|
|
市場調査レポート
商品コード
1366407
抗体薬物複合体 (ADC) の世界市場 (~2028年):製品タイプ ・リンカータイプ ・ペイロードタイプ ・標的・疾患・地域別Antibody Drug Conjugates (ADC) Market by Product (Kadcyla, Enhertu, Padcev, Polivy), Linker Type (Cleavable, Non-Cleavable), Payload Type (Calicheamicin, MMAE), Target (HER2, CD30, CD22), Disease, Region - Global Forecast to 2028 |
||||||
カスタマイズ可能
|
|||||||
| 抗体薬物複合体 (ADC) の世界市場 (~2028年):製品タイプ ・リンカータイプ ・ペイロードタイプ ・標的・疾患・地域別 |
|
出版日: 2023年10月05日
発行: MarketsandMarkets
ページ情報: 英文 273 Pages
納期: 即納可能
|
- 全表示
- 概要
- 目次
世界の抗体薬物複合体 (ADC) の市場規模は、2023年の97億米ドルから、予測期間中は15.2%のCAGRで推移し、2028年には198億米ドルの規模に成長すると予測されています。
癌の有病率の上昇、臨床研究中のロバストな製品、産業連携の増加などの要因が同市場の成長を促進する見通しです。
| 調査範囲 | |
|---|---|
| 調査対象年 | 2021-2028年 |
| 基準年 | 2022年 |
| 予測期間 | 2023-2028年 |
| 単位 | 金額 (米ドル) |
| 部門別 | 製品タイプ・疾患タイプ・リンカータイプ・標的・ペイロードタイプ別 |
| 対象地域 | 北米・欧州・アジア太平洋・ラテンアメリカ・中東&アフリカ |
抗体薬物複合体 (ADC) の市場ではKadcylaの部門が圧倒的シェアを占める
製品別で見ると、Kadcylaの部門が2022年に最大のシェアを示しています。乳癌治療にKadcylaが広く使用されており、市場成長を促進すると考えられています。また、PolivyとAdcertisのさまざまな適応症に対する最近の承認も今後数年で市場をさらに押し上げる可能性が高いです。
疾患タイプ別では、乳癌の部門が2022年に最大のシェアを占める
疾患タイプ別では、乳癌の部門が最大のシェアを示しました。同部門は、乳癌の有病率の増加や乳癌に対するADCの需要の高まりなど、さまざまな要因から圧倒的なシェアを占めています。バイオシミラー医薬品の上市も市場成長にさらに好影響を与える見通しです。
アジア太平洋地域がより速いペースで成長する見通し
地域別では、アジア太平洋地域が予測期間中に高いCAGRで成長すると予測されています。中国と日本におけるADCの政府承認の増加の加え、インドでは乳癌治療用のバイオシミラーUjviraが発売され、同地域の市場成長に弾みがつくと考えられています。
当レポートでは、世界の抗体薬物複合体 (ADC) の市場を調査し、市場概要、市場影響因子および市場機会の分析、技術・特許動向、法規制環境、市場規模の推移・予測、各種区分・地域別の詳細分析、競合情勢、主要企業のプロファイルなどをまとめています。
目次
第1章 イントロダクション
第2章 調査手法
第3章 エグゼクティブサマリー
第4章 重要考察
第5章 市場概要
- 市場力学
- 促進要因
- 抑制要因
- 機会
- 課題
- パイプライン分析
- バリューチェーン分析
- エコシステム分析
- 技術分析
- 規制上の評価
- ポーターのファイブフォース分析
- 特許分析
- 顧客の事業に影響を与える動向/ディスラプション
- 価格分析
- 主要な会議とイベント
- 主要なステークホルダーと購入基準
第6章 抗体薬物複合体 (ADC) 市場:製品別
- KADCYLA
- ENHERTU
- ADCETRIS
- PADCEV
- TRODELVY
- POLIVY
- その他
第7章 抗体薬物複合体 (ADC) 市場:リンカータイプ別
- 切断可能リンカー
- 切断不可能リンカー
第8章 抗体薬物複合体 (ADC) 市場:標的タイプ別
- HER2
- CD22
- CD30
- その他
第9章 抗体薬物複合体 (ADC) 市場:ペイロードタイプ別
- モノメチルオーリスタチンE
- カリチアマイシン
- メイタンシノイド
- その他
第10章 抗体薬物複合体 (ADC) 市場:疾患タイプ別
- 乳癌
- 血液癌
- その他
第11章 抗体薬物複合体 (ADC) 市場:地域別
- 北米
- 欧州
- アジア太平洋
- ラテンアメリカ
- 中東・アフリカ
第12章 競合情勢
- 主要企業の採用戦略
- 収益シェア分析
- 市場シェア分析
- 主要企業の企業評価マトリックス
- 競合ベンチマーキング
- スタートアップ/中小企業の企業評価マトリクス
- スタートアップ/中小企業の競合ベンチマーキング
- 競合シナリオ・動向
第13章 企業プロファイル
- 主要企業
- F. HOFFMANN-LA ROCHE LTD.
- DAIICHI SANKYO COMPANY, LIMITED
- SEAGEN INC.
- GILEAD SCIENCES, INC.
- TAKEDA PHARMACEUTICAL COMPANY LIMITED
- PFIZER INC.
- ASTELLAS PHARMA INC.
- ASTRAZENECA
- ADC THERAPEUTICS SA
- IMMUNOGEN, INC.
- ZYDUS GROUP
- その他の企業
- ABBVIE INC.
- AMBRX
- LEGOCHEM BIOSCIENCES, INC.
- BYONDIS
- PROFOUNDBIO
- REMEGEN
- SUTRO BIOPHARMA, INC.
- LEPU BIOPHARMA CO., LTD.
- ZYMEWORKS INC.
- MERSANA THERAPEUTICS
- DUALITY BIOLOGICS
- LANOVA MEDICINES
- EXELIXIS, INC.
- BIONECURE THERAPEUTICS INC.
- TRIPARTITE THERAPEUTICS, INC.
第14章 付録
The global antibody drug conjugates market size is projected to reach USD 19.8 billion by 2028 from USD 9.7 billion in 2023, at a CAGR of 15.2% during the forecast period. Factors such as the rising prevalence of cancer, robust products under clinical studies, and increased industrial collaborations are likely to drive market growth. For instance, in August 2023, ImmunoGen, Inc. entered into an exclusive collaboration with Takeda Pharmaceutical Company Limited to develop and commercialize ELAHERE in Japan.
| Scope of the Report | |
|---|---|
| Years Considered for the Study | 2021-2028 |
| Base Year | 2022 |
| Forecast Period | 2023-2028 |
| Units Considered | Value (USD) billion |
| Segments | By Product, By Disease Type, By Linker type, By Target, By Payload Type |
| Regions covered | North America, Europe, Asia Pacific, Latin America, and the Middle East and Africa. |
"The Kadcyla segment held the dominant share in the antibody drug conjugates market."
Based on product, the global antibody drug conjugates market is segmented into Kadcyla, Enhertu, Adcetris, Padcev, Trodelvy, Polivy and others. The Kadcyla segment accounted for the largest share of the market in 2022. Extensive use of Kadcyla for breast cancer treatment is likely to drive the growth of
the market. Recent approvals for various disease indications for Polivy and Adcertis are further likely to uplift the market in the coming years.
"Breast cancer segment accounted for the largest share of the disease type segment in 2022."
Based on disease type, the antibody drug conjugates market is segmented into breast cancer, lung cancer and other diseases. In 2022, the breast cancer segment accounted for the largest share of the antibody drug conjugates market. The segment held the dominant share in the market owing to various factors such as the increasing prevalence of breast cancer coupled with the rising demand for ADCs for breast cancer. The launch of biosimilar is further likely to have a positive impact on the market growth.
"Asia Pacific region is likely to grow at a faster pace."
The antibody-drug conjugates market is segmented into North America, Europe, Asia Pacific, Latin America and Middle East & Africa. Asia Pacific region is anticipated to grow at a significant CAGR during the forecast period. Rising approvals from the government for ADCs in China and Japan. The launch of the biosimilar Ujvira for the treatment of breast cancer in India is likely to give momentum to the market growth in the region.
The primary interviews conducted for this report can be categorized as follows:
- By Respondent: Supply Side- 80%, and Demand Side - 20%
- By Designation (Supply Side): Managers - 45%, CXOs & Directors - 30%, Executives- 25%
- By Region: North America -40%, Europe -25%, Asia-Pacific -20%, Latin America -10%, and Middle East and Africa -5%
List of Companies Profiled in the Report:
- F. Hoffmann-La Roche Ltd (Switzerland)
- Daiichi Sankyo Company, Limited (Japan)
- Seagen Inc. (US)
- Gilead Sciences, Inc. (US)
- Takeda Pharmaceutical Company Limited (Japan)
- Pfizer Inc. (US),
- Astellas Pharma Inc (Japan)
- AstraZeneca (UK)
- ADC Therapeutics SA (Switzerland)
- ImmunoGen, Inc. (US)
- Zydus Group (India)
- Abbvie Inc. (US)
- Ambrx (US)
- LegoChem Biosciences, Inc. (South Korea)
- Byondis (Netherlands)
- ProfoundBio (China)
- RemeGen (China)
- Sutro Biopharma, Inc. (US)
- Lepu Biopharma CO., Ltd. (China)
- Zymeworks Inc. (Canada)
- Mersana Therapeutics (US)
- Duality Biologics (China)
- LaNova Medicines (China)
- Exelixis, Inc. (US)
- BiOneCure Therapeutics Inc. (US)
- Tripartite Therapeutics, Inc. (Taiwan).
Research Coverage:
This report provides a detailed picture of the antibody drug conjugates market. It aims to estimate the size and future growth potential of the market across different segments, such as product, route of administration, disease type, application, type and region. The report also includes an in-depth competitive analysis of the key market players, along with their company profiles, recent developments, and key market strategies.
Key Benefits of Buying the Report:
The report will help market leaders/new entrants by providing them with the closest approximations of the revenue numbers for the overall antibody drug conjugates market and its subsegments. It will also help stakeholders better understand the competitive landscape and gain more insights to better position their business and make suitable go-to-market strategies. This report will enable stakeholders to understand the market's pulse and provide them with information on the key market drivers, restraints, opportunities, and challenges.
The report provides insights on the following pointers:
- Analysis of key drivers (Rising incidence of cancer, Increased investment and collaborations by key market players for the development of novel ADCs, Growing number of ADCs in clinical trials, Regulatory support for ADC development ), restraints ( High manufacturing costs, Side effects associated with ADCs, High attrition rate in product development), opportunities (Adoption of combination therapies, High growth in emerging economies, Emergence of advanced ADCs) and challenges (Technical complexities) are influencing the growth of antibody drug conjugates market.
- Product Development/Innovation: Detailed insights on newly launched products of the antibody drug conjugates market.
- Market Development: Comprehensive information about lucrative markets - the report analyses the antibody drug conjugates market across varied regions.
- Market Diversification: Exhaustive information about new services, untapped geographies, recent developments, and investments in the antibody drug conjugates market.
- Competitive Assessment: In-depth assessment of market shares, growth strategies, and service offerings of leading players include F. Hoffmann-La Roche Ltd (Switzerland), Daiichi Sankyo Company, Limited (Japan), Seagen Inc. (US), Gilead Sciences, Inc. (US), Takeda Pharmaceutical Company Limited (Japan), Pfizer Inc. (US), Astellas Pharma Inc (Japan), AstraZeneca (UK), ADC Therapeutics SA (Switzerland), ImmunoGen, Inc. (US), Zydus Group (India) and among others in the antibody drug conjugates market.
TABLE OF CONTENTS
1 INTRODUCTION
- 1.1 STUDY OBJECTIVES
- 1.2 MARKET DEFINITION
- 1.2.1 INCLUSIONS AND EXCLUSIONS
- 1.3 MARKET SCOPE
- 1.3.1 MARKETS COVERED
- 1.3.2 YEARS CONSIDERED
- 1.3.3 CURRENCY CONSIDERED
- 1.4 RESEARCH LIMITATIONS
- 1.5 STAKEHOLDERS
- 1.6 RECESSION IMPACT
2 RESEARCH METHODOLOGY
- 2.1 RESEARCH DATA
- FIGURE 1 RESEARCH DESIGN
- 2.1.1 SECONDARY DATA
- 2.1.2 PRIMARY DATA
- FIGURE 2 ANTIBODY DRUG CONJUGATES MARKET: BREAKDOWN OF PRIMARIES
- 2.2 MARKET SIZE ESTIMATION
- FIGURE 3 ANTIBODY DRUG CONJUGATES MARKET: MARKET SIZE ESTIMATION FOR SUPPLY-SIDE ANALYSIS (2022)
- FIGURE 4 MARKET SIZE ESTIMATION: APPROACH 1 (REVENUE SHARE ANALYSIS)
- FIGURE 5 ANTIBODY DRUG CONJUGATES MARKET: REVENUE SHARE ANALYSIS OF F-HOFFMAN LA ROCHE LTD.
- 2.2.1 PRIMARY INSIGHTS
- FIGURE 6 MARKET VALIDATION FROM PRIMARY EXPERTS
- 2.2.2 SEGMENT ASSESSMENT METHODOLOGY
- FIGURE 7 MARKET SIZE ESTIMATION METHODOLOGY: TOP-DOWN APPROACH
- 2.3 GROWTH RATE ASSUMPTIONS
- FIGURE 8 ANTIBODY DRUG CONJUGATES MARKET: CAGR PROJECTION ANALYSIS
- FIGURE 9 ANTIBODY DRUG CONJUGATES MARKET: GROWTH ANALYSIS OF DRIVERS, RESTRAINTS, CHALLENGES, AND OPPORTUNITIES
- 2.4 MARKET BREAKDOWN AND DATA TRIANGULATION
- FIGURE 10 DATA TRIANGULATION METHODOLOGY
- 2.5 STUDY ASSUMPTIONS
- 2.6 RISK ANALYSIS
- 2.7 RECESSION IMPACT ANALYSIS
- TABLE 1 GLOBAL INFLATION RATE PROJECTION, 2024-2028 (% GROWTH)
- TABLE 2 US HEALTH EXPENDITURE, 2019-2022 (USD MILLION)
- TABLE 3 US HEALTH EXPENDITURE, 2023-2027 (USD MILLION)
3 EXECUTIVE SUMMARY
- FIGURE 11 ANTIBODY DRUG CONJUGATES MARKET, BY PRODUCT, 2023 VS. 2028 (USD MILLION)
- FIGURE 12 ANTIBODY DRUG CONJUGATES MARKET, BY DISEASE TYPE, 2023 VS. 2028 (USD MILLION)
- FIGURE 13 ANTIBODY DRUG CONJUGATES MARKET, BY LINKER TYPE, 2023 VS. 2028 (USD MILLION)
- FIGURE 14 ANTIBODY DRUG CONJUGATES MARKET, BY TARGET TYPE, 2023 VS. 2028 (USD MILLION)
- FIGURE 15 ANTIBODY DRUG CONJUGATES MARKET, BY PAYLOAD TYPE, 2023 VS. 2028 (USD MILLION)
- FIGURE 16 GEOGRAPHICAL SNAPSHOT OF ANTIBODY DRUG CONJUGATES MARKET
4 PREMIUM INSIGHTS
- 4.1 ANTIBODY DRUG CONJUGATES MARKET OVERVIEW
- FIGURE 17 RISING PREVALENCE OF CANCER TO DRIVE MARKET GROWTH DURING FORECAST PERIOD
- 4.2 NORTH AMERICA: ANTIBODY DRUG CONJUGATES MARKET, BY DISEASE TYPE AND COUNTRY (2022)
- FIGURE 18 BREAST CANCER SEGMENT ACCOUNTED FOR LARGEST SHARE OF NORTH AMERICAN ANTIBODY DRUG CONJUGATES MARKET IN 2022
- 4.3 ANTIBODY DRUG CONJUGATES MARKET SHARE, BY PRODUCT, 2023 VS. 2028
- FIGURE 19 KADCYLA SEGMENT TO DOMINATE MARKET DURING FORECAST PERIOD
- 4.4 ANTIBODY DRUG CONJUGATES: GEOGRAPHIC GROWTH OPPORTUNITIES
- FIGURE 20 ASIA PACIFIC COUNTRIES TO REGISTER HIGHER GROWTH RATES FROM 2023 TO 2028
5 MARKET OVERVIEW
- 5.1 INTRODUCTION
- 5.2 MARKET DYNAMICS
- FIGURE 21 ANTIBODY DRUG CONJUGATES MARKET: DRIVERS, RESTRAINTS, OPPORTUNITIES, AND CHALLENGES
- TABLE 4 ANTIBODY DRUG CONJUGATES MARKET: IMPACT ANALYSIS OF DRIVERS, RESTRAINTS, OPPORTUNITIES, AND CHALLENGES
- 5.2.1 DRIVERS
- 5.2.1.1 Rising incidence of cancer
- TABLE 5 GLOBAL INCREASE IN NUMBER OF CANCER PATIENTS, 2015 VS. 2018 VS. 2035
- 5.2.1.2 Increasing investments for ADC development
- 5.2.1.3 Growing number of ADCs in clinical trials
- TABLE 6 ASSET RANKING FOR LEADING ADC DEVELOPERS (2019 VS. 2023)
- 5.2.1.4 Favorable regulatory support
- 5.2.2 RESTRAINTS
- 5.2.2.1 High manufacturing costs
- 5.2.2.2 Side effects associated with ADCs
- 5.2.2.3 High attrition rate in product development
- TABLE 7 LIST OF DISCONTINUED ANTIBODY DRUG CONJUGATES (2020-2022)
- 5.2.3 OPPORTUNITIES
- 5.2.3.1 Adoption of combination therapies
- 5.2.3.2 High growth in emerging economies
- 5.2.3.3 Emergence of advanced ADCs
- 5.2.4 CHALLENGES
- 5.2.4.1 Technical complexities
- 5.3 PIPELINE ANALYSIS
- FIGURE 22 ANTIBODY DRUG CONJUGATES MARKET: CLINICAL TRIALS
- 5.4 VALUE CHAIN ANALYSIS
- FIGURE 23 ANTIBODY DRUG CONJUGATES MARKET: VALUE CHAIN ANALYSIS
- 5.5 ECOSYSTEM ANALYSIS
- 5.5.1 ROLE IN ECOSYSTEM
- 5.6 TECHNOLOGY ANALYSIS
- TABLE 8 LEADING TECHNOLOGICAL ADVANCEMENTS FOR ADC GENERATIONS
- 5.7 REGULATORY ASSESSMENT
- 5.7.1 FDA REGULATIONS ON ANTIBODY DRUG CONJUGATES
- 5.7.1.1 CLINICAL PHARMACOLOGY CONSIDERATIONS
- 5.7.2 REGULATORY BODIES, GOVERNMENT AGENCIES, AND OTHER ORGANIZATIONS
- TABLE 9 NORTH AMERICA: LIST OF REGULATORY BODIES, GOVERNMENT AGENCIES, AND OTHER ORGANIZATIONS
- TABLE 10 EUROPE: LIST OF REGULATORY BODIES, GOVERNMENT AGENCIES, AND OTHER ORGANIZATIONS
- TABLE 11 ASIA PACIFIC: LIST OF REGULATORY BODIES, GOVERNMENT AGENCIES, AND OTHER ORGANIZATIONS
- TABLE 12 LATIN AMERICA: LIST OF REGULATORY BODIES, GOVERNMENT AGENCIES, AND OTHER ORGANIZATIONS
- TABLE 13 MIDDLE EAST & AFRICA: LIST OF REGULATORY BODIES, GOVERNMENT AGENCIES, AND OTHER ORGANIZATIONS
- 5.7.1 FDA REGULATIONS ON ANTIBODY DRUG CONJUGATES
- 5.8 PORTER'S FIVE FORCES ANALYSIS
- TABLE 14 ANTIBODY DRUG CONJUGATES MARKET: PORTER'S FIVE FORCES ANALYSIS
- 5.8.1 INTENSITY OF COMPETITIVE RIVALRY
- 5.8.2 BARGAINING POWER OF SUPPLIERS
- 5.8.3 BARGAINING POWER OF BUYERS
- 5.8.4 THREAT OF SUBSTITUTES
- 5.8.5 THREAT OF NEW ENTRANTS
- 5.9 PATENT ANALYSIS
- 5.9.1 LIST OF MAJOR PATENTS
- 5.10 TRENDS/DISRUPTIONS IMPACTING CUSTOMERS' BUSINESSES
- FIGURE 24 GROWING USE OF ACDS FOR ONCOLOGY AND OTHER CHRONIC DISEASES TO DRIVE MARKET
- 5.11 PRICING ANALYSIS
- TABLE 15 ANTIBODY DRUG CONJUGATES MARKET: PRICING ANALYSIS OF ADC PRODUCTS, BY REGION
- TABLE 16 ANTIBODY DRUG CONJUGATES MARKET: PRICING ANALYSIS OF ADC PRODUCTS, BY KEY PLAYERS
- 5.12 KEY CONFERENCES AND EVENTS
- TABLE 17 ANTIBODY DRUG CONJUGATES MARKET: DETAILED LIST OF EVENTS AND CONFERENCES (2023-2024)
- 5.13 KEY STAKEHOLDERS AND BUYING CRITERIA
- FIGURE 25 KEY STAKEHOLDERS IN PHARMACEUTICAL COMPANIES AND INFLUENCE ON BUYING PROCESS
- FIGURE 26 KEY BUYING CRITERIA FOR ADC PRODUCTS AMONG END USERS
6 ANTIBODY DRUG CONJUGATES MARKET, BY PRODUCT
- 6.1 INTRODUCTION
- TABLE 18 ANTIBODY DRUG CONJUGATES MARKET, BY PRODUCT, 2021-2028 (USD MILLION)
- 6.2 KADCYLA
- 6.2.1 LAUNCH OF BIOSIMILARS TO DRIVE MARKET
- TABLE 19 ANTIBODY DRUG CONJUGATES MARKET FOR KADCYLA, BY REGION, 2021-2028 (USD MILLION)
- TABLE 20 NORTH AMERICA: ANTIBODY DRUG CONJUGATES MARKET FOR KADCYLA, BY COUNTRY, 2021-2028 (USD MILLION)
- TABLE 21 EUROPE: ANTIBODY DRUG CONJUGATES MARKET FOR KADCYLA, BY COUNTRY, 2021-2028 (USD MILLION)
- TABLE 22 ASIA PACIFIC: ANTIBODY DRUG CONJUGATES MARKET FOR KADCYLA, BY COUNTRY, 2021-2028 (USD MILLION)
- TABLE 23 LATIN AMERICA: ANTIBODY DRUG CONJUGATES MARKET FOR KADCYLA, BY COUNTRY, 2021-2028 (USD MILLION)
- 6.3 ENHERTU
- 6.3.1 RISING INCIDENCE OF BREAST CANCER TO PROPEL MARKET
- TABLE 24 ANTIBODY DRUG CONJUGATES MARKET FOR ENHERTU, BY REGION, 2021-2028 (USD MILLION)
- TABLE 25 NORTH AMERICA: ANTIBODY DRUG CONJUGATES MARKET FOR ENHERTU, BY COUNTRY, 2021-2028 (USD MILLION)
- TABLE 26 EUROPE: ANTIBODY DRUG CONJUGATES MARKET FOR ENHERTU, BY COUNTRY, 2021-2028 (USD MILLION)
- TABLE 27 ASIA PACIFIC: ANTIBODY DRUG CONJUGATES MARKET FOR ENHERTU, BY COUNTRY, 2021-2028 (USD MILLION)
- TABLE 28 LATIN AMERICA: ANTIBODY DRUG CONJUGATES MARKET FOR ENHERTU, BY COUNTRY, 2021-2028 (USD MILLION)
- 6.4 ADCETRIS
- 6.4.1 GROWING CASES OF HODGKIN LYMPHOMA TO DRIVE MARKET
- TABLE 29 ANTIBODY DRUG CONJUGATES MARKET FOR ADCETRIS, BY REGION, 2021-2028 (USD MILLION)
- TABLE 30 NORTH AMERICA: ANTIBODY DRUG CONJUGATES MARKET FOR ADCETRIS, BY COUNTRY, 2021-2028 (USD MILLION)
- TABLE 31 EUROPE: ANTIBODY DRUG CONJUGATES MARKET FOR ADCETRIS, BY COUNTRY, 2021-2028 (USD MILLION)
- TABLE 32 ASIA PACIFIC: ANTIBODY DRUG CONJUGATES MARKET FOR ADCETRIS, BY COUNTRY, 2021-2028 (USD MILLION)
- TABLE 33 LATIN AMERICA: ANTIBODY DRUG CONJUGATES MARKET FOR ADCETRIS, BY COUNTRY, 2021-2028 (USD MILLION)
- 6.5 PADCEV
- 6.5.1 RISING INCIDENCE OF UROTHELIAL CANCER TO DRIVE MARKET
- TABLE 34 ANTIBODY DRUG CONJUGATES MARKET FOR PADCEV MARKET, BY REGION, 2021-2028 (USD MILLION)
- TABLE 35 NORTH AMERICA: ANTIBODY DRUG CONJUGATES MARKET FOR PADCEV, BY COUNTRY, 2021-2028 (USD MILLION)
- TABLE 36 EUROPE: ANTIBODY DRUG CONJUGATES MARKET FOR PADCEV, BY COUNTRY, 2021-2028 (USD MILLION)
- TABLE 37 ASIA PACIFIC: ANTIBODY DRUG CONJUGATES MARKET FOR PADCEV, BY COUNTRY, 2021-2028 (USD MILLION)
- TABLE 38 LATIN AMERICA: ANTIBODY DRUG CONJUGATES MARKET FOR PADCEV, BY COUNTRY, 2021-2028 (USD MILLION)
- 6.6 TRODELVY
- 6.6.1 INCREASING GOVERNMENT APPROVALS FOR BREAST CANCER TO DRIVE MARKET
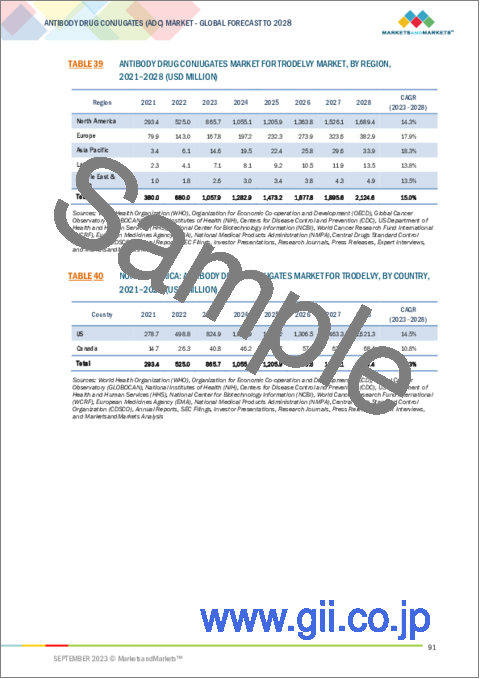
- TABLE 39 ANTIBODY DRUG CONJUGATES MARKET FOR TRODELVY MARKET, BY REGION, 2021-2028 (USD MILLION)
- TABLE 40 NORTH AMERICA: ANTIBODY DRUG CONJUGATES MARKET FOR TRODELVY, BY COUNTRY, 2021-2028 (USD MILLION)
- TABLE 41 EUROPE: ANTIBODY DRUG CONJUGATES MARKET FOR TRODELVY, BY COUNTRY, 2021-2028 (USD MILLION)
- TABLE 42 ASIA PACIFIC: ANTIBODY DRUG CONJUGATES MARKET FOR TRODELVY, BY COUNTRY, 2021-2028 (USD MILLION)
- TABLE 43 LATIN AMERICA: ANTIBODY DRUG CONJUGATES MARKET FOR TRODELVY, BY COUNTRY, 2021-2028 (USD MILLION)
- 6.7 POLIVY
- 6.7.1 RISING PREVALENCE OF NON-HODGKIN LYMPHOMA TO PROPEL MARKET
- TABLE 44 ANTIBODY DRUG CONJUGATES MARKET FOR POLIVY MARKET, BY REGION, 2021-2028 (USD MILLION)
- TABLE 45 NORTH AMERICA: ANTIBODY DRUG CONJUGATES MARKET FOR POLIVY, BY COUNTRY, 2021-2028 (USD MILLION)
- TABLE 46 EUROPE: ANTIBODY DRUG CONJUGATES MARKET FOR POLIVY, BY COUNTRY, 2021-2028 (USD MILLION)
- TABLE 47 ASIA PACIFIC: ANTIBODY DRUG CONJUGATES MARKET FOR POLIVY, BY COUNTRY, 2021-2028 (USD MILLION)
- TABLE 48 LATIN AMERICA: ANTIBODY DRUG CONJUGATES MARKET FOR POLIVY, BY COUNTRY, 2021-2028 (USD MILLION)
- 6.8 OTHER PRODUCTS
- TABLE 49 ANTIBODY DRUG CONJUGATES MARKET FOR OTHER PRODUCTS, BY REGION, 2021-2028 (USD MILLION)
- TABLE 50 NORTH AMERICA: ANTIBODY DRUG CONJUGATES MARKET FOR OTHER PRODUCTS, BY COUNTRY, 2021-2028 (USD MILLION)
- TABLE 51 EUROPE: ANTIBODY DRUG CONJUGATES MARKET FOR OTHER PRODUCTS, BY COUNTRY, 2021-2028 (USD MILLION)
- TABLE 52 ASIA PACIFIC: ANTIBODY DRUG CONJUGATES MARKET FOR OTHER PRODUCTS, BY COUNTRY, 2021-2028 (USD MILLION)
- TABLE 53 LATIN AMERICA: ANTIBODY DRUG CONJUGATES MARKET FOR OTHER PRODUCTS, BY COUNTRY, 2021-2028 (USD MILLION)
7 ANTIBODY DRUG CONJUGATES MARKET, BY LINKER TYPE
- 7.1 INTRODUCTION
- TABLE 54 ANTIBODY DRUG CONJUGATES MARKET, BY LINKER TYPE, 2021-2028 (USD MILLION)
- 7.2 CLEAVABLE LINKERS
- 7.2.1 HIGH COMPATIBILITY WITH BROAD RANGE OF DRUGS TO DRIVE MARKET
- TABLE 55 ANTIBODY DRUG CONJUGATES MARKET FOR CLEAVABLE LINKERS, BY REGION, 2021-2028 (USD MILLION)
- TABLE 56 NORTH AMERICA: ANTIBODY DRUG CONJUGATES MARKET FOR CLEAVABLE LINKERS, BY COUNTRY, 2021-2028 (USD MILLION)
- TABLE 57 EUROPE: ANTIBODY DRUG CONJUGATES MARKET FOR CLEAVABLE LINKERS, BY COUNTRY, 2021-2028 (USD MILLION)
- TABLE 58 ASIA PACIFIC: ANTIBODY DRUG CONJUGATES MARKET FOR CLEAVABLE LINKERS, BY COUNTRY, 2021-2028 (USD MILLION)
- TABLE 59 LATIN AMERICA: ANTIBODY DRUG CONJUGATES MARKET FOR CLEAVABLE LINKERS, BY COUNTRY, 2021-2028 (USD MILLION)
- 7.3 NON-CLEAVABLE LINKERS
- 7.3.1 LOW TOXICITY LEVELS TO SUPPORT MARKET GROWTH
- TABLE 60 ANTIBODY DRUG CONJUGATES MARKET FOR NON-CLEAVABLE LINKERS, BY REGION, 2021-2028 (USD MILLION)
- TABLE 61 NORTH AMERICA: ANTIBODY DRUG CONJUGATES MARKET FOR NON-CLEAVABLE LINKERS, BY COUNTRY, 2021-2028 (USD MILLION)
- TABLE 62 EUROPE: ANTIBODY DRUG CONJUGATES MARKET FOR NON-CLEAVABLE LINKERS, BY COUNTRY, 2021-2028 (USD MILLION)
- TABLE 63 ASIA PACIFIC: ANTIBODY DRUG CONJUGATES MARKET FOR NON-CLEAVABLE LINKERS, BY COUNTRY, 2021-2028 (USD MILLION)
- TABLE 64 LATIN AMERICA: ANTIBODY DRUG CONJUGATES MARKET FOR NON-CLEAVABLE LINKERS, BY COUNTRY, 2021-2028 (USD MILLION)
8 ANTIBODY DRUG CONJUGATES MARKET, BY TARGET TYPE
- 8.1 INTRODUCTION
- TABLE 65 ANTIBODY DRUG CONJUGATES MARKET, BY TARGET TYPE, 2021-2028 (USD MILLION)
- 8.2 HER2
- 8.2.1 RISING PREVALENCE OF BREAST CANCER TO DRIVE MARKET
- TABLE 66 ANTIBODY DRUG CONJUGATES MARKET FOR HER2, BY REGION, 2021-2028 (USD MILLION)
- TABLE 67 NORTH AMERICA: ANTIBODY DRUG CONJUGATES MARKET FOR HER2, BY COUNTRY, 2021-2028 (USD MILLION)
- TABLE 68 EUROPE: ANTIBODY DRUG CONJUGATES MARKET FOR HER2, BY COUNTRY, 2021-2028 (USD MILLION)
- TABLE 69 ASIA PACIFIC: ANTIBODY DRUG CONJUGATES MARKET FOR HER2, BY COUNTRY, 2021-2028 (USD MILLION)
- TABLE 70 LATIN AMERICA: ANTIBODY DRUG CONJUGATES MARKET FOR HER2, BY COUNTRY, 2021-2028 (USD MILLION)
- 8.3 CD22
- 8.3.1 INCREASING CASES OF B-CELL LYMPHOMAS TO DRIVE MARKET
- TABLE 71 ANTIBODY DRUG CONJUGATES MARKET FOR CD22, BY REGION, 2021-2028 (USD MILLION)
- TABLE 72 NORTH AMERICA: ANTIBODY DRUG CONJUGATES MARKET FOR CD22, BY COUNTRY, 2021-2028 (USD MILLION)
- TABLE 73 EUROPE: ANTIBODY DRUG CONJUGATES MARKET FOR CD22, BY COUNTRY, 2021-2028 (USD MILLION)
- TABLE 74 ASIA PACIFIC: ANTIBODY DRUG CONJUGATES MARKET FOR CD22, BY COUNTRY, 2021-2028 (USD MILLION)
- TABLE 75 LATIN AMERICA: ANTIBODY DRUG CONJUGATES MARKET FOR CD22, BY COUNTRY, 2021-2028 (USD MILLION)
- 8.4 CD30
- 8.4.1 INCREASING CLINICAL TRIALS FOR PRODUCT LAUNCHES TO SUPPORT MARKET GROWTH
- TABLE 76 ANTIBODY DRUG CONJUGATES MARKET FOR CD30, BY REGION, 2021-2028 (USD MILLION)
- TABLE 77 NORTH AMERICA: ANTIBODY DRUG CONJUGATES MARKET FOR CD30, BY COUNTRY, 2021-2028 (USD MILLION)
- TABLE 78 EUROPE: ANTIBODY DRUG CONJUGATES MARKET FOR CD30, BY COUNTRY, 2021-2028 (USD MILLION)
- TABLE 79 ASIA PACIFIC: ANTIBODY DRUG CONJUGATES MARKET FOR CD30, BY COUNTRY, 2021-2028 (USD MILLION)
- TABLE 80 LATIN AMERICA: ANTIBODY DRUG CONJUGATES MARKET FOR CD30, BY COUNTRY, 2021-2028 (USD MILLION)
- 8.5 OTHER TARGET TYPES
- TABLE 81 ANTIBODY DRUG CONJUGATES MARKET FOR OTHER TARGET TYPES, BY REGION, 2021-2028 (USD MILLION)
- TABLE 82 NORTH AMERICA: ANTIBODY DRUG CONJUGATES MARKET FOR OTHER TARGET TYPES, BY COUNTRY, 2021-2028 (USD MILLION)
- TABLE 83 EUROPE: ANTIBODY DRUG CONJUGATES MARKET FOR OTHER TARGET TYPES, BY COUNTRY, 2021-2028 (USD MILLION)
- TABLE 84 ASIA PACIFIC: ANTIBODY DRUG CONJUGATES MARKET FOR OTHER TARGET TYPES, BY COUNTRY, 2021-2028 (USD MILLION)
- TABLE 85 LATIN AMERICA: ANTIBODY DRUG CONJUGATES MARKET FOR OTHER TARGET TYPES, BY COUNTRY, 2021-2028 (USD MILLION)
9 ANTIBODY DRUG CONJUGATES MARKET, BY PAYLOAD TYPE
- 9.1 INTRODUCTION
- TABLE 86 ANTIBODY DRUG CONJUGATES MARKET, BY PAYLOAD TYPE, 2021-2028 (USD MILLION)
- 9.2 MONOMETHYL AURISTATIN E
- 9.2.1 CYTOTOXIC PAYLOAD FOR ADC DEVELOPMENT TO DRIVE MARKET
- TABLE 87 ANTIBODY DRUG CONJUGATES MARKET FOR MONOMETHYL AURISTATIN E, BY REGION, 2021-2028 (USD MILLION)
- TABLE 88 NORTH AMERICA: ANTIBODY DRUG CONJUGATES MARKET FOR MONOMETHYL AURISTATIN E, BY COUNTRY, 2021-2028 (USD MILLION)
- TABLE 89 EUROPE: ANTIBODY DRUG CONJUGATES MARKET FOR MONOMETHYL AURISTATIN E, BY COUNTRY, 2021-2028 (USD MILLION)
- TABLE 90 ASIA PACIFIC: ANTIBODY DRUG CONJUGATES MARKET FOR MONOMETHYL AURISTATIN E, BY COUNTRY, 2021-2028 (USD MILLION)
- TABLE 91 LATIN AMERICA: ANTIBODY DRUG CONJUGATES MARKET FOR MONOMETHYL AURISTATIN E, BY COUNTRY, 2021-2028 (USD MILLION)
- 9.3 CALICHEAMICIN
- 9.3.1 LAUNCH OF CALICHEAMICIN-BASED ADCS TO PROPEL MARKET
- TABLE 92 ANTIBODY DRUG CONJUGATES MARKET FOR CALICHEAMICIN, BY REGION, 2021-2028 (USD MILLION)
- TABLE 93 NORTH AMERICA: ANTIBODY DRUG CONJUGATES MARKET FOR CALICHEAMICIN, BY COUNTRY, 2021-2028 (USD MILLION)
- TABLE 94 EUROPE: ANTIBODY DRUG CONJUGATES MARKET FOR CALICHEAMICIN, BY COUNTRY, 2021-2028 (USD MILLION)
- TABLE 95 ASIA PACIFIC: ANTIBODY DRUG CONJUGATES MARKET FOR CALICHEAMICIN, BY COUNTRY, 2021-2028 (USD MILLION)
- TABLE 96 LATIN AMERICA: ANTIBODY DRUG CONJUGATES MARKET FOR CALICHEAMICIN, BY COUNTRY, 2021-2028 (USD MILLION)
- 9.4 MAYTANSINOIDS
- 9.4.1 INNOVATIVE PRODUCT PIPELINE TO SUPPORT MARKET GROWTH
- TABLE 97 ANTIBODY DRUG CONJUGATES MARKET FOR MAYTANSINOIDS, BY REGION, 2021-2028 (USD MILLION)
- TABLE 98 NORTH AMERICA: ANTIBODY DRUG CONJUGATES MARKET FOR MAYTANSINOIDS, BY COUNTRY, 2021-2028 (USD MILLION)
- TABLE 99 EUROPE: ANTIBODY DRUG CONJUGATES MARKET FOR MAYTANSINOIDS, BY COUNTRY, 2021-2028 (USD MILLION)
- TABLE 100 ASIA PACIFIC: ANTIBODY DRUG CONJUGATES MARKET FOR MAYTANSINOIDS, BY COUNTRY, 2021-2028 (USD MILLION)
- TABLE 101 LATIN AMERICA: ANTIBODY DRUG CONJUGATES MARKET FOR MAYTANSINOIDS, BY COUNTRY, 2021-2028 (USD MILLION)
- 9.5 OTHER PAYLOAD TYPES
- TABLE 102 ANTIBODY DRUG CONJUGATES MARKET FOR OTHER PAYLOAD TYPES, BY REGION, 2021-2028 (USD MILLION)
- TABLE 103 NORTH AMERICA: ANTIBODY DRUG CONJUGATES MARKET FOR OTHER PAYLOAD TYPES, BY COUNTRY, 2021-2028 (USD MILLION)
- TABLE 104 EUROPE: ANTIBODY DRUG CONJUGATES MARKET FOR OTHER PAYLOAD TYPES, BY COUNTRY, 2021-2028 (USD MILLION)
- TABLE 105 ASIA PACIFIC: ANTIBODY DRUG CONJUGATES MARKET FOR OTHER PAYLOAD TYPES, BY COUNTRY, 2021-2028 (USD MILLION)
- TABLE 106 LATIN AMERICA: ANTIBODY DRUG CONJUGATES MARKET FOR OTHER PAYLOAD TYPES, BY COUNTRY, 2021-2028 (USD MILLION)
10 ANTIBODY DRUG CONJUGATES MARKET, BY DISEASE TYPE
- 10.1 INTRODUCTION
- TABLE 107 ANTIBODY DRUG CONJUGATES MARKET, BY DISEASE TYPE, 2021-2028 (USD MILLION)
- 10.2 BREAST CANCER
- 10.2.1 RISING CASES OF INFLAMMATORY BREAST CANCER TO DRIVE MARKET
- TABLE 108 PROJECTION OF BREAST CANCER RATES
- TABLE 109 ANTIBODY DRUG CONJUGATES MARKET FOR BREAST CANCER, BY REGION, 2021-2028 (USD MILLION)
- TABLE 110 NORTH AMERICA: ANTIBODY DRUG CONJUGATES MARKET FOR BREAST CANCER, BY COUNTRY, 2021-2028 (USD MILLION)
- TABLE 111 EUROPE: ANTIBODY DRUG CONJUGATES MARKET FOR BREAST CANCER, BY COUNTRY, 2021-2028 (USD MILLION)
- TABLE 112 ASIA PACIFIC: ANTIBODY DRUG CONJUGATES MARKET FOR BREAST CANCER, BY COUNTRY, 2021-2028 (USD MILLION)
- TABLE 113 LATIN AMERICA: ANTIBODY DRUG CONJUGATES MARKET FOR BREAST CANCER, BY COUNTRY, 2021-2028 (USD MILLION)
- 10.3 BLOOD CANCER
- 10.3.1 INCREASING FOCUS ON CLINICAL TRIALS FOR ADVANCED CONJUGATES TO PROPEL MARKET
- TABLE 114 GLOBAL INCIDENCE OF BLOOD CANCER
- TABLE 115 ESTIMATED NEW BLOOD CANCER CASES IN US (2023)
- TABLE 116 ANTIBODY DRUG CONJUGATES MARKET FOR BLOOD CANCER, BY REGION, 2021-2028 (USD MILLION)
- TABLE 117 NORTH AMERICA: ANTIBODY DRUG CONJUGATES MARKET FOR BLOOD CANCER, BY COUNTRY, 2021-2028 (USD MILLION)
- TABLE 118 EUROPE: ANTIBODY DRUG CONJUGATES MARKET FOR BLOOD CANCER, BY COUNTRY, 2021-2028 (USD MILLION)
- TABLE 119 ASIA PACIFIC: ANTIBODY DRUG CONJUGATES MARKET FOR BLOOD CANCER, BY COUNTRY, 2021-2028 (USD MILLION)
- TABLE 120 LATIN AMERICA: ANTIBODY DRUG CONJUGATES MARKET FOR BLOOD CANCER, BY COUNTRY, 2021-2028 (USD MILLION)
- 10.4 OTHER DISEASE TYPES
- TABLE 121 ANTIBODY DRUG CONJUGATES MARKET FOR OTHER DISEASE TYPES, BY REGION, 2021-2028 (USD MILLION)
- TABLE 122 NORTH AMERICA: ANTIBODY DRUG CONJUGATES MARKET FOR OTHER DISEASE TYPES, BY COUNTRY, 2021-2028 (USD MILLION)
- TABLE 123 EUROPE: ANTIBODY DRUG CONJUGATES MARKET FOR OTHER DISEASE TYPES, BY COUNTRY, 2021-2028 (USD MILLION)
- TABLE 124 ASIA PACIFIC: ANTIBODY DRUG CONJUGATES MARKET FOR OTHER DISEASE TYPES, BY COUNTRY, 2021-2028 (USD MILLION)
- TABLE 125 LATIN AMERICA: ANTIBODY DRUG CONJUGATES MARKET FOR OTHER DISEASE TYPES, BY COUNTRY, 2021-2028 (USD MILLION)
11 ANTIBODY DRUG CONJUGATES MARKET, BY REGION
- 11.1 INTRODUCTION
- TABLE 126 ANTIBODY DRUG CONJUGATES MARKET, BY REGION, 2021-2028 (USD MILLION)
- 11.2 NORTH AMERICA
- FIGURE 27 NORTH AMERICA: ANTIBODY DRUG CONJUGATES MARKET SNAPSHOT
- TABLE 127 NORTH AMERICA: ANTIBODY DRUG CONJUGATES MARKET, BY COUNTRY, 2021-2028 (USD MILLION)
- TABLE 128 NORTH AMERICA: ANTIBODY DRUG CONJUGATES MARKET, BY PRODUCT, 2021-2028 (USD MILLION)
- TABLE 129 NORTH AMERICA: ANTIBODY DRUG CONJUGATES MARKET, BY LINKER TYPE, 2021-2028 (USD MILLION)
- TABLE 130 NORTH AMERICA: ANTIBODY DRUG CONJUGATES MARKET, BY PAYLOAD TYPE, 2021-2028 (USD MILLION)
- TABLE 131 NORTH AMERICA: ANTIBODY DRUG CONJUGATES MARKET, BY TARGET TYPE, 2021-2028 (USD MILLION)
- TABLE 132 NORTH AMERICA: ANTIBODY DRUG CONJUGATES MARKET, BY DISEASE TYPE, 2021-2028 (USD MILLION)
- 11.2.1 US
- 11.2.1.1 Rising regulatory approvals for ADCs to drive market
- TABLE 133 US: ANTIBODY DRUG CONJUGATES MARKET, BY PRODUCT, 2021-2028 (USD MILLION)
- TABLE 134 US: ANTIBODY DRUG CONJUGATES MARKET, BY LINKER TYPE, 2021-2028 (USD MILLION)
- TABLE 135 US: ANTIBODY DRUG CONJUGATES MARKET, BY PAYLOAD TYPE, 2021-2028 (USD MILLION)
- TABLE 136 US: ANTIBODY DRUG CONJUGATES MARKET, BY TARGET TYPE, 2021-2028 (USD MILLION)
- TABLE 137 US: ANTIBODY DRUG CONJUGATES MARKET, BY DISEASE TYPE, 2021-2028 (USD MILLION)
- 11.2.2 CANADA
- 11.2.2.1 High R&D investments for novel ADCs to propel market
- TABLE 138 CANADA: ANTIBODY DRUG CONJUGATES MARKET, BY PRODUCT, 2021-2028 (USD MILLION)
- TABLE 139 CANADA: ANTIBODY DRUG CONJUGATES MARKET, BY LINKER TYPE, 2021-2028 (USD MILLION)
- TABLE 140 CANADA: ANTIBODY DRUG CONJUGATES MARKET, BY PAYLOAD TYPE, 2021-2028 (USD MILLION)
- TABLE 141 CANADA: ANTIBODY DRUG CONJUGATES MARKET, BY TARGET TYPE, 2021-2028 (USD MILLION)
- TABLE 142 CANADA: ANTIBODY DRUG CONJUGATES MARKET, BY DISEASE TYPE, 2021-2028 (USD MILLION)
- 11.2.3 NORTH AMERICA: RECESSION IMPACT
- 11.3 EUROPE
- TABLE 143 EUROPE: ANTIBODY DRUG CONJUGATES MARKET, BY COUNTRY, 2021-2028 (USD MILLION)
- TABLE 144 EUROPE: ANTIBODY DRUG CONJUGATES MARKET, BY PRODUCT, 2021-2028 (USD MILLION)
- TABLE 145 EUROPE: ANTIBODY DRUG CONJUGATES MARKET, BY LINKER TYPE, 2021-2028 (USD MILLION)
- TABLE 146 EUROPE: ANTIBODY DRUG CONJUGATES MARKET, BY PAYLOAD TYPE, 2021-2028 (USD MILLION)
- TABLE 147 EUROPE: ANTIBODY DRUG CONJUGATES MARKET, BY TARGET TYPE, 2021-2028 (USD MILLION)
- TABLE 148 EUROPE: ANTIBODY DRUG CONJUGATES MARKET, BY DISEASE TYPE, 2021-2028 (USD MILLION)
- 11.3.1 GERMANY
- 11.3.1.1 Increasing collaborations among market players for cancer therapeutics to propel market
- TABLE 149 GERMANY: ANTIBODY DRUG CONJUGATES MARKET, BY PRODUCT, 2021-2028 (USD MILLION)
- TABLE 150 GERMANY: ANTIBODY DRUG CONJUGATES MARKET, BY LINKER TYPE, 2021-2028 (USD MILLION)
- TABLE 151 GERMANY: ANTIBODY DRUG CONJUGATES MARKET, BY PAYLOAD TYPE, 2021-2028 (USD MILLION)
- TABLE 152 GERMANY: ANTIBODY DRUG CONJUGATES MARKET, BY TARGET TYPE, 2021-2028 (USD MILLION)
- TABLE 153 GERMANY: ANTIBODY DRUG CONJUGATES MARKET, BY DISEASE TYPE, 2021-2028 (USD MILLION)
- 11.3.2 FRANCE
- 11.3.2.1 Growing demand for personalized therapeutics to drive market
- TABLE 154 FRANCE: ANTIBODY DRUG CONJUGATES MARKET, BY PRODUCT, 2021-2028 (USD MILLION)
- TABLE 155 FRANCE: ANTIBODY DRUG CONJUGATES MARKET, BY LINKER TYPE, 2021-2028 (USD MILLION)
- TABLE 156 FRANCE: ANTIBODY DRUG CONJUGATES MARKET, BY PAYLOAD TYPE, 2021-2028 (USD MILLION)
- TABLE 157 FRANCE: ANTIBODY DRUG CONJUGATES MARKET, BY TARGET TYPE, 2021-2028 (USD MILLION)
- TABLE 158 FRANCE: ANTIBODY DRUG CONJUGATES MARKET, BY DISEASE TYPE, 2021-2028 (USD MILLION)
- 11.3.3 UK
- 11.3.3.1 Increasing cancer burden to support market growth
- TABLE 159 UK: ANTIBODY DRUG CONJUGATES MARKET, BY PRODUCT, 2021-2028 (USD MILLION)
- TABLE 160 UK: ANTIBODY DRUG CONJUGATES MARKET, BY LINKER TYPE, 2021-2028 (USD MILLION)
- TABLE 161 UK: ANTIBODY DRUG CONJUGATES MARKET, BY PAYLOAD TYPE, 2021-2028 (USD MILLION)
- TABLE 162 UK: ANTIBODY DRUG CONJUGATES MARKET, BY TARGET TYPE, 2021-2028 (USD MILLION)
- TABLE 163 UK: ANTIBODY DRUG CONJUGATES MARKET, BY DISEASE TYPE, 2021-2028 (USD MILLION)
- 11.3.4 ITALY
- 11.3.4.1 Rising industrial collaborations for ADC production to drive market
- TABLE 164 ITALY: ANTIBODY DRUG CONJUGATES MARKET, BY PRODUCT, 2021-2028 (USD MILLION)
- TABLE 165 ITALY: ANTIBODY DRUG CONJUGATES MARKET, BY LINKER TYPE, 2021-2028 (USD MILLION)
- TABLE 166 ITALY: ANTIBODY DRUG CONJUGATES MARKET, BY PAYLOAD TYPE, 2021-2028 (USD MILLION)
- TABLE 167 ITALY: ANTIBODY DRUG CONJUGATES MARKET, BY TARGET TYPE, 2021-2028 (USD MILLION)
- TABLE 168 ITALY: ANTIBODY DRUG CONJUGATES MARKET, BY DISEASE TYPE, 2021-2028 (USD MILLION)
- 11.3.5 SPAIN
- 11.3.5.1 Rising focus on drug discovery to support market growth
- TABLE 169 SPAIN: ANTIBODY DRUG CONJUGATES MARKET, BY PRODUCT, 2021-2028 (USD MILLION)
- TABLE 170 SPAIN: ANTIBODY DRUG CONJUGATES MARKET, BY LINKER TYPE, 2021-2028 (USD MILLION)
- TABLE 171 SPAIN: ANTIBODY DRUG CONJUGATES MARKET, BY PAYLOAD TYPE, 2021-2028 (USD MILLION)
- TABLE 172 SPAIN: ANTIBODY DRUG CONJUGATES MARKET, BY TARGET, 2021-2028 (USD MILLION)
- TABLE 173 SPAIN: ANTIBODY DRUG CONJUGATES MARKET, BY DISEASE TYPE, 2021-2028 (USD MILLION)
- 11.3.6 REST OF EUROPE
- TABLE 174 REST OF EUROPE: ANTIBODY DRUG CONJUGATES MARKET, BY PRODUCT, 2021-2028 (USD MILLION)
- TABLE 175 REST OF EUROPE: ANTIBODY DRUG CONJUGATES MARKET, BY LINKER TYPE, 2021-2028 (USD MILLION)
- TABLE 176 REST OF EUROPE: ANTIBODY DRUG CONJUGATES MARKET, BY PAYLOAD TYPE, 2021-2028 (USD MILLION)
- TABLE 177 REST OF EUROPE: ANTIBODY DRUG CONJUGATES MARKET, BY TARGET TYPE, 2021-2028 (USD MILLION)
- TABLE 178 REST OF EUROPE: ANTIBODY DRUG CONJUGATES MARKET, BY DISEASE TYPE, 2021-2028 (USD MILLION)
- 11.3.7 EUROPE: RECESSION IMPACT
- 11.4 ASIA PACIFIC
- FIGURE 28 ASIA PACIFIC: ANTIBODY DRUG CONJUGATES MARKET SNAPSHOT
- TABLE 179 ASIA PACIFIC: ANTIBODY DRUG CONJUGATES MARKET, BY COUNTRY, 2021-2028 (USD MILLION)
- TABLE 180 ASIA PACIFIC: ANTIBODY DRUG CONJUGATES MARKET, BY PRODUCT, 2021-2028 (USD MILLION)
- TABLE 181 ASIA PACIFIC: ANTIBODY DRUG CONJUGATES MARKET, BY LINKER TYPE, 2021-2028 (USD MILLION)
- TABLE 182 ASIA PACIFIC: ANTIBODY DRUG CONJUGATES MARKET, BY PAYLOAD TYPE, 2021-2028 (USD MILLION)
- TABLE 183 ASIA PACIFIC: ANTIBODY DRUG CONJUGATES MARKET, BY TARGET TYPE, 2021-2028 (USD MILLION)
- TABLE 184 ASIA PACIFIC: ANTIBODY DRUG CONJUGATES MARKET, BY DISEASE TYPE, 2021-2028 (USD MILLION)
- 11.4.1 CHINA
- 11.4.1.1 Rising initiatives for advanced therapies to drive market
- TABLE 185 CHINA: ANTIBODY DRUG CONJUGATES MARKET, BY PRODUCT, 2021-2028 (USD MILLION)
- TABLE 186 CHINA: ANTIBODY DRUG CONJUGATES MARKET, BY LINKER TYPE, 2021-2028 (USD MILLION)
- TABLE 187 CHINA: ANTIBODY DRUG CONJUGATES MARKET, BY PAYLOAD TYPE, 2021-2028 (USD MILLION)
- TABLE 188 CHINA: ANTIBODY DRUG CONJUGATES MARKET, BY TARGET TYPE, 2021-2028 (USD MILLION)
- TABLE 189 CHINA: ANTIBODY DRUG CONJUGATES MARKET, BY DISEASE TYPE, 2021-2028 (USD MILLION)
- 11.4.2 JAPAN
- 11.4.2.1 Favorable regulatory support to propel market
- TABLE 190 JAPAN: ANTIBODY DRUG CONJUGATES MARKET, BY PRODUCT, 2021-2028 (USD MILLION)
- TABLE 191 JAPAN: ANTIBODY DRUG CONJUGATES MARKET, BY LINKER TYPE, 2021-2028 (USD MILLION)
- TABLE 192 JAPAN: ANTIBODY DRUG CONJUGATES MARKET, BY PAYLOAD TYPE, 2021-2028 (USD MILLION)
- TABLE 193 JAPAN: ANTIBODY DRUG CONJUGATES MARKET, BY TARGET TYPE, 2021-2028 (USD MILLION)
- TABLE 194 JAPAN: ANTIBODY DRUG CONJUGATES MARKET, BY DISEASE TYPE, 2021-2028 (USD MILLION)
- 11.4.3 INDIA
- 11.4.3.1 Emergence of innovative biosimilars to drive market
- TABLE 195 INDIA: ANTIBODY DRUG CONJUGATES MARKET, BY PRODUCT, 2021-2028 (USD MILLION)
- TABLE 196 INDIA: ANTIBODY DRUG CONJUGATES MARKET, BY LINKER TYPE, 2021-2028 (USD MILLION)
- TABLE 197 INDIA: ANTIBODY DRUG CONJUGATES MARKET, BY PAYLOAD TYPE, 2021-2028 (USD MILLION)
- TABLE 198 INDIA: ANTIBODY DRUG CONJUGATES MARKET, BY TARGET TYPE, 2021-2028 (USD MILLION)
- TABLE 199 INDIA: ANTIBODY DRUG CONJUGATES MARKET, BY DISEASE TYPE, 2021-2028 (USD MILLION)
- 11.4.4 REST OF ASIA PACIFIC
- TABLE 200 REST OF ASIA PACIFIC: ANTIBODY DRUG CONJUGATES MARKET, BY PRODUCT, 2021-2028 (USD MILLION)
- TABLE 201 REST OF ASIA PACIFIC: ANTIBODY DRUG CONJUGATES MARKET, BY LINKER TYPE, 2021-2028 (USD MILLION)
- TABLE 202 REST OF ASIA PACIFIC: ANTIBODY DRUG CONJUGATES MARKET, BY PAYLOAD TYPE, 2021-2028 (USD MILLION)
- TABLE 203 REST OF ASIA PACIFIC: ANTIBODY DRUG CONJUGATES MARKET, BY TARGET TYPE, 2021-2028 (USD MILLION)
- TABLE 204 REST OF ASIA PACIFIC: ANTIBODY DRUG CONJUGATES MARKET, BY DISEASE TYPE, 2021-2028 (USD MILLION)
- 11.4.5 ASIA PACIFIC: RECESSION IMPACT
- 11.5 LATIN AMERICA
- TABLE 205 LATIN AMERICA: ANTIBODY DRUG CONJUGATES MARKET, BY COUNTRY, 2021-2028 (USD MILLION)
- TABLE 206 LATIN AMERICA: ANTIBODY DRUG CONJUGATES MARKET, BY PRODUCT, 2021-2028 (USD MILLION)
- TABLE 207 LATIN AMERICA: ANTIBODY DRUG CONJUGATES MARKET, BY LINKER TYPE, 2021-2028 (USD MILLION)
- TABLE 208 LATIN AMERICA: ANTIBODY DRUG CONJUGATES MARKET, BY PAYLOAD TYPE, 2021-2028 (USD MILLION)
- TABLE 209 LATIN AMERICA: ANTIBODY DRUG CONJUGATES MARKET, BY TARGET TYPE, 2021-2028 (USD MILLION)
- TABLE 210 LATIN AMERICA: ANTIBODY DRUG CONJUGATES MARKET, BY DISEASE TYPE, 2021-2028 (USD MILLION)
- 11.5.1 BRAZIL
- 11.5.1.1 Regulatory approvals for breast cancer therapeutics to drive market
- TABLE 211 BRAZIL: ANTIBODY DRUG CONJUGATES MARKET, BY PRODUCT, 2021-2028 (USD MILLION)
- TABLE 212 BRAZIL: ANTIBODY DRUG CONJUGATES MARKET, BY LINKER TYPE, 2021-2028 (USD MILLION)
- TABLE 213 BRAZIL: ANTIBODY DRUG CONJUGATES MARKET, BY PAYLOAD TYPE, 2021-2028 (USD MILLION)
- TABLE 214 BRAZIL: ANTIBODY DRUG CONJUGATES MARKET, BY TARGET, 2021-2028 (USD MILLION)
- TABLE 215 BRAZIL: ANTIBODY DRUG CONJUGATES MARKET, BY DISEASE TYPE, 2021-2028 (USD MILLION)
- 11.5.2 REST OF LATIN AMERICA
- TABLE 216 REST OF LATIN AMERICA: ANTIBODY DRUG CONJUGATES MARKET, BY PRODUCT, 2021-2028 (USD MILLION)
- TABLE 217 REST OF LATIN AMERICA: ANTIBODY DRUG CONJUGATES MARKET, BY LINKER TYPE, 2021-2028 (USD MILLION)
- TABLE 218 REST OF LATIN AMERICA: ANTIBODY DRUG CONJUGATES MARKET, BY PAYLOAD TYPE, 2021-2028 (USD MILLION)
- TABLE 219 REST OF LATIN AMERICA: ANTIBODY DRUG CONJUGATES MARKET, BY TARGET TYPE, 2021-2028 (USD MILLION)
- TABLE 220 REST OF LATIN AMERICA: ANTIBODY DRUG CONJUGATES MARKET, BY DISEASE TYPE, 2021-2028 (USD MILLION)
- 11.5.3 LATIN AMERICA: RECESSION IMPACT
- 11.6 MIDDLE EAST & AFRICA
- 11.6.1 RISING PRODUCT LAUNCHES FOR CANCER THERAPEUTICS TO SUPPORT MARKET GROWTH
- TABLE 221 MIDDLE EAST & AFRICA: ANTIBODY DRUG CONJUGATES MARKET, BY PRODUCT, 2021-2028 (USD MILLION)
- TABLE 222 MIDDLE EAST & AFRICA: ANTIBODY DRUG CONJUGATES MARKET, BY LINKER TYPE, 2021-2028 (USD MILLION)
- TABLE 223 MIDDLE EAST & AFRICA: ANTIBODY DRUG CONJUGATES MARKET, BY PAYLOAD TYPE, 2021-2028 (USD MILLION)
- TABLE 224 MIDDLE EAST & AFRICA: ANTIBODY DRUG CONJUGATES MARKET, BY TARGET TYPE, 2021-2028 (USD MILLION)
- TABLE 225 MIDDLE EAST & AFRICA: ANTIBODY DRUG CONJUGATES MARKET, BY DISEASE TYPE, 2021-2028 (USD MILLION)
- 11.7 MIDDLE EAST & AFRICA: RECESSION IMPACT
12 COMPETITIVE LANDSCAPE
- 12.1 INTRODUCTION
- 12.2 STRATEGIES ADOPTED BY KEY PLAYERS
- TABLE 226 ANTIBODY DRUG CONJUGATES MARKET: STRATEGIES ADOPTED BY KEY PLAYERS
- 12.3 REVENUE SHARE ANALYSIS
- FIGURE 29 REVENUE SHARE ANALYSIS OF KEY PLAYERS (2019-2022)
- 12.4 MARKET SHARE ANALYSIS
- FIGURE 30 MARKET SHARE ANALYSIS OF KEY PLAYERS (2022)
- TABLE 227 ANTIBODY DRUG CONJUGATES MARKET: INTENSITY OF COMPETITIVE RIVALRY
- 12.5 COMPANY EVALUATION MATRIX FOR KEY PLAYERS
- FIGURE 31 ANTIBODY DRUG CONJUGATES MARKET: COMPANY EVALUATION MATRIX (2022)
- 12.5.1 STARS
- 12.5.2 EMERGING LEADERS
- 12.5.3 PERVASIVE PLAYERS
- 12.5.4 PARTICIPANTS
- 12.6 COMPETITIVE BENCHMARKING OF 25 PLAYERS
- 12.6.1 PRODUCT FOOTPRINT OF 11 COMPANIES
- TABLE 228 ANTIBODY DRUG CONJUGATES MARKET: COMPANY FOOTPRINT ANALYSIS OF KEY PLAYERS
- TABLE 229 ANTIBODY DRUG CONJUGATES MARKET: PRODUCT FOOTPRINT ANALYSIS OF KEY PLAYERS
- 12.6.2 REGIONAL FOOTPRINT OF 26 COMPANIES
- TABLE 230 ANTIBODY DRUG CONJUGATES MARKET: REGIONAL FOOTPRINT ANALYSIS OF KEY PLAYERS
- 12.7 COMPANY EVALUATION MATRIX FOR STARTUPS/SMES
- FIGURE 32 ANTIBODY DRUG CONJUGATES MARKET: COMPANY EVALUATION MATRIX FOR STARTUPS/SMES (2022)
- 12.7.1 PROGRESSIVE COMPANIES
- 12.7.2 STARTING BLOCKS
- 12.7.3 RESPONSIVE COMPANIES
- 12.7.4 DYNAMIC COMPANIES
- 12.8 COMPETITIVE BENCHMARKING OF STARTUPS/SMES
- TABLE 231 ANTIBODY DRUG CONJUGATES MARKET: DETAILED LIST OF KEY STARTUPS/SMES
- TABLE 232 ANTIBODY DRUG CONJUGATES MARKET: COMPETITIVE BENCHMARKING OF STARTUPS/SMES
- 12.9 COMPETITIVE SCENARIO AND TRENDS
- 12.9.1 PRODUCT LAUNCHES
- TABLE 233 ANTIBODY DRUG CONJUGATES MARKET: PRODUCT LAUNCHES (JANUARY 2020-JUNE 2023)
- 12.9.2 DEALS
- TABLE 234 ANTIBODY DRUG CONJUGATES MARKET: DEALS (JANUARY 2020-JUNE 2023)
13 COMPANY PROFILES
(Business Overview, Products/Solutions/Services Offered, Recent Developments, MnM view (Key strengths/Right to win, Strategic choices made, Weakness/competitive threats)**
- 13.1 KEY PLAYERS
- 13.1.1 F. HOFFMANN-LA ROCHE LTD.
- TABLE 235 F. HOFFMANN-LA ROCHE LTD.: BUSINESS OVERVIEW
- FIGURE 33 F. HOFFMANN-LA ROCHE LTD.: COMPANY SNAPSHOT (2022)
- 13.1.2 DAIICHI SANKYO COMPANY, LIMITED
- TABLE 236 DAIICHI SANKYO COMPANY, LIMITED: BUSINESS OVERVIEW
- FIGURE 34 DAIICHI SANKYO COMPANY, LIMITED: COMPANY SNAPSHOT (2022)
- 13.1.3 SEAGEN INC.
- TABLE 237 SEAGEN INC.: BUSINESS OVERVIEW
- FIGURE 35 SEAGEN INC.: COMPANY SNAPSHOT (2022)
- 13.1.4 GILEAD SCIENCES, INC.
- TABLE 238 GILEAD SCIENCES, INC.: BUSINESS OVERVIEW
- FIGURE 36 GILEAD SCIENCES, INC.: COMPANY SNAPSHOT (2022)
- 13.1.5 TAKEDA PHARMACEUTICAL COMPANY LIMITED
- TABLE 239 TAKEDA PHARMACEUTICAL COMPANY LIMITED: BUSINESS OVERVIEW
- FIGURE 37 TAKEDA PHARMACEUTICAL COMPANY LIMITED: COMPANY SNAPSHOT (2022)
- 13.1.6 PFIZER INC.
- TABLE 240 PFIZER INC.: BUSINESS OVERVIEW
- FIGURE 38 PFIZER INC.: COMPANY SNAPSHOT (2022)
- 13.1.7 ASTELLAS PHARMA INC.
- TABLE 241 ASTELLAS PHARMA INC.: BUSINESS OVERVIEW
- FIGURE 39 ASTELLAS PHARMA INC: COMPANY SNAPSHOT (2022)
- 13.1.8 ASTRAZENECA
- TABLE 242 ASTRAZENECA: BUSINESS OVERVIEW
- FIGURE 40 ASTRAZENECA: COMPANY SNAPSHOT (2022)
- 13.1.9 ADC THERAPEUTICS SA
- TABLE 243 ADC THERAPEUTICS SA: BUSINESS OVERVIEW
- FIGURE 41 ADC THERAPEUTICS SA: COMPANY SNAPSHOT (2022)
- 13.1.10 IMMUNOGEN, INC.
- TABLE 244 IMMUNOGEN, INC.: BUSINESS OVERVIEW
- FIGURE 42 IMMUNOGEN, INC.: COMPANY SNAPSHOT (2022)
- 13.1.11 ZYDUS GROUP
- TABLE 245 ZYDUS GROUP: BUSINESS OVERVIEW
- FIGURE 43 ZYDUS GROUP: COMPANY SNAPSHOT (2022)
- 13.2 OTHER PLAYERS
- 13.2.1 ABBVIE INC.
- TABLE 246 ABBVIE INC.: BUSINESS OVERVIEW
- 13.2.2 AMBRX
- TABLE 247 AMBRX: BUSINESS OVERVIEW
- 13.2.3 LEGOCHEM BIOSCIENCES, INC.
- TABLE 248 LEGOCHEM BIOSCIENCES, INC.: BUSINESS OVERVIEW
- 13.2.4 BYONDIS
- TABLE 249 BYONDIS: BUSINESS OVERVIEW
- 13.2.5 PROFOUNDBIO
- TABLE 250 PROFOUNDBIO: BUSINESS OVERVIEW
- 13.2.6 REMEGEN
- TABLE 251 REMEGEN: BUSINESS OVERVIEW
- 13.2.7 SUTRO BIOPHARMA, INC.
- TABLE 252 SUTRO BIOPHARMA, INC.: BUSINESS OVERVIEW
- 13.2.8 LEPU BIOPHARMA CO., LTD.
- TABLE 253 LEPU BIOPHARMA CO., LTD.: BUSINESS OVERVIEW
- 13.2.9 ZYMEWORKS INC.
- TABLE 254 ZYMEWORKS INC.: BUSINESS OVERVIEW
- 13.2.10 MERSANA THERAPEUTICS
- TABLE 255 MERSANA THERAPEUTICS: BUSINESS OVERVIEW
- 13.2.11 DUALITY BIOLOGICS
- TABLE 256 DUALITY BIOLOGICS: BUSINESS OVERVIEW
- 13.2.12 LANOVA MEDICINES
- TABLE 257 LANOVA MEDICINES: BUSINESS OVERVIEW
- 13.2.13 EXELIXIS, INC.
- TABLE 258 EXELIXIS, INC.: BUSINESS OVERVIEW
- 13.2.14 BIONECURE THERAPEUTICS INC.
- TABLE 259 BIONECURE THERAPEUTICS INC.: BUSINESS OVERVIEW
- 13.2.15 TRIPARTITE THERAPEUTICS, INC.
- TABLE 260 TRIPARTITE THERAPEUTICS, INC.: BUSINESS OVERVIEW
- *Details on Business Overview, Products/Solutions/Services Offered, Recent Developments, MnM view (Key strengths/Right to win, Strategic choices made, Weakness/competitive threats)** might not be captured in case of unlisted companies.
14 APPENDIX
- 14.1 DISCUSSION GUIDE
- 14.2 KNOWLEDGESTORE: MARKETSANDMARKETS' SUBSCRIPTION PORTAL
- 14.3 CUSTOMIZATION OPTIONS
- 14.4 RELATED REPORTS
- 14.5 AUTHOR DETAILS