 300 BRAZIL: NON-FUSION DEVICES MARKET, BY TYPE, 2021-2028 (USD MILLION)
- TABLE 301 BRAZIL: SPINE BONE STIMULATORS MARKET, BY TYPE, 2021-2028 (USD MILLION)
- 10.5.3 MEXICO
- 10.5.3.1 Fragmented healthcare system to create challenges for market players
- TABLE 302 MEXICO: SPINAL IMPLANTS MARKET, BY PRODUCT, 2021-2028 (USD MILLION)
- TABLE 303 MEXICO: THORACIC & LUMBAR FUSION DEVICES MARKET, BY TYPE, 2021-2028 (USD MILLION)
- TABLE 304 MEXICO: CERVICAL FUSION DEVICES MARKET, BY TYPE, 2021-2028 (USD MILLION)
- TABLE 305 MEXICO: SPINE BIOLOGICS MARKET, BY TYPE, 2021-2028 (USD MILLION)
- TABLE 306 MEXICO: VCF TREATMENT DEVICES MARKET, BY TYPE, 2021-2028 (USD MILLION)
- TABLE 307 MEXICO: SPINAL DECOMPRESSION DEVICES MARKET, BY PROCEDURE, 2021-2028 (USD MILLION)
- TABLE 308 MEXICO: NON-FUSION DEVICES MARKET, BY TYPE, 2021-2028 (USD MILLION)
- TABLE 309 MEXICO: SPINE BONE STIMULATORS MARKET, BY TYPE, 2021-2028 (USD MILLION)
- 10.5.4 REST OF LATIN AMERICA
- TABLE 310 REST OF LATIN AMERICA: SPINAL IMPLANTS MARKET, BY PRODUCT, 2021-2028 (USD MILLION)
- TABLE 311 REST OF LATIN AMERICA: THORACIC & LUMBAR FUSION DEVICES MARKET, BY TYPE, 2021-2028 (USD MILLION)
- TABLE 312 REST OF LATIN AMERICA: CERVICAL FUSION DEVICES MARKET, BY TYPE, 2021-2028 (USD MILLION)
- TABLE 313 REST OF LATIN AMERICA: SPINE BIOLOGICS MARKET, BY TYPE, 2021-2028 (USD MILLION)
- TABLE 314 REST OF LATIN AMERICA: VCF TREATMENT DEVICES MARKET, BY TYPE, 2021-2028 (USD MILLION)
- TABLE 315 REST OF LATIN AMERICA: SPINAL DECOMPRESSION DEVICES MARKET, BY PROCEDURE, 2021-2028 (USD MILLION)
- TABLE 316 REST OF LATIN AMERICA: NON-FUSION DEVICES MARKET, BY TYPE, 2021-2028 (USD MILLION)
- TABLE 317 REST OF LATIN AMERICA: SPINE BONE STIMULATORS MARKET, BY TYPE, 2021-2028 (USD MILLION)
- 10.6 MIDDLE EAST & AFRICA
- 10.6.1 MEA TO OFFER PROMISING OPPORTUNITIES FOR PLAYERS
- 10.6.2 MIDDLE EAST & AFRICA: RECESSION IMPACT
- TABLE 318 MIDDLE EAST & AFRICA: SPINAL IMPLANTS MARKET, BY PRODUCT, 2021-2028 (USD MILLION)
- TABLE 319 MIDDLE EAST & AFRICA: THORACIC & LUMBAR FUSION DEVICES MARKET, BY TYPE, 2021-2028 (USD MILLION)
- TABLE 320 MIDDLE EAST & AFRICA: INTERBODY THORACIC & LUMBAR FUSION DEVICES MARKET, BY APPROACH, 2021-2028 (USD MILLION)
- TABLE 321 MIDDLE EAST & AFRICA: INTERBODY THORACIC & LUMBAR FUSION DEVICES MARKET, BY MATERIAL, 2021-2028 (USD MILLION)
- TABLE 322 MIDDLE EAST & AFRICA: CERVICAL FUSION DEVICES MARKET, BY TYPE, 2021-2028 (USD MILLION)
- TABLE 323 MIDDLE EAST & AFRICA: ANTERIOR CERVICAL FUSION DEVICES MARKET, BY TYPE, 2021-2028 (USD MILLION)
- TABLE 324 MIDDLE EAST & AFRICA: POSTERIOR CERVICAL FUSION DEVICES MARKET, BY TYPE, 2021-2028 (USD MILLION)
- TABLE 325 MIDDLE EAST & AFRICA: SPINE BIOLOGICS MARKET, BY TYPE, 2021-2028 (USD MILLION)
- TABLE 326 MIDDLE EAST & AFRICA: VCF TREATMENT DEVICES MARKET, BY TYPE, 2021-2028 (USD MILLION)
- TABLE 327 MIDDLE EAST & AFRICA: SPINAL DECOMPRESSION DEVICES MARKET, BY PROCEDURE, 2021-2028 (USD MILLION)
- TABLE 328 MIDDLE EAST & AFRICA: NON-FUSION DEVICES MARKET, BY TYPE, 2021-2028 (USD MILLION)
- TABLE 329 MIDDLE EAST & AFRICA: DYNAMIC STABILIZATION DEVICES MARKET, BY TYPE, 2021-2028 (USD MILLION)
- TABLE 330 MIDDLE EAST & AFRICA: ARTIFICIAL DISCS MARKET, BY TYPE, 2021-2028 (USD MILLION)
- TABLE 331 MIDDLE EAST & AFRICA: SPINE BONE STIMULATORS MARKET, BY TYPE, 2021-2028 (USD MILLION)
- TABLE 332 MIDDLE EAST & AFRICA: NONINVASIVE SPINE BONE STIMULATORS MARKET, BY TYPE, 2021-2028 (USD MILLION)
- TABLE 333 MIDDLE EAST & AFRICA: SPINAL IMPLANTS MARKET, BY TYPE OF SURGERY, 2021-2028 (USD MILLION)
- TABLE 334 MIDDLE EAST & AFRICA: SPINAL IMPLANTS MARKET, BY APPLICATION, 2021-2028 (USD MILLION)
- TABLE 335 MIDDLE EAST & AFRICA: SPINAL IMPLANTS MARKET, BY END USER, 2021-2028 (USD MILLION)
- 10.6.3 GCC COUNTRIES
11 COMPETITIVE LANDSCAPE
- 11.1 OVERVIEW
- 11.2 KEY PLAYER STRATEGIES/RIGHT TO WIN
- 11.2.1 OVERVIEW OF STRATEGIES ADOPTED BY SPINAL IMPLANT PLAYERS
- 11.3 REVENUE SHARE ANALYSIS
- FIGURE 27 REVENUE SHARE ANALYSIS OF TOP PLAYERS IN SPINAL IMPLANTS MARKET, 2018-2022 (USD MILLION)
- 11.4 MARKET SHARE ANALYSIS
- 11.4.1 GLOBAL SPINAL IMPLANTS/SURGICAL DEVICES MARKET SHARE, BY KEY PLAYER, 2022
- FIGURE 28 SPINAL IMPLANTS/SURGICAL DEVICES MARKET SHARE ANALYSIS, 2022
- 11.4.2 GLOBAL SPINE BIOLOGICS MARKET SHARE, BY KEY PLAYER, 2022
- FIGURE 29 SPINE BIOLOGICS MARKET SHARE ANALYSIS, 2022
- 11.4.3 GLOBAL SPINE BONE STIMULATORS MARKET SHARE, BY KEY PLAYER, 2022
- FIGURE 30 SPINE BONE STIMULATORS MARKET SHARE ANALYSIS, 2022
- FIGURE 31 SPINAL IMPLANTS MARKET (2022)
- 11.5 COMPANY EVALUATION MATRIX
- 11.5.1 STARS
- 11.5.2 EMERGING LEADERS
- 11.5.3 PERVASIVE PLAYERS
- 11.5.4 PARTICIPANTS
- FIGURE 32 SPINAL IMPLANTS MARKET: COMPANY EVALUATION MATRIX, 2022
- 11.5.5 COMPANY FOOTPRINT
- TABLE 336 PRODUCT FOOTPRINT (17 COMPANIES)
- TABLE 337 REGIONAL FOOTPRINT (17 COMPANIES)
- TABLE 338 COMPANY FOOTPRINT (17 COMPANIES)
- 11.6 STARTUP/SME EVALUATION MATRIX
- 11.6.1 PROGRESSIVE COMPANIES
- 11.6.2 STARTING BLOCKS
- 11.6.3 RESPONSIVE COMPANIES
- 11.6.4 DYNAMIC COMPANIES
- FIGURE 33 SPINAL IMPLANTS MARKET: STARTUP/SME EVALUATION MATRIX, 2022
- 11.6.5 COMPANY BENCHMARKING
- TABLE 339 SPINAL IMPLANTS MARKET: DETAILED LIST OF KEY STARTUP/SME PLAYERS
- TABLE 340 SPINAL IMPLANTS MARKET: COMPETITIVE BENCHMARKING OF STARTUP/SME PLAYERS
- 11.7 COMPETITIVE SCENARIOS AND TRENDS
- TABLE 341 KEY PRODUCT LAUNCHES & APPROVALS (JANUARY 2021 TO OCTOBER 2023)
- TABLE 342 KEY DEALS
12 COMPANY PROFILES
- 12.1 KEY PLAYERS
- (Business overview, Services/Solutions offered, Recent developments, MnM view, Right to win, Strategic choices made, and Weaknesses and Competitive threats)**
- 12.1.1 MEDTRONIC PLC
- TABLE 343 MEDTRONIC PLC: COMPANY OVERVIEW
- FIGURE 34 MEDTRONIC PLC: COMPANY SNAPSHOT (2023)
- 12.1.2 JOHNSON & JOHNSON MEDTECH
- TABLE 344 JOHNSON & JOHNSON MEDTECH: COMPANY OVERVIEW
- FIGURE 35 JOHNSON & JOHNSON MEDTECH: COMPANY SNAPSHOT (2022)
- 12.1.3 NUVASIVE, INC.
- TABLE 345 NUVASIVE, INC: COMPANY OVERVIEW
- FIGURE 36 NUVASIVE, INC: COMPANY SNAPSHOT (2022)
- 12.1.4 STRYKER CORPORATION
- TABLE 346 STRYKER CORPORATION: COMPANY OVERVIEW
- FIGURE 37 STRYKER CORPORATION: COMPANY SNAPSHOT (2022)
- 12.1.5 GLOBUS MEDICAL, INC.
- TABLE 347 GLOBUS MEDICAL, INC.: COMPANY OVERVIEW
- FIGURE 38 GLOBUS MEDICAL, INC.: COMPANY SNAPSHOT (2022)
- 12.1.6 ZIMMER BIOMET HOLDINGS, INC.
- TABLE 348 ZIMMER BIOMET HOLDINGS, INC.: COMPANY OVERVIEW
- FIGURE 39 ZIMVIE, INC.: COMPANY SNAPSHOT (2022)
- 12.1.7 ORTHOFIX INTERNATIONAL N.V.
- TABLE 349 ORTHOFIX INTERNATIONAL N.V.: COMPANY OVERVIEW
- FIGURE 40 ORTHOFIX INTERNATIONAL N.V.: COMPANY SNAPSHOT (2022)
- 12.1.8 ULRICH GMBH & CO. KG
- TABLE 350 ULRICH GMBH & CO. KG: COMPANY OVERVIEW
- 12.1.9 B. BRAUN MELSUNGEN AG
- FIGURE 41 B. BRAUN MELSUNGEN AG: COMPANY SNAPSHOT (2022)
- 12.1.10 INTEGRA LIFESCIENCES HOLDINGS CORPORATION
- TABLE 351 INTEGRA LIFESCIENCES HOLDINGS CORPORATION: COMPANY OVERVIEW
- FIGURE 42 INTEGRA LIFESCIENCES HOLDINGS CORPORATION: COMPANY SNAPSHOT (2023)
- 12.1.11 BOSTON SCIENTIFIC CORPORATION
- TABLE 352 BOSTON SCIENTIFIC CORPORATION: COMPANY OVERVIEW
- FIGURE 43 BOSTON SCIENTIFIC CORPORATION: COMPANY SNAPSHOT (2022)
- 12.1.12 ABBOTT LABORATORIES
- TABLE 353 ABBOTT LABORATORIES: COMPANY OVERVIEW
- FIGURE 44 ABBOTT LABORATORIES: COMPANY SNAPSHOT (2022)
- 12.1.13 SPINEART
- TABLE 354 SPINEART: COMPANY OVERVIEW
- 12.1.14 CENTINEL SPINE LLC
- TABLE 355 CENTINEL SPINE LLC: BUSINESS OVERVIEW
- 12.1.15 PREMIA SPINE
- TABLE 356 PREMIA SPINE: BUSINESS OVERVIEW
- 12.1.16 EXACTECH, INC.
- TABLE 357 EXACTECH, INC.: BUSINESS OVERVIEW
- 12.1.17 LIFE SPINE, INC.
- TABLE 358 LIFE SPINE, INC.: BUSINESS OVERVIEW
- 12.1.18 ATEC SPINE
- TABLE 359 ATEC SPINE: BUSINESS OVERVIEW
- FIGURE 45 ATEC SPINE: COMPANY SNAPSHOT (2022)
- 12.2 OTHER PLAYERS
- 12.2.1 KUROS BIOSCIENCES LTD.
- 12.2.2 BIOVENTUS LLC
- 12.2.3 COLFAX CORPORATION
- 12.2.4 AESCULAP INC.
- 12.2.5 IMPLANET
- 12.2.6 CAMBER SPINE TECHNOLOGIES
- 12.2.7 CAPTIVA SPINE, INC.
- 12.2.8 MEDACTA INTERNATIONAL
- 12.2.9 AURORA SPINE CORPORATION
- *Details on Business overview, Services/Solutions offered, Recent developments, MnM view, Right to win, Strategic choices made, and Weaknesses and Competitive threats might not be captured in case of unlisted companies.
13 APPENDIX
- 13.1 DISCUSSION GUIDE
- 13.2 KNOWLEDGESTORE: MARKETSANDMARKETS' SUBSCRIPTION PORTAL
- 13.3 CUSTOMIZATION OPTIONS
- 13.4 RELATED REPORTS
- 13.5 AUTHOR DETAILS