|
|
市場調査レポート
商品コード
1366415
シスタチンC測定の世界市場:製品別、測定法別、用途別、サンプルタイプ別、エンドユーザー別、地域別-2028年までの予測Cystatin C Assay Market by Product (Analyzers, Kits, Reagents), Method (ELISA, PETIA, IFA, CLIA, PENIA), Application (Diagnostics, Research), Sample Type (Blood, Urine), End User (Hospitals, Clinical Laboratories) & Region - Global Forecast to 2028 |
||||||
カスタマイズ可能
|
|||||||
| シスタチンC測定の世界市場:製品別、測定法別、用途別、サンプルタイプ別、エンドユーザー別、地域別-2028年までの予測 |
|
出版日: 2023年10月12日
発行: MarketsandMarkets
ページ情報: 英文 263 Pages
納期: 即納可能
|
- 全表示
- 概要
- 目次
世界のシスタチンC測定の市場規模は、2023年の3億7,700万米ドルから2028年には5億4,000万米ドルに達すると予測され、予測期間中のCAGRは7.5%と見込まれています。
この市場の成長の主な原動力は、腎臓病の危険因子である糖尿病と高血圧の有病率が上昇し、早期かつ正確な腎機能評価の必要性が高まったことによる糖尿病人口の増加です。しかし、コストと手頃な価格に関する課題は、この市場の成長を脅かす可能性があります。
| レポート対象範囲 | |
|---|---|
| 対象期間 | 2021–2028 |
| 基準年 | 2022 |
| 予測期間/td> | 2023–2028 |
| 単位 | 金額 (USD) million |
| セグメント | 製品別、測定法別、用途別、サンプルタイプ別、エンドユーザー別、地域別 |
| 対象地域 | 北米、欧州、アジア太平洋、その他地域 |
米国は予測期間中に最も高いCAGRで成長すると予測されています。米国は、シスタチンC分析装置を含む医療機器や診断装置の重要な市場であり、そのような分析装置の需要はヘルスケアニーズや検査実務によって牽引されています。腎臓病の有病率、臨床ガイドライン、検査室のインフラ、技術の進歩などの要因が米国におけるシスタチンC分析装置の採用に影響を与えています。
欧州市場は予測期間中に3番目に高い成長率を記録すると予測されています。この市場の成長の主な原動力は、65歳以上の人口比率が高く、高齢化が著しいことです。高齢者層は糖尿病性腎症や慢性腎疾患のような慢性疾患に罹患しやすく、診断検査が必要となることが多いです。さらに、自動分析装置やポイントオブケア検査オプションの利用可能性を含め、検査技術の進歩が続いているため、欧州ではシスタチンC検査の利用しやすさと効率が向上しています。
当レポートでは、世界のシスタチンC測定市場について調査し、製品別、測定法別、用途別、サンプルタイプ別、エンドユーザー別、地域別動向、および市場に参入する企業のプロファイルなどをまとめています。
目次
第1章 イントロダクション
第2章 調査手法
第3章 エグゼクティブサマリー
第4章 重要考察
第5章 市場概要
- イントロダクション
- 市場力学
- 規制の概要
- 技術分析
- 貿易分析
- 特許分析
- バリューチェーン分析
- サプライチェーン分析
- シスタチンC測定市場のエコシステム分析
- ポーターのファイブフォース分析
- 主要な会議とイベント(2023年~2024年)
- 価格分析
- 主要な利害関係者と購入基準
- 顧客のビジネスに影響を与える動向/混乱
第6章 シスタチンC測定市場、製品別
- イントロダクション
- キット
- アナライザー
第7章 シスタチンC測定市場、測定法別
- イントロダクション
- 酵素免疫測定法
- 粒子増強比濁免疫測定法
- 免疫蛍光アッセイ
- 化学発光免疫測定法
- 粒子増強比濁免疫測定法
- その他
第8章 シスタチンC測定市場、サンプルタイプ別
- イントロダクション
- 血液
- 尿
第9章 シスタチンC測定市場、用途別
- イントロダクション
- 診断
- 研究
第10章 シスタチンC測定市場、エンドユーザー別
- イントロダクション
- 病院
- 臨床検査室
- 製薬会社およびバイオテクノロジー会社、CRO、および学術研究機関
第11章 シスタチンC測定市場、地域別
- イントロダクション
- 北米
- 欧州
- アジア太平洋
- その他の地域
第12章 競合情勢
- 概要
- 主要参入企業が採用した戦略
- 市場ランキング分析、2022年
- 収益シェア分析、2022年
- 企業評価マトリックス
- 中小企業/スタートアップ企業の評価マトリックス
- 競合ベンチマーキング
- 競合シナリオ
第13章 企業プロファイル
- 主要参入企業
- ROCHE DIAGNOSTICS LIMITED
- ABBOTT
- THERMO FISHER SCIENTIFIC INC.
- SIEMENS HEALTHCARE GMBH
- BIO-TECHNE
- AGILENT TECHNOLOGIES, INC.
- GENTIAN DIAGNOSTICS ASA
- ABCAM PLC
- GETEIN BIOTECH, INC.
- SINO BIOLOGICAL, INC.
- EUROLYSER DIAGNOSTICA GMBH
- DIAZYME LABORATORIES, INC
- KAMIYA BIOMEDICAL COMPANY
- RANDOX LABORATORIES LTD.
- DIASYS DIAGNOSTIC SYSTEMS GMBH
- CEPHAM LIFE SCIENCES
- その他の企業
- ETHOS BIOSCIENCES
- IMMUNODIAGNOSTICS LIMITED
- SEKISUI DIAGNOSTICS
- AALTO SCIENTIFIC, LTD.
- RAYBIOTECH LIFE, INC.
- ARBOR ASSAYS
- CUSABIO TECHNOLOGY LLC
- PROTEINTECH GROUP, INC.
- ZHEJIANG KANGTE BIOTECHNOLOGY CO., LTD.
第14章 付録
The global cystatin C assay market is projected to reach USD 540 million by 2028 from USD 377 million in 2023, at a CAGR of 7.5% during the forecast period. The growth of this market is majorly driven by Increasing number of diabetes population as increasing prevalence of diabetes and hypertension, both risk factors for kidney disease, has heightened the need for early and accurate kidney function assessments.. However, Challenges associated with cost and affordability may threat the growth of this market.
| Scope of the Report | |
|---|---|
| Years Considered for the Study | 2021–2028 |
| Base Year | 2022 |
| Forecast Period | 2023–2028 |
| Units Considered | Value (USD) million |
| Segments | Product, Method, Application, Sample Type, End User and Region |
| Regions covered | North America, Europe, APAC, Rest of the World |
"US' Analyzer in the product segment to witness the highest growth during the forecast period in the north American market."
Based north american region analyzer' portion, the cystatin C assay market is segmented into US and Cananda. US is projected to grow at the highest CAGR during the forecast period. The United States is a significant market for medical devices and diagnostic equipment, including cystatin C analyzers, the demand for such analyzers is driven by healthcare needs and laboratory practices. Factors such as the prevalence of kidney diseases, clinical guidelines, laboratory infrastructure, and technological advancements influence the adoption of cystatin C analyzers in US.
"Europe is estimated to register the third highest CAGR during the forecast period."
In this report, the cystatin C assay market is segmented into four major regional segments: North America, Europe, Asia Pacific, Rest of the world. The market in Europe is projected to register the third highest growth rate during the forecast period. The growth in this market is primarily driven by the presence of significant aging population, with a higher proportion of individuals aged 65 and above. The aging demographic is more prone to chronic conditions, such as diabetes nephopathy, and chronic nephrological conditions , which often require diagnostic tesing moreover, ongoing advancements in assay technologies, including the availability of automated analyzers and point-of-care testing options, have improved the accessibility and efficiency of cystatin C testing in Europe.
Breakdown of supply-side primary interviews, by company type, designation, and region:
- By Company Type: Tier 1 (48%), Tier 2 (34%), and Tier 3 (18%)
- By Designation: C-level (33%), Director-level (40%), and Others (27%)
- By Region: North America (36%), Europe (28%), AsiaPacific (20%), Rest of the World (16%)
List of Companies Profiled in the Report
- Gentian Diagnostics ASA (Norway)
- Getein Biotech, Inc. (China)
- Abbott (US)
- Roche Diagnostics Limited.(Switzerland)
- Siemens Healthcare GmbH (Germany)
- Thermo Fisher Scientific Inc. (US)
- Agilent Technologies, Inc. (US)
- Abcam plc. (UK)
- Sino Biological, Inc. (China)
- Bio-Techne (US)
- Eurolyser Diagnostica GmbH (Austria)
- Diazyme Laboratories, Inc (US)
- KAMIYA BIOMEDICAL COMPANY (US)
- Randox Laboratories Ltd. (UK)
- DiaSys Diagnostic Systems GmbH (Germany)
- Cepham Life Sciences (US)
- ETHOS BIOSCIENCES (US)
- ImmunoDiagnostics Limited.(Cananda)
- SEKISUI Diagnostics (US)
- Aalto Scientific, Ltd (US)
- RayBiotech Life, Inc. (US)
- Arbor Assays (US)
- CUSABIO TECHNOLOGY LLC (US)
- Proteintech Group, Inc. (US)
- Zhejiang Kangte Biotechnology Co., Ltd.(China)
Research Coverage:
This report studies the cystatin C assay market based on product and region. The report also analyzes factors (such as drivers, opportunities, and challenges, restraints) affecting the market growth. It evaluates the opportunities and challenges in the market for stakeholders and provides details of the competitive landscape for market leaders. The report also studies micromarkets with respect to their growth trends, prospects, and contributions to the total cystatin C assay market. The report forecasts the revenue of the market segments with respect to five major regions.
Reasons to Buy the Report:
The report provides insights on the following pointers:
- Market Penetration: Comprehensive information on bio decontamination products offered by the top 25 players in the cystatin C assay market. The report analyzes the cystatin C assay market by product, method, application, sample type, end user and region.
- Market Drivers: Comprehensive information about the factors and conditions that stimulate and influence the growth and performance of a specific market.
- Market Development: Comprehensive information about lucrative emerging markets. The report analyzes the markets for various securement devices across key geographic regions.
- Market Diversification: Exhaustive information about untapped geographies, recent developments, and investments in the cystatin C assay market.
- Competitive Assessment: In-depth assessment of market shares and strategies of the leading players in the cystatin C assay market.
TABLE OF CONTENTS
1 INTRODUCTION
- 1.1 STUDY OBJECTIVES
- 1.2 MARKET DEFINITION
- 1.2.1 INCLUSIONS & EXCLUSIONS
- 1.3 MARKET SCOPE
- 1.3.1 REGIONAL SCOPE
- 1.3.2 YEARS CONSIDERED
- 1.4 CURRENCY CONSIDERED
- 1.5 UNITS CONSIDERED
- 1.6 STAKEHOLDERS
- 1.7 RECESSION IMPACT
2 RESEARCH METHODOLOGY
- 2.1 RESEARCH DATA
- FIGURE 1 RESEARCH DESIGN
- 2.1.1 SECONDARY DATA
- 2.1.1.1 Secondary sources
- 2.1.2 PRIMARY DATA
- 2.1.2.1 Key data from primary sources
- 2.1.2.2 Key industry insights
- FIGURE 2 BREAKDOWN OF PRIMARY INTERVIEWS: SUPPLY-SIDE AND DEMAND-SIDE PARTICIPANTS
- FIGURE 3 BREAKDOWN OF PRIMARY INTERVIEWS
- 2.2 MARKET SIZE ESTIMATION
- 2.2.1 BOTTOM-UP APPROACH
- FIGURE 4 MARKET SIZE ESTIMATION: BOTTOM-UP APPROACH
- 2.2.2 BOTTOM-UP APPROACH: NUMBER OF TEST-BASED ANALYSES
- FIGURE 5 MARKET SIZE ESTIMATION: TOP-DOWN APPROACH
- FIGURE 6 CAGR PROJECTIONS: SUPPLY-SIDE ANALYSIS
- 2.3 MARKET BREAKDOWN AND DATA TRIANGULATION
- FIGURE 7 DATA TRIANGULATION METHODOLOGY
- 2.4 RESEARCH ASSUMPTIONS
- 2.5 RISK ASSESSMENT
- TABLE 1 RISK ASSESSMENT: CYSTATIN C ASSAY MARKET
- 2.6 RESEARCH LIMITATIONS
- 2.6.1 METHODOLOGY-RELATED LIMITATIONS
- 2.6.2 SCOPE-RELATED LIMITATIONS
- 2.7 GROWTH RATE ASSUMPTIONS
- 2.8 RECESSION IMPACT ANALYSIS
3 EXECUTIVE SUMMARY
- FIGURE 8 CYSTATIN C ASSAY MARKET, BY PRODUCT, 2023 VS. 2028 (USD MILLION)
- FIGURE 9 CYSTATIN C ASSAY MARKET, BY METHOD, 2023 VS. 2028 (USD MILLION)
- FIGURE 10 CYSTATIN C ASSAY MARKET, BY APPLICATION, 2023 VS. 2028 (USD MILLION)
- FIGURE 11 CYSTATIN C ASSAY MARKET, BY SAMPLE TYPE, 2023 VS. 2028 (USD MILLION)
- FIGURE 12 CYSTATIN C ASSAY MARKET, BY END USER, 2023 VS. 2028 (USD MILLION)
- FIGURE 13 REGIONAL SNAPSHOT: CYSTATIN C ASSAY MARKET
4 PREMIUM INSIGHTS
- 4.1 ATTRACTIVE OPPORTUNITIES FOR PLAYERS IN CYSTATIN C ASSAY MARKET
- FIGURE 14 INCREASING PREVALENCE OF KIDNEY DISEASES AND GROWING GERIATRIC POPULATION TO DRIVE MARKET
- 4.2 NORTH AMERICA: CYSTATIN C ASSAY MARKET, BY PRODUCT
- FIGURE 15 KITS ACCOUNTED FOR LARGEST SHARE, BY PRODUCT, IN NORTH AMERICA
- 4.3 CYSTATIN C ASSAY MARKET: REGIONAL MIX
- FIGURE 16 ASIA PACIFIC MARKET TO WITNESS FASTEST GROWTH DURING FORECAST PERIOD
- 4.4 CYSTATIN C ASSAY MARKET: DEVELOPED VS. EMERGING ECONOMIES
- FIGURE 17 DEVELOPING ECONOMIES TO REGISTER HIGHER GROWTH DURING FORECAST PERIOD
- 4.5 REGIONAL GROWTH OPPORTUNITIES IN CYSTATIN C ASSAY MARKET
- FIGURE 18 CHINA TO REGISTER HIGHEST GROWTH RATE DURING FORECAST PERIOD
5 MARKET OVERVIEW
- 5.1 INTRODUCTION
- 5.2 MARKET DYNAMICS
- FIGURE 19 CYSTATIN C ASSAY MARKET: DRIVERS, RESTRAINTS, OPPORTUNITIES, AND CHALLENGES
- 5.2.1 DRIVERS
- 5.2.1.1 Rising prevalence of kidney diseases
- 5.2.1.2 Growing geriatric population
- TABLE 2 GERIATRIC POPULATION, BY REGION, 2015 VS. 2030 VS. 2050 (MILLION)
- TABLE 3 ESTIMATED INCREASE IN GERIATRIC POPULATION, BY REGION (2019-2050)
- 5.2.1.3 Recent advancements in chemiluminescence immunoassay technologies
- 5.2.1.4 Growth in biotechnology and biopharmaceutical industries
- 5.2.1.5 Increasing adoption of POC testing
- TABLE 4 POC ANALYSIS DEVICES
- 5.2.1.6 Supportive government policies
- 5.2.2 RESTRAINTS
- 5.2.2.1 Stringent requirements for approval of cystatin C assay instruments and consumables
- 5.2.2.2 High development costs of cystatin C assays
- TABLE 5 CYSTATIN C CONSUMABLES AND INSTRUMENTS
- 5.2.3 OPPORTUNITIES
- 5.2.3.1 Growth opportunities in emerging economies
- 5.2.3.2 Importance of companion diagnostics
- TABLE 6 APPROVED AND LAUNCHED COMPANION DIAGNOSTIC ASSAYS
- 5.2.3.3 Development of condition-specific biomarkers and tests
- 5.2.4 CHALLENGES
- 5.2.4.1 Dearth of skilled professionals
- 5.3 REGULATORY OVERVIEW
- 5.3.1 US
- FIGURE 20 US: REGULATORY PROCESS FOR IVD DEVICES
- 5.3.2 CANADA
- FIGURE 21 CANADA: REGULATORY PROCESS FOR IVD DEVICES IN CANADA
- 5.3.3 EUROPE
- TABLE 7 EUROPE: CLASSIFICATION OF IVD DEVICES
- 5.3.4 JAPAN
- 5.3.4.1 Japan: Regulatory process for IVD devices
- TABLE 8 JAPAN: TIME, COST, AND COMPLEXITY OF REGISTRATION PROCESS
- 5.3.5 CHINA
- TABLE 9 CHINA: TIME, COST, AND COMPLEXITY OF REGISTRATION PROCESS
- 5.3.6 INDIA
- 5.3.6.1 India: Regulatory process for IVD devices
- 5.3.7 INDONESIA
- TABLE 10 INDONESIA: REGISTRATION PROCESS FOR IVD DEVICES
- 5.3.8 RUSSIA
- TABLE 11 RUSSIA: CLASSIFICATION OF IVD DEVICES
- 5.3.9 SAUDI ARABIA
- TABLE 12 SAUDI ARABIA: TIME, COST, AND COMPLEXITY OF REGISTRATION PROCESS
- 5.3.10 MEXICO
- 5.3.10.1 Mexico: Regulatory process for IVD devices
- TABLE 13 MEXICO: TIME, COST, AND COMPLEXITY OF REGISTRATION PROCESS
- 5.3.11 BRAZIL
- 5.3.11.1 Brazil: Regulatory process for IVD devices
- 5.3.12 SOUTH KOREA
- TABLE 14 SOUTH KOREA: TIME, COST, AND COMPLEXITY OF REGISTRATION PROCESS
- 5.3.13 MIDDLE EAST
- 5.3.14 AFRICA
- 5.4 TECHNOLOGY ANALYSIS
- TABLE 15 NON-GFR DETERMINANTS OF SERUM CREATININE AND CYSTATIN C
- TABLE 16 RECENT PRODUCT LAUNCHES WITH ADVANCED TECHNOLOGIES IN CYSTATIN C ASSAY MARKET
- 5.5 TRADE ANALYSIS
- 5.5.1 TRADE ANALYSIS FOR IMMUNOASSAYS
- TABLE 17 IMPORT DATA FOR HS CODE 902750, BY COUNTRY, 2018-2022 (USD THOUSAND)
- TABLE 18 EXPORT DATA FOR HS CODE 902750, BY COUNTRY, 2018-2022 (USD THOUSAND)
- 5.6 PATENT ANALYSIS
- 5.7 VALUE CHAIN ANALYSIS
- FIGURE 22 VALUE CHAIN ANALYSIS: MAJOR VALUE ADDED DURING MANUFACTURING AND ASSEMBLY PHASES
- 5.8 SUPPLY CHAIN ANALYSIS
- FIGURE 23 DIRECT DISTRIBUTION-PREFERRED STRATEGY FOR PROMINENT COMPANIES
- 5.9 ECOSYSTEM ANALYSIS OF CYSTATIN C ASSAY MARKET
- FIGURE 24 ECOSYSTEM ANALYSIS OF CYSTATIN C ASSAY MARKET
- 5.9.1 ROLE OF COMPANIES IN ECOSYSTEM
- 5.9.2 KEY PLAYERS OPERATING IN CYSTATIN C ASSAY MARKET
- 5.10 PORTER'S FIVE FORCES ANALYSIS
- TABLE 19 IMPACT OF PORTER'S FIVE FORCES ON CYSTATIN C ASSAY MARKET
- 5.10.1 INTENSITY OF COMPETITIVE RIVALRY
- 5.10.2 BARGAINING POWER OF SUPPLIERS
- 5.10.3 BARGAINING POWER OF BUYERS
- 5.10.4 THREAT OF SUBSTITUTES
- 5.10.5 THREAT OF NEW ENTRANTS
- 5.11 KEY CONFERENCES & EVENTS (2023-2024)
- TABLE 20 LIST OF CONFERENCES AND EVENTS (2023-2024)
- 5.12 PRICING ANALYSIS
- TABLE 21 CYSTATIN C ASSAY: PRICE RANGE FOR CYSTATIN C ASSAY PRODUCTS
- TABLE 22 AVERAGE SELLING PRICE OF PRODUCTS OFFERED BY KEY PLAYERS (2022)
- 5.13 KEY STAKEHOLDERS & BUYING CRITERIA
- 5.13.1 KEY STAKEHOLDERS IN BUYING PROCESS
- FIGURE 25 INFLUENCE OF STAKEHOLDERS ON BUYING PROCESS, BY END USER
- TABLE 23 INFLUENCE OF STAKEHOLDERS ON BUYING PROCESS FOR END USERS
- 5.13.2 BUYING CRITERIA
- FIGURE 26 KEY BUYING CRITERIA FOR TOP END USERS
- TABLE 24 KEY BUYING CRITERIA, BY END USER
- 5.14 TRENDS/DISRUPTIONS IMPACTING CUSTOMER BUSINESS
6 CYSTATIN C ASSAY MARKET, BY PRODUCT
- 6.1 INTRODUCTION
- TABLE 25 CYSTATIN C ASSAY MARKET, BY PRODUCT, 2021-2028 (USD MILLION)
- 6.2 KITS
- 6.2.1 WIDE USE OF KITS IN CLINICAL LABORATORIES, HEALTHCARE SETTINGS, AND RESEARCH INSTITUTIONS TO DRIVE MARKET
- TABLE 26 CYSTATIN C ASSAY KITS AVAILABLE IN MARKET
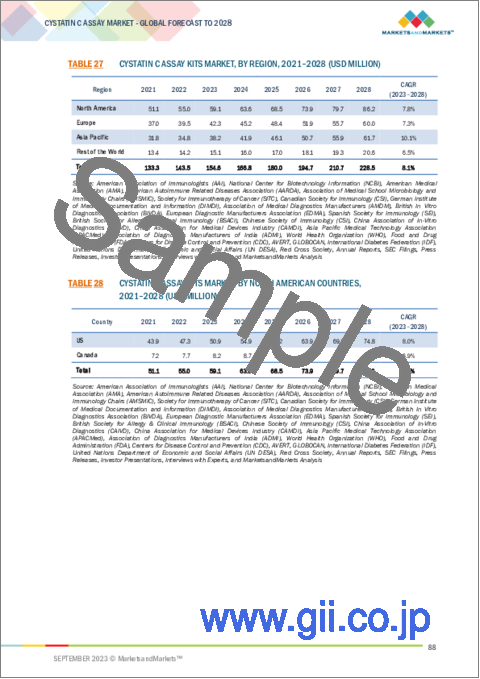
- TABLE 27 CYSTATIN C ASSAY KITS MARKET, BY REGION, 2021-2028 (USD MILLION)
- TABLE 28 CYSTATIN C ASSAY KITS MARKET, BY NORTH AMERICAN COUNTRIES, 2021-2028 (USD MILLION)
- TABLE 29 CYSTATIN C ASSAY KITS MARKET, BY EUROPEAN COUNTRIES, 2021-2028 (USD MILLION)
- TABLE 30 CYSTATIN C ASSAY KITS MARKET, BY ASIA PACIFIC COUNTRIES, 2021-2028 (USD MILLION)
- 6.2.2 REAGENTS
- 6.2.2.1 Wide use in diagnostic, research, and high-throughput screening to drive market
- TABLE 31 CYSTATIN C ASSAY REAGENTS AVAILABLE IN MARKET
- TABLE 32 CYSTATIN C REAGENTS MARKET, BY REGION, 2021-2028 (USD MILLION)
- TABLE 33 CYSTATIN C ASSAY REAGENTS MARKET, BY NORTH AMERICAN COUNTRIES, 2021-2028 (USD MILLION)
- TABLE 34 CYSTATIN C ASSAY REAGENTS MARKET, BY EUROPEAN COUNTRIES, 2021-2028 (USD MILLION)
- TABLE 35 CYSTATIN C ASSAY REAGENTS MARKET, BY ASIA PACIFIC COUNTRIES, 2021-2028 (USD MILLION)
- 6.3 ANALYZERS
- 6.3.1 INCREASING NUMBER OF HOSPITALS AND CLINICAL LABORATORIES TO DRIVE MARKET
- TABLE 36 CYSTATIN C ASSAY ANALYZERS AVAILABLE IN MARKET
- TABLE 37 CYSTATIN C ASSAY ANALYZERS MARKET, BY REGION, 2021-2028 (USD MILLION)
- TABLE 38 CYSTATIN C ASSAY ANALYZERS MARKET, BY NORTH AMERICAN COUNTRIES, 2021-2028 (USD MILLION)
- TABLE 39 CYSTATIN C ASSAY ANALYZERS MARKET, BY EUROPEAN COUNTRIES, 2021-2028 (USD MILLION)
- TABLE 40 CYSTATIN C ASSAY ANALYZERS MARKET, BY ASIA PACIFIC COUNTRIES, 2021-2028 (USD MILLION)
7 CYSTATIN C ASSAY MARKET, BY METHOD
- 7.1 INTRODUCTION
- TABLE 41 CYSTATIN C ASSAY MARKET, BY METHOD, 2021-2028 (USD MILLION)
- 7.2 ENZYME-LINKED IMMUNOSORBENT ASSAY
- 7.2.1 WIDE ROLE IN DIAGNOSIS, PROGNOSIS, AND MONITORING OF VARIOUS RENAL DISEASES TO DRIVE MARKET
- TABLE 42 ENZYME-LINKED IMMUNOSORBENT ASSAY: CYSTATIN C ASSAY MARKET, BY REGION, 2021-2028 (USD MILLION)
- TABLE 43 ENZYME-LINKED IMMUNOSORBENT ASSAY: CYSTATIN C ASSAY MARKET, BY NORTH AMERICAN COUNTRIES, 2021-2028 (USD MILLION)
- TABLE 44 ENZYME-LINKED IMMUNOSORBENT ASSAY: CYSTATIN C ASSAY MARKET, BY EUROPEAN COUNTRIES, 2021-2028 (USD MILLION)
- TABLE 45 ENZYME-LINKED IMMUNOSORBENT ASSAY: CYSTATIN C ASSAY MARKET, BY ASIA PACIFIC COUNTRIES, 2021-2028 (USD MILLION)
- 7.3 PARTICLE-ENHANCED TURBIDIMETRIC IMMUNOASSAY
- 7.3.1 APPLICATION OF PETIA TECHNOLOGY IN ALL CLINICAL ANALYZERS TO DRIVE MARKET
- TABLE 46 PARTICLE-ENHANCED TURBIDIMETRIC IMMUNOASSAY: CYSTATIN C ASSAY MARKET, BY REGION, 2021-2028 (USD MILLION)
- TABLE 47 PARTICLE-ENHANCED TURBIDIMETRIC IMMUNOASSAY: CYSTATIN C ASSAY MARKET, BY NORTH AMERICAN COUNTRIES, 2021-2028 (USD MILLION)
- TABLE 48 PARTICLE-ENHANCED TURBIDIMETRIC IMMUNOASSAY: CYSTATIN C ASSAY MARKET, BY EUROPEAN COUNTRIES, 2021-2028 (USD MILLION)
- TABLE 49 PARTICLE-ENHANCED TURBIDIMETRIC IMMUNOASSAY: CYSTATIN C ASSAY MARKET, BY ASIA PACIFIC COUNTRIES, 2021-2028 (USD MILLION)
- 7.4 IMMUNOFLUORESCENCE ASSAY
- 7.4.1 WIDE USE OF IMMUNOFLUORESCENCE ASSAY IN DIAGNOSIS OF ANTIBODIES IMMUNOLOGY AND CELL BIOLOGY RESEARCH TO DRIVE MARKET
- TABLE 50 IMMUNOFLUORESCENCE ASSAY: CYSTATIN C ASSAY MARKET, BY REGION, 2021-2028 (USD MILLION)
- TABLE 51 IMMUNOFLUORESCENCE ASSAY: CYSTATIN C ASSAY MARKET, BY NORTH AMERICAN COUNTRIES, 2021-2028 (USD MILLION)
- TABLE 52 IMMUNOFLUORESCENCE ASSAY: CYSTATIN C ASSAY MARKET, BY EUROPEAN COUNTRIES, 2021-2028 (USD MILLION)
- TABLE 53 IMMUNOFLUORESCENCE ASSAY: CYSTATIN C ASSAY MARKET, BY ASIA PACIFIC COUNTRIES, 2021-2028 (USD MILLION)
- 7.5 CHEMILUMINESCENT IMMUNOASSAY
- 7.5.1 ACCESSIBILITY TO MANY CLINICAL LABORATORIES TO INCREASE DEMAND
- TABLE 54 CHEMILUMINESCENT IMMUNOASSAY: CYSTATIN C ASSAY MARKET, BY REGION, 2021-2028 (USD MILLION)
- TABLE 55 CHEMILUMINESCENT IMMUNOASSAY: CYSTATIN C ASSAY MARKET, BY NORTH AMERICAN COUNTRIES, 2021-2028 (USD MILLION)
- TABLE 56 CHEMILUMINESCENT IMMUNOASSAY: CYSTATIN C ASSAY MARKET, BY EUROPEAN COUNTRIES, 2021-2028 (USD MILLION)
- TABLE 57 CHEMILUMINESCENT IMMUNOASSAY: CYSTATIN C ASSAY MARKET, BY ASIA PACIFIC COUNTRIES, 2021-2028 (USD MILLION)
- 7.6 PARTICLE-ENHANCED NEPHELOMETRIC IMMUNOASSAY
- 7.6.1 PRECISION AND AUTOMATION CAPABILITIES IN CLINICAL LABORATORIES TO DRIVE MARKET
- TABLE 58 PARTICLE-ENHANCED NEPHELOMETRIC IMMUNOASSAY: CYSTATIN C ASSAY MARKET, BY REGION, 2021-2028 (USD MILLION)
- TABLE 59 PARTICLE-ENHANCED NEPHELOMETRIC IMMUNOASSAY: CYSTATIN C ASSAY MARKET, BY NORTH AMERICAN COUNTRIES, 2021-2028 (USD MILLION)
- TABLE 60 PARTICLE-ENHANCED NEPHELOMETRIC IMMUNOASSAY: CYSTATIN C ASSAY MARKET, BY EUROPEAN COUNTRIES, 2021-2028 (USD MILLION)
- TABLE 61 PARTICLE-ENHANCED NEPHELOMETRIC IMMUNOASSAY: CYSTATIN C ASSAY MARKET, BY ASIA PACIFIC COUNTRIES, 2021-2028 (USD MILLION)
- 7.7 OTHER METHODS
- TABLE 62 OTHER METHODS: CYSTATIN C ASSAY MARKET, BY REGION, 2021-2028 (USD MILLION)
- TABLE 63 OTHER METHODS: CYSTATIN C ASSAY MARKET, BY NORTH AMERICAN COUNTRIES, 2021-2028 (USD MILLION)
- TABLE 64 OTHER METHODS: CYSTATIN C ASSAY MARKET, BY EUROPEAN COUNTRIES, 2021-2028 (USD MILLION)
- TABLE 65 OTHER METHODS: CYSTATIN C ASSAY MARKET, BY ASIA PACIFIC COUNTRIES, 2021-2028 (USD MILLION)
8 CYSTATIN C ASSAY MARKET, BY SAMPLE TYPE
- 8.1 INTRODUCTION
- TABLE 66 CYSTATIN C ASSAY MARKET, BY SAMPLE TYPE, 2021-2028 (USD MILLION)
- 8.2 BLOOD
- 8.2.1 RISING PREVALENCE OF CHRONIC CONDITIONS TO DRIVE MARKET
- TABLE 67 BLOOD: CYSTATIN C ASSAY MARKET, BY REGION, 2021-2028 (USD MILLION)
- TABLE 68 BLOOD: CYSTATIN C ASSAY MARKET, BY NORTH AMERICAN COUNTRIES, 2021-2028 (USD MILLION)
- TABLE 69 BLOOD: CYSTATIN C ASSAY MARKET, BY EUROPEAN COUNTRIES, 2021-2028 (USD MILLION)
- TABLE 70 BLOOD: CYSTATIN C ASSAY MARKET, BY ASIA PACIFIC COUNTRIES, 2021-2028 (USD MILLION)
- 8.3 URINE
- 8.3.1 RESEARCH APPLICATION OF URINE CYSTATIN C IN CLINICAL INVESTIGATION TO DRIVE MARKET
- TABLE 71 URINE: CYSTATIN C ASSAY MARKET, BY REGION, 2021-2028 (USD MILLION)
- TABLE 72 URINE: CYSTATIN C ASSAY MARKET, BY NORTH AMERICAN COUNTRIES, 2021-2028 (USD MILLION)
- TABLE 73 URINE: CYSTATIN C ASSAY MARKET, BY EUROPEAN COUNTRIES, 2021-2028 (USD MILLION)
- TABLE 74 URINE: CYSTATIN C ASSAY MARKET, BY ASIA PACIFIC COUNTRIES, 2021-2028 (USD MILLION)
9 CYSTATIN C ASSAY MARKET, BY APPLICATION
- 9.1 INTRODUCTION
- TABLE 75 CYSTATIN C ASSAY MARKET, BY APPLICATION, 2021-2028 (USD MILLION)
- 9.2 DIAGNOSTICS
- 9.2.1 WIDE USE OF CYSTATIN C ASSAYS IN DIAGNOSTICS TO DRIVE MARKET
- TABLE 76 DIAGNOSTICS: CYSTATIN C ASSAY MARKET, BY REGION, 2021-2028 (USD MILLION)
- TABLE 77 DIAGNOSTICS: CYSTATIN C ASSAY MARKET, BY NORTH AMERICAN COUNTRIES, 2021-2028 (USD MILLION)
- TABLE 78 DIAGNOSTICS: CYSTATIN C ASSAY MARKET, BY EUROPEAN COUNTRIES, 2021-2028 (USD MILLION)
- TABLE 79 DIAGNOSTICS: CYSTATIN C ASSAY MARKET, BY ASIA PACIFIC COUNTRIES, 2021-2028 (USD MILLION)
- 9.3 RESEARCH
- 9.3.1 USE OF CYSTATIN C ASSAYS IN SCIENTIFIC STUDIES, CLINICAL TRIALS, AND BIOMARKER RESEARCH TO BOOST MARKET
- TABLE 80 RESEARCH: CYSTATIN C ASSAY MARKET, BY REGION, 2021-2028 (USD MILLION)
- TABLE 81 RESEARCH: CYSTATIN C ASSAY MARKET, BY NORTH AMERICAN COUNTRIES, 2021-2028 (USD MILLION)
- TABLE 82 RESEARCH: CYSTATIN C ASSAY MARKET, BY EUROPEAN COUNTRIES, 2021-2028 (USD MILLION)
- TABLE 83 RESEARCH: CYSTATIN C ASSAY MARKET, BY ASIA PACIFIC COUNTRIES, 2021-2028 (USD MILLION)
10 CYSTATIN C ASSAY MARKET, BY END USER
- 10.1 INTRODUCTION
- TABLE 85 CYSTATIN C ASSAY MARKET, BY END USER, 2021-2028 (USD MILLION)
- 10.2 HOSPITALS
- 10.2.1 GROWING PATIENT POPULATION TO DRIVE MARKET
- TABLE 86 CYSTATIN C ASSAY MARKET FOR HOSPITALS, BY REGION, 2021-2028 (USD MILLION)
- TABLE 87 CYSTATIN C ASSAY MARKET FOR HOSPITALS, BY NORTH AMERICAN COUNTRIES, 2021-2028 (USD MILLION)
- TABLE 88 CYSTATIN C ASSAY MARKET FOR HOSPITALS, BY EUROPEAN COUNTRIES, 2021-2028 (USD MILLION)
- TABLE 89 CYSTATIN C ASSAY MARKET FOR HOSPITALS, BY ASIA PACIFIC COUNTRIES, 2021-2028 (USD MILLION)
- 10.3 CLINICAL LABORATORIES
- 10.3.1 RISING CLINICAL TEST VOLUMES AND REQUIREMENTS TO BOOST USE OF CYSTATIN C ASSAYS
- TABLE 90 CYSTATIN C ASSAY MARKET FOR CLINICAL LABORATORIES, BY REGION, 2021-2028 (USD MILLION)
- TABLE 91 CYSTATIN C ASSAY MARKET FOR CLINICAL LABORATORIES, BY NORTH AMERICAN COUNTRIES, 2021-2028 (USD MILLION)
- TABLE 92 CYSTATIN C ASSAY MARKET FOR CLINICAL LABORATORIES, BY EUROPEAN COUNTRIES, 2021-2028 (USD MILLION)
- TABLE 93 CYSTATIN C ASSAY MARKET FOR CLINICAL LABORATORIES, BY ASIA PACIFIC COUNTRIES, 2021-2028 (USD MILLION)
- 10.4 PHARMACEUTICAL & BIOTECHNOLOGY COMPANIES, CONTRACT RESEARCH ORGANIZATIONS, AND ACADEMIC RESEARCH INSTITUTES
- 10.4.1 GROWING DRUG DISCOVERY AND CLINICAL STUDY AND RISING NUMBER OF COLLEGES & UNIVERSITIES TO DRIVE DEMAND
- TABLE 94 CYSTATIN C ASSAY MARKET FOR PHARMACEUTICAL & BIOTECHNOLOGY COMPANIES, CROS, AND ACADEMIC RESEARCH INSTITUTES, BY REGION, 2021-2028 (USD MILLION)
- TABLE 95 CYSTATIN C ASSAY MARKET FOR PHARMACEUTICAL & BIOTECHNOLOGY COMPANIES, CROS, AND ACADEMIC RESEARCH INSTITUTES, BY NORTH AMERICAN COUNTRIES, 2021-2028 (USD MILLION)
- TABLE 96 CYSTATIN C ASSAY MARKET FOR PHARMACEUTICAL & BIOTECHNOLOGY COMPANIES, CROS, AND ACADEMIC RESEARCH INSTITUTES, BY EUROPEAN COUNTRIES, 2021-2028 (USD MILLION)
- TABLE 97 CYSTATIN C ASSAY MARKET FOR PHARMACEUTICAL & BIOTECHNOLOGY COMPANIES, CROS, AND ACADEMIC RESEARCH INSTITUTES, BY ASIA PACIFIC COUNTRIES, 2021-2028 (USD MILLION)
11 CYSTATIN C ASSAY MARKET, BY REGION
- 11.1 INTRODUCTION
- TABLE 98 CYSTATIN C ASSAY MARKET, BY REGION, 2021-2028 (USD MILLION)
- 11.2 NORTH AMERICA
- FIGURE 27 NORTH AMERICA: CYSTATIN C ASSAY MARKET SNAPSHOT
- TABLE 99 NORTH AMERICA: CYSTATIN C ASSAY MARKET, BY COUNTRY, 2021-2028 (USD MILLION)
- TABLE 100 NORTH AMERICA: CYSTATIN C ASSAY MARKET, BY PRODUCT, 2021-2028 (USD MILLION)
- TABLE 101 NORTH AMERICA: CYSTATIN C ASSAY MARKET, BY METHOD, 2021-2028 (USD MILLION)
- TABLE 102 NORTH AMERICA: CYSTATIN C ASSAY MARKET, BY APPLICATION, 2021-2028 (USD MILLION)
- TABLE 103 NORTH AMERICA: CYSTATIN C ASSAY MARKET, BY SAMPLE TYPE, 2021-2028 (USD MILLION)
- TABLE 104 NORTH AMERICA: CYSTATIN C ASSAY MARKET, BY END USER, 2021-2028 (USD MILLION)
- 11.2.1 RECESSION IMPACT ON NORTH AMERICA
- 11.2.2 US
- 11.2.2.1 US to account for largest market share in North America
- TABLE 105 US: KEY MACRO INDICATORS
- TABLE 106 US: CYSTATIN C ASSAY MARKET, BY PRODUCT, 2021-2028 (USD MILLION)
- TABLE 107 US: CYSTATIN C ASSAY MARKET, BY METHOD, 2021-2028 (USD MILLION)
- TABLE 108 US: CYSTATIN C ASSAY MARKET, BY APPLICATION, 2021-2028 (USD MILLION)
- TABLE 109 US: CYSTATIN C ASSAY MARKET, BY SAMPLE TYPE, 2021-2028 (USD MILLION)
- TABLE 110 US: CYSTATIN C ASSAY MARKET, BY END USER, 2021-2028 (USD MILLION)
- 11.2.3 CANADA
- 11.2.3.1 Government initiatives to support market growth
- TABLE 111 CANADA: KEY MACRO INDICATORS
- TABLE 112 CANADA: CYSTATIN C ASSAY MARKET, BY PRODUCT, 2021-2028 (USD MILLION)
- TABLE 113 CANADA: CYSTATIN C ASSAY MARKET, BY METHOD, 2021-2028 (USD MILLION)
- TABLE 114 CANADA: CYSTATIN C ASSAY MARKET, BY APPLICATION, 2021-2028 (USD MILLION)
- TABLE 115 CANADA: CYSTATIN C ASSAY MARKET, BY SAMPLE TYPE, 2021-2028 (USD MILLION)
- TABLE 116 CANADA: CYSTATIN C ASSAY MARKET, BY END USER, 2021-2028 (USD MILLION)
- 11.3 EUROPE
- TABLE 117 EUROPE: HEALTHCARE EXPENDITURE, BY COUNTRY (% OF GDP)
- 11.3.1 RECESSION IMPACT ON EUROPE
- TABLE 118 EUROPE: CYSTATIN C ASSAY MARKET, BY COUNTRY, 2021-2028 (USD MILLION)
- TABLE 119 EUROPE: CYSTATIN C ASSAY MARKET, BY PRODUCT, 2021-2028 (USD MILLION)
- TABLE 120 EUROPE: CYSTATIN C ASSAY MARKET, BY METHOD, 2021-2028 (USD MILLION)
- TABLE 121 EUROPE: CYSTATIN C ASSAY MARKET, BY APPLICATION, 2021-2028 (USD MILLION)
- TABLE 122 EUROPE: CYSTATIN C ASSAY MARKET, BY SAMPLE TYPE, 2021-2028 (USD MILLION)
- TABLE 123 EUROPE: CYSTATIN C ASSAY MARKET, BY END USER, 2021-2028 (USD MILLION)
- 11.3.2 GERMANY
- 11.3.2.1 High healthcare spending to drive market
- TABLE 124 GERMANY: KEY MACRO INDICATORS
- TABLE 125 GERMANY: CYSTATIN C ASSAY MARKET, BY PRODUCT, 2021-2028 (USD MILLION)
- TABLE 126 GERMANY: CYSTATIN C ASSAY MARKET, BY METHOD, 2021-2028 (USD MILLION)
- TABLE 127 GERMANY: CYSTATIN C ASSAY MARKET, BY APPLICATION, 2021-2028 (USD MILLION)
- TABLE 128 GERMANY: CYSTATIN C ASSAY MARKET, BY SAMPLE TYPE, 2021-2028 (USD MILLION)
- TABLE 129 GERMANY: CYSTATIN C ASSAY MARKET, BY END USER, 2021-2028 (USD MILLION)
- 11.3.3 FRANCE
- 11.3.3.1 Presence of favorable reimbursement policies to make market lucrative
- TABLE 130 FRANCE: KEY MACRO INDICATORS
- TABLE 131 FRANCE: CYSTATIN C ASSAY MARKET, BY PRODUCT, 2021-2028 (USD MILLION)
- TABLE 132 FRANCE: CYSTATIN C ASSAY MARKET, BY METHOD, 2021-2028 (USD MILLION)
- TABLE 133 FRANCE: CYSTATIN C ASSAY MARKET, BY APPLICATION, 2021-2028 (USD MILLION)
- TABLE 134 FRANCE: CYSTATIN C ASSAY MARKET, BY SAMPLE TYPE, 2021-2028 (USD MILLION)
- TABLE 135 FRANCE: CYSTATIN C ASSAY MARKET, BY END USER, 2021-2028 (USD MILLION)
- 11.3.4 UK
- 11.3.4.1 Government support for disease diagnostics and favorable investment scenario to drive market
- TABLE 136 UK: CYSTATIN C ASSAY MARKET, BY PRODUCT, 2021-2028 (USD MILLION)
- TABLE 137 UK: CYSTATIN C ASSAY MARKET, BY METHOD, 2021-2028 (USD MILLION)
- TABLE 138 UK: CYSTATIN C ASSAY MARKET, BY APPLICATION, 2021-2028 (USD MILLION)
- TABLE 139 UK: CYSTATIN C ASSAY MARKET, BY SAMPLE TYPE, 2021-2028 (USD MILLION)
- TABLE 140 UK: CYSTATIN C ASSAY MARKET, BY END USER, 2021-2028 (USD MILLION)
- 11.3.5 ITALY
- 11.3.5.1 Growing geriatric population and increasing support for research to boost market growth
- TABLE 141 ITALY: CYSTATIN C ASSAY MARKET, BY PRODUCT, 2021-2028 (USD MILLION)
- TABLE 142 ITALY: CYSTATIN C ASSAY MARKET, BY METHOD, 2021-2028 (USD MILLION)
- TABLE 143 ITALY: CYSTATIN C ASSAY MARKET, BY APPLICATION, 2021-2028 (USD MILLION)
- TABLE 144 ITALY: CYSTATIN C ASSAY MARKET, BY SAMPLE TYPE, 2021-2028 (USD MILLION)
- TABLE 145 ITALY: CYSTATIN C ASSAY MARKET, BY END USER, 2021-2028 (USD MILLION)
- 11.3.6 SPAIN
- 11.3.6.1 Presence of well-established network of research centers, universities, and hospitals to drive market
- TABLE 146 SPAIN: CYSTATIN C ASSAY MARKET, BY PRODUCT, 2021-2028 (USD MILLION)
- TABLE 147 SPAIN: CYSTATIN C ASSAY MARKET, BY METHOD, 2021-2028 (USD MILLION)
- TABLE 148 SPAIN: CYSTATIN C ASSAY MARKET, BY APPLICATION, 2021-2028 (USD MILLION)
- TABLE 149 SPAIN: CYSTATIN C ASSAY MARKET, BY SAMPLE TYPE, 2021-2028 (USD MILLION)
- TABLE 150 SPAIN: CYSTATIN C ASSAY MARKET, BY END USER, 2021-2028 (USD MILLION)
- 11.3.7 REST OF EUROPE
- TABLE 151 REST OF EUROPE: HEALTHCARE EXPENDITURE, BY COUNTRY, 2010 VS. 2021 (% OF GDP)
- TABLE 152 REST OF EUROPE: CYSTATIN C ASSAY MARKET, BY PRODUCT, 2021-2028 (USD MILLION)
- TABLE 153 REST OF EUROPE: CYSTATIN C ASSAY MARKET, BY METHOD, 2021-2028 (USD MILLION)
- TABLE 154 REST OF EUROPE: CYSTATIN C ASSAY MARKET, BY APPLICATION, 2021-2028 (USD MILLION)
- TABLE 155 REST OF EUROPE: CYSTATIN C ASSAY MARKET, BY SAMPLE TYPE, 2021-2028 (USD MILLION)
- TABLE 156 REST OF EUROPE: CYSTATIN C ASSAY MARKET, BY END USER, 2021-2028 (USD MILLION)
- 11.4 ASIA PACIFIC
- 11.4.1 RECESSION IMPACT ON ASIA PACIFIC
- FIGURE 28 ASIA PACIFIC: CYSTATIN C ASSAY MARKET SNAPSHOT
- TABLE 157 ASIA PACIFIC: CYSTATIN C ASSAY MARKET, BY COUNTRY, 2021-2028 (USD MILLION)
- TABLE 158 ASIA PACIFIC: CYSTATIN C ASSAY MARKET, BY PRODUCT, 2021-2028 (USD MILLION)
- TABLE 159 ASIA PACIFIC: CYSTATIN C ASSAY MARKET, BY METHOD, 2021-2028 (USD MILLION)
- TABLE 160 ASIA PACIFIC: CYSTATIN C ASSAY MARKET, BY APPLICATION, 2021-2028 (USD MILLION)
- TABLE 161 ASIA PACIFIC: CYSTATIN C ASSAY MARKET, BY SAMPLE TYPE, 2021-2028 (USD MILLION)
- TABLE 162 ASIA PACIFIC: CYSTATIN C ASSAY MARKET, BY END USER, 2021-2028 (USD MILLION)
- 11.4.2 CHINA
- 11.4.2.1 High prevalence of diabetes and chronic kidney disease to drive market
- TABLE 163 CHINA: KEY MACRO INDICATORS
- TABLE 164 CHINA: CYSTATIN C ASSAY MARKET, BY PRODUCT, 2021-2028 (USD MILLION)
- TABLE 165 CHINA: CYSTATIN C ASSAY MARKET, BY METHOD, 2021-2028 (USD MILLION)
- TABLE 166 CHINA: CYSTATIN C ASSAY MARKET, BY APPLICATION, 2021-2028 (USD MILLION)
- TABLE 167 CHINA: CYSTATIN C ASSAY MARKET, BY SAMPLE TYPE, 2021-2028 (USD MILLION)
- TABLE 168 CHINA: CYSTATIN C ASSAY MARKET, BY END USER, 2021-2028 (USD MILLION)
- 11.4.3 INDIA
- 11.4.3.1 Growing medical tourism and healthcare infrastructure to drive market
- TABLE 169 INDIA: KEY MACRO INDICATORS
- TABLE 170 INDIA: CYSTATIN C ASSAY MARKET, BY PRODUCT, 2021-2028 (USD MILLION)
- TABLE 171 INDIA: CYSTATIN C ASSAY MARKET, BY METHOD, 2021-2028 (USD MILLION)
- TABLE 172 INDIA: CYSTATIN C ASSAY MARKET, BY APPLICATION, 2021-2028 (USD MILLION)
- TABLE 173 INDIA: CYSTATIN C ASSAY MARKET, BY SAMPLE TYPE, 2021-2028 (USD MILLION)
- TABLE 174 INDIA: CYSTATIN C ASSAY MARKET, BY END USER, 2021-2028 (USD MILLION)
- 11.4.4 JAPAN
- 11.4.4.1 Investments in healthcare technology and research to support growth
- TABLE 175 JAPAN: KEY MACRO INDICATORS
- TABLE 176 JAPAN: CYSTATIN C ASSAY MARKET, BY PRODUCT, 2021-2028 (USD MILLION)
- TABLE 177 JAPAN: CYSTATIN C ASSAY MARKET, BY METHOD, 2021-2028 (USD MILLION)
- TABLE 178 JAPAN: CYSTATIN C ASSAY MARKET, BY APPLICATION, 2021-2028 (USD MILLION)
- TABLE 179 JAPAN: CYSTATIN C ASSAY MARKET, BY SAMPLE TYPE, 2021-2028 (USD MILLION)
- TABLE 180 JAPAN: CYSTATIN C ASSAY MARKET, BY END USER, 2021-2028 (USD MILLION)
- 11.4.5 REST OF ASIA PACIFIC
- TABLE 181 REST OF ASIA PACIFIC: DIABETES PREVALENCE (% OF POPULATION AGED 20-79), BY COUNTRY
- TABLE 182 REST OF ASIA PACIFIC: CYSTATIN C ASSAY MARKET, BY PRODUCT, 2021-2028 (USD MILLION)
- TABLE 183 REST OF ASIA PACIFIC: CYSTATIN C ASSAY MARKET, BY METHOD, 2021-2028 (USD MILLION)
- TABLE 184 REST OF ASIA PACIFIC: CYSTATIN C ASSAY MARKET, BY APPLICATION, 2021-2028 (USD MILLION)
- TABLE 185 REST OF ASIA PACIFIC: CYSTATIN C ASSAY MARKET, BY SAMPLE TYPE, 2021-2028 (USD MILLION)
- TABLE 186 REST OF ASIA PACIFIC: CYSTATIN C ASSAY MARKET, BY END USER, 2021-2028 (USD MILLION)
- 11.5 REST OF THE WORLD
- TABLE 187 REST OF THE WORLD: POPULATION AGED 65 AND ABOVE (% OF TOTAL POPULATION)
- 11.5.1 RECESSION IMPACT ON REST OF THE WORLD
- TABLE 188 REST OF THE WORLD: CYSTATIN C ASSAY MARKET, BY PRODUCT, 2021-2028 (USD MILLION)
- TABLE 189 REST OF THE WORLD: CYSTATIN C ASSAY MARKET, BY METHOD, 2021-2028 (USD MILLION)
- TABLE 190 REST OF THE WORLD: CYSTATIN C ASSAY MARKET, BY APPLICATION, 2021-2028 (USD MILLION)
- TABLE 191 REST OF THE WORLD: CYSTATIN C ASSAY MARKET, BY SAMPLE TYPE, 2021-2028 (USD MILLION)
- TABLE 192 REST OF THE WORLD: CYSTATIN C ASSAY MARKET, BY END USER, 2021-2028 (USD MILLION)
12 COMPETITIVE LANDSCAPE
- 12.1 OVERVIEW
- 12.2 STRATEGIES ADOPTED BY KEY PLAYERS
- TABLE 193 OVERVIEW OF STRATEGIES ADOPTED BY KEY CYSTATIN C ASSAY MARKET PLAYERS
- 12.3 MARKET RANKING ANALYSIS, 2022
- FIGURE 29 CYSTATIN C ASSAY MARKET RANKING ANALYSIS, 2022
- 12.4 REVENUE SHARE ANALYSIS, 2022
- FIGURE 30 REVENUE ANALYSIS OF TOP FOUR PUBLIC MARKET PLAYERS
- 12.5 COMPANY EVALUATION MATRIX
- 12.5.1 STARS
- 12.5.2 PERVASIVE PLAYERS
- 12.5.3 PARTICIPANTS
- 12.5.4 EMERGING LEADERS
- FIGURE 31 CYSTATIN C ASSAY MARKET: COMPANY EVALUATION MATRIX (2022)
- 12.6 SMES/STARTUPS EVALUATION MATRIX
- 12.6.1 PROGRESSIVE COMPANIES
- 12.6.2 STARTING BLOCKS
- 12.6.3 RESPONSIVE COMPANIES
- 12.6.4 DYNAMIC COMPANIES
- FIGURE 32 CYSTATIN C ASSAY MARKET: COMPANY EVALUATION MATRIX FOR STARTUPS/SMES (2022)
- 12.7 COMPETITIVE BENCHMARKING
- 12.7.1 COMPANY FOOTPRINT ANALYSIS
- TABLE 194 COMPANY FOOTPRINT ANALYSIS
- TABLE 195 COMPANY PRODUCT FOOTPRINT
- TABLE 196 COMPANY REGIONAL FOOTPRINT
- TABLE 197 CYSTATIN C ASSAY MARKET: DETAILED LIST OF KEY SMES/STARTUPS
- 12.8 COMPETITIVE SCENARIO
- 12.8.1 PRODUCT LAUNCHES AND APPROVALS
- TABLE 198 PRODUCT LAUNCHES AND APPROVALS, 2022
- 12.8.2 DEALS
- TABLE 199 DEALS, 2021
13 COMPANY PROFILES
- 13.1 KEY PLAYERS
- (Business Overview, Products Offered, Recent Developments, and MnM View (Key strengths/Right to Win, Strategic Choices Made, and Weaknesses and Competitive Threats))**
- 13.1.1 ROCHE DIAGNOSTICS LIMITED
- TABLE 200 ROCHE DIAGNOSTICS LIMITED: BUSINESS OVERVIEW
- FIGURE 33 ROCHE DIAGNOSTICS LIMITED.: COMPANY SNAPSHOT
- 13.1.2 ABBOTT
- TABLE 201 ABBOTT: BUSINESS OVERVIEW
- FIGURE 34 ABBOTT: COMPANY SNAPSHOT
- 13.1.3 THERMO FISHER SCIENTIFIC INC.
- TABLE 202 THERMO FISHER SCIENTIFIC INC.: BUSINESS OVERVIEW
- FIGURE 35 THERMO FISHER SCIENTIFIC INC.: COMPANY SNAPSHOT
- 13.1.4 SIEMENS HEALTHCARE GMBH
- TABLE 203 SIEMENS HEALTHCARE GMBH: BUSINESS OVERVIEW
- FIGURE 36 SIEMENS HEALTHCARE GMBH: COMPANY SNAPSHOT
- 13.1.5 BIO-TECHNE
- TABLE 204 BIO-TECHNE: BUSINESS OVERVIEW
- FIGURE 37 BIO-TECHNE: COMPANY SNAPSHOT
- 13.1.6 AGILENT TECHNOLOGIES, INC.
- TABLE 205 AGILENT TECHNOLOGIES, INC.: BUSINESS OVERVIEW
- FIGURE 38 AGILENT TECHNOLOGIES, INC.: COMPANY SNAPSHOT
- 13.1.7 GENTIAN DIAGNOSTICS ASA
- TABLE 206 GENTIAN DIAGNOSTICS ASA: BUSINESS OVERVIEW
- FIGURE 39 GENTIAN DIAGNOSTICS ASA: COMPANY SNAPSHOT
- 13.1.8 ABCAM PLC
- TABLE 207 ABCAM PLC.: BUSINESS OVERVIEW
- FIGURE 40 ABCAM PLC.: COMPANY SNAPSHOT
- 13.1.9 GETEIN BIOTECH, INC.
- TABLE 208 GETEIN BIOTECH, INC.: BUSINESS OVERVIEW
- FIGURE 41 GETEIN BIOTECH, INC.: COMPANY SNAPSHOT
- 13.1.10 SINO BIOLOGICAL, INC.
- TABLE 209 SINO BIOLOGICAL, INC.: BUSINESS OVERVIEW
- FIGURE 42 SINO BIOLOGICAL, INC.: COMPANY SNAPSHOT
- 13.1.11 EUROLYSER DIAGNOSTICA GMBH
- TABLE 210 EUROLYSER DIAGNOSTICA GMBH: BUSINESS OVERVIEW
- 13.1.12 DIAZYME LABORATORIES, INC
- TABLE 211 DIAZYME LABORATORIES, INC.: BUSINESS OVERVIEW
- 13.1.13 KAMIYA BIOMEDICAL COMPANY
- TABLE 212 KAMIYA BIOMEDICAL COMPANY: BUSINESS OVERVIEW
- 13.1.14 RANDOX LABORATORIES LTD.
- TABLE 213 RANDOX LABORATORIES LTD..: BUSINESS OVERVIEW
- 13.1.15 DIASYS DIAGNOSTIC SYSTEMS GMBH
- TABLE 214 DIASYS DIAGNOSTIC SYSTEMS GMBH: BUSINESS OVERVIEW
- 13.1.16 CEPHAM LIFE SCIENCES
- TABLE 215 CEPHAM LIFE SCIENCES: BUSINESS OVERVIEW
- 13.2 OTHER PLAYERS
- 13.2.1 ETHOS BIOSCIENCES
- 13.2.2 IMMUNODIAGNOSTICS LIMITED
- 13.2.3 SEKISUI DIAGNOSTICS
- 13.2.4 AALTO SCIENTIFIC, LTD.
- 13.2.5 RAYBIOTECH LIFE, INC.
- 13.2.6 ARBOR ASSAYS
- 13.2.7 CUSABIO TECHNOLOGY LLC
- 13.2.8 PROTEINTECH GROUP, INC.
- 13.2.9 ZHEJIANG KANGTE BIOTECHNOLOGY CO., LTD.
- *Details on Business Overview, Products Offered, Recent Developments, and MnM View (Key strengths/Right to Win, Strategic Choices Made, and Weaknesses and Competitive Threats) might not be captured in case of unlisted companies.
14 APPENDIX
- 14.1 INSIGHTS FROM INDUSTRY EXPERTS
- 14.2 DISCUSSION GUIDE
- 14.3 KNOWLEDGESTORE: MARKETSANDMARKETS' SUBSCRIPTION PORTAL
- 14.4 CUSTOMIZATION OPTIONS
- 14.5 RELATED REPORTS
- 14.6 AUTHOR DETAILS