|
|
市場調査レポート
商品コード
1311268
マイクロカテーテルの世界市場:タイプ (デリバリー・診断・吸引・ステアラブル)・設計 (シングル・デュアル)・用途 (心血管・神経血管・末梢血管・腫瘍)・エンドユーザー (病院・外来外科センター) 別Microcatheters Market by Type (Delivery, Diagnostic, Aspiration, Steerable), Design (Single, Dual), Application (Cardiovascular, Neurovascular, Peripheral Vascular, Oncology), End-User (Hospitals, Ambulatory Surgical Centers) - Global Forecast to 2028 |
||||||
カスタマイズ可能
|
|||||||
| マイクロカテーテルの世界市場:タイプ (デリバリー・診断・吸引・ステアラブル)・設計 (シングル・デュアル)・用途 (心血管・神経血管・末梢血管・腫瘍)・エンドユーザー (病院・外来外科センター) 別 |
|
出版日: 2023年07月07日
発行: MarketsandMarkets
ページ情報: 英文 225 Pages
納期: 即納可能
|
- 全表示
- 概要
- 目次
世界のマイクロカテーテルの市場規模は、2023年の8億7,400万米ドルから、予測期間中は5.5%のCAGRで推移し、2028年には11億4,200万米ドルの規模に成長すると予測されています。
低侵襲性、正確なナビゲーション、標的治療など、マイクロカテーテルが提供する利点の増加が予測期間中の市場成長を後押しすると予想されています。
製品タイプ別で見ると、ステアラブルマイクロカテーテルの部門が予測期間中に最大の最高のCAGRを記録すると予測されています。ステアラブルマイクロカテーテルは、インターベンショナルラジオロジー、神経血管インターベンション、末梢血管インターベンションなどの低侵襲手術で使用されることが多いです。これらの処置は、従来の開腹手術に比べ、患者の外傷の軽減、入院期間の短縮、回復時間の短縮、ヘルスケアコストの削減などの利点があります。体内の複雑な血管系内をマイクロカテーテルで移動・操縦する能力は、正確で的を絞った治療を可能にすることから、ステアラブルマイクロカテーテルの成長をさらに後押しています。
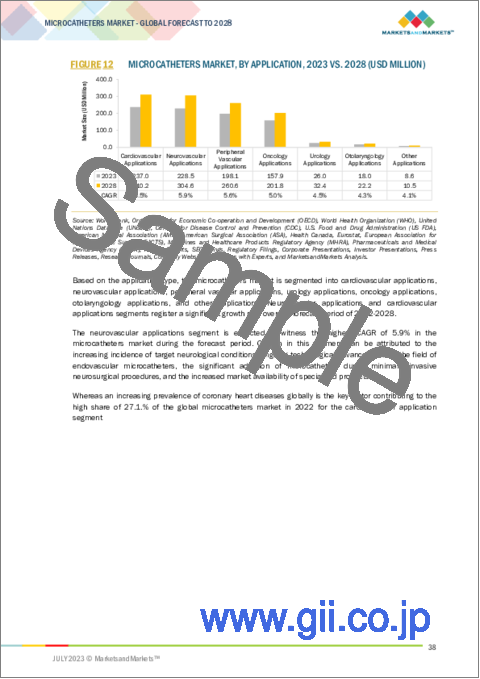
用途別では、神経血管用途の部門が予測期間中に最大のCAGRを記録する見通しです。材料、設計、コーティングの改良を含むマイクロカテーテル技術の継続的な進歩により、神経血管用マイクロカテーテルのナビゲーション能力と送達能力が向上しています。このような進歩により、医師は頭蓋内の困難な場所に、より高い精度と制御性で到達することができるようになり、治療可能な病変の範囲が拡大し、手術成績が向上しています。また、政府、ヘルスケア機関、研究機関は、神経血管ヘルスケアインフラの改善に投資しています。このような投資も、医療施設における神経血管用マイクロカテーテルの成長と採用に寄与しています。
エンドユーザー別では、病院・外科センター・専門クリニックの部門が予測期間中に最大の成長を示すと予測されています。外来外科センターが予測期間中は大きな市場シェアを占める見通しです。外来外科センターは、入院の必要性を最小限に抑える即日手術の提供に重点を置いています。マイクロカテーテルは、低侵襲手術を可能にする上で重要な役割を果たしており、従来の開腹手術に比べて回復時間が短く、痛みが軽減され、低コストであるため、好まれることが多いです。
地域別では、アジア太平洋地域が予測期間中に大きな成長を記録する見通しです。アジア太平洋諸国では、経済成長、人口増加、ヘルスケアへのアクセス向上などを背景に、ヘルスケア支出が増加しています。このため、低侵襲手術をサポートし、患者の予後を改善するために、マイクロカテーテルを含む先進医療技術への投資が拡大しています。また、同地域は高齢者人口が多く、慢性疾患にかかりやすく、専門的な医療介入が必要です。マイクロカテーテルは、心血管疾患、神経血管障害、その他の加齢関連疾患の管理に広く使用されています。高齢化人口の増加も同地域のマイクロカテーテル需要を刺激しています。さらに、同地域では、心血管疾患、癌、神経血管障害などの慢性疾患の有病率が上昇しています。マイクロカテーテルはこれらの疾患の診断、治療、管理において重要な役割を担っており、同地域における需要増につながっています。
当レポートでは、世界のマイクロカテーテルの市場を調査し、市場概要、市場影響因子および市場機会の分析、技術・特許の動向、ケーススタディ、関連法規制、市場規模の推移・予測、各種区分・地域/主要国別の詳細分析、競合環境、主要企業のプロファイルなどをまとめています。
目次
第1章 イントロダクション
第2章 調査手法
第3章 エグゼクティブサマリー
第4章 重要考察
第5章 市場概要
- 市場力学
- 促進要因
- 抑制要因
- 機会
- 課題
- 主なステークホルダー・購入基準
- ポーターのファイブフォース分析
- 規制分析
- 価格分析
- 償還シナリオ分析
- エコシステムマッピング
- バリューチェーン分析
- サプライチェーン分析
- 特許分析
- 技術分析
- ケーススタディ分析
- 主な会議とイベント
- 顧客の事業に影響を与える動向/ディスラプション
- 景気後退がマイクロカテーテル市場に与える影響
第6章 マイクロカテーテル市場:製品別
- デリバリーマイクロカテーテル
- 吸引マイクロカテーテル
- 診断用マイクロカテーテル
- ステアラブルマイクロカテーテル
第7章 マイクロカテーテル市場:製品設計別
- シングルルーメンマイクロカテーテル
- デュアルおよびマルチルーメンマイクロカテーテル
第8章 マイクロカテーテル市場:用途別
- 心臓血管
- 神経血管
- 末梢血管
- 泌尿器
- 腫瘍
- 耳鼻
- その他
第9章 マイクロカテーテル市場:エンドユーザー別
- 病院・外科センター・専門クリニック
- 外来治療センター
第10章 マイクロカテーテル市場:地域別
- 北米
- 欧州
- アジア太平洋
- ラテンアメリカ
- 中東・アフリカ
第11章 競合情勢
- 概要
- 大手企業の採用戦略
- 主要企業の収益シェア分析
- 市場ランキング分析
- 主要企業の企業評価マトリックス
- スタートアップ/中小企業の企業評価マトリックス
- 企業のフットプリント
- 競合シナリオと動向
第12章 企業プロファイル
- 主要企業
- MEDTRONIC
- BOSTON SCIENTIFIC CORPORATION
- TERUMO MEDICAL CORPORATION
- TELEFLEX INCORPORATED
- MERIT MEDICAL SYSTEMS, INC.
- ASAHI INTECC CO., LTD.
- STRYKER
- SURMODICS, INC.
- CARDINAL HEALTH, INC.
- JOHNSON & JOHNSON
- GUERBET LLC
- LEPU MEDICAL TECHNOLOGY(BEIJING)CO., LTD
- BECTON, DICKINSON AND COMPANY
- PENUMBRA, INC.
- KANEKA CORPORATION
- ANGIODYNAMICS, INC.
- BIOCARDIA INC.
- その他の企業
- MILLAR, INC.
- BAYLIS MEDICAL COMPANY, INC.
- EMBOLX, INC.
- ACANDIS GMBH
- ACROSTAK
- COOK MEDICAL
- REFLOW MEDICAL, INC.
- TZ MEDICAL, INC.
- ORBUSNEICH MEDICAL
- SPARTAN MICRO, INC.
- TRANSIT SCIENTIFIC
- TOKAI MEDICAL PRODUCTS
- LIBATAPE PHARMACEUTICAL CO., LTD
第13章 付録
The global microcatheter market is projected to reach USD 1,142 million by 2028 from USD 874 million in 2023, growing at a CAGR of 5.5% during the forecast period. The rise in advantages offered by microcatheter like Minimally Invasive, precise navigation, and targeted therapy among several others to be used are major factors anticipated to boost the market growth in the forecasting years.
"Steerable Microcatheters segment to register highest CAGR over the forecast period of 2023-2028"
Based on the product type, the microcatheter market is segmented into Delivery Microcatheter, Aspiration Microcatheter, Diagnostic Microcatheter, and Steerable Microcatheter. Steerable Microcatheters segment to register a highest CAGR over the forecast period of 2023-2028. Steerable microcatheters are often used in minimally invasive procedures, such as interventional radiology, neurovascular interventions, and peripheral vascular interventions. These procedures offer advantages over traditional open surgeries, including reduced patient trauma, shorter hospital stays, faster recovery times, and lower healthcare costs. The ability to navigate and steer microcatheters within the body's intricate vasculature allows for precise and targeted treatments, further supporting the growth of steerable microcatheters.
"The Neurovascular application segment to register highest CAGR over the forecast period of 2023-2028"
Based on the application, the Microcatheters market is segmented into Cardiovascular, Neurovascular, Peripheral Vascular, Urology, Otolaryngology, and other applications. Ongoing advancements in microcatheter technology, including improvements in materials, designs, and coatings, enhance the navigational capabilities and deliverability of neurovascular microcatheters. These advancements enable physicians to reach challenging intracranial locations with greater precision and control, expanding the range of treatable lesions and improving procedural outcomes. Governments, healthcare organizations, and research institutions are investing in improving neurovascular healthcare infrastructure. This includes the availability of advanced neuro-interventional suites and the adoption of state-of-the-art neurovascular devices, including microcatheters. Such investments contribute to the growth and adoption of neurovascular microcatheters in medical facilities.
"The single-lumen microcatheter segment accounted for the largest share of the microcatheter market in 2022-2028"
Based on the design, the microcatheter market is segmented into single-lumen microcatheter and Dual and Multi-lumen Microcatheter. The dual and multi-lumen microcatheter segment design segment is estimated to hold a significant market share of the microcatheter market during the forecast period.
Duel and multi-lumen microcatheters are medical devices used in various interventional procedures, particularly in the field of minimally invasive surgery. They consist of small, flexible tubes with multiple lumens (channels) that allow the simultaneous delivery of fluids, medications, and medical instruments to the target site. The drivers for using duel and multi-lumen microcatheters include:
Enhanced Procedure Efficiency: By incorporating multiple lumens within a single catheter, duel and multi-lumen microcatheters can streamline procedures by reducing the need for multiple catheter insertions. This can save time and improve overall procedural efficiency.
"Hospitals, Surgical Centers, and Specialty Clinics' segment to register for the highest growth rate of the microcatheters market in 2022-2028"
The major end users in the microcatheters market are hospitals, surgical centers & specialty clinics, and ambulatory surgical centers. The Ambulatory surgical center's end-user segment is estimated to hold a significant market share of the microcatheter market during the forecast period. Ambulatory surgical centers focus on providing same-day surgical procedures that minimize the need for hospitalization. Microcatheters play a vital role in enabling minimally invasive procedures, which are often preferred in ASCs due to their shorter recovery times, reduced pain, and lower cost compared to traditional open surgeries.
Technological Advances: The advancements in microcatheter technology have made these devices more versatile, reliable, and user-friendly. Improved catheter materials, enhanced flexibility, and smaller profiles allow for precise navigation through complex anatomical structures, making microcatheters suitable for a broader range of procedures performed in ASCs.
"Asia Pacific to register significant growth in the market during the forecast period."
In 2022, the APAC region is expected to register significant growth in the market during the forecast period. Asia Pacific comprises India, China, Japan, Australia, South Korea, and RoAPAC. The Asia-Pacific (APAC) region has witnessed a significant increase in the market for microcatheters. Several factors have contributed to this growth:
APAC countries have been experiencing rising healthcare expenditure, driven by economic growth, increasing population, and improved access to healthcare. This has led to greater investment in advanced medical technologies, including microcatheters, to support minimally invasive procedures and improve patient outcomes.
The APAC region has a large aging population, which is more prone to chronic diseases and requires specialized medical interventions. Microcatheters are extensively used in the management of cardiovascular diseases, neurovascular disorders, and other age-related conditions. The growing aging population has fueled the demand for microcatheters in APAC.
APAC countries have seen a rise in the prevalence of chronic diseases such as cardiovascular diseases, cancer, and neurovascular disorders. Microcatheters play a crucial role in the diagnosis, treatment, and management of these conditions, leading to an increased demand for these devices in the region.
A breakdown of the primary participants referred to for this report is provided below:
- By Company Type: Tier 1-30%, Tier 2-42%, and Tier 3- 28%
- By Designation: C-level-10%, Director-level-76%, and Others-14%
- By Region: North America-40%, Europe-30%, Asia Pacific-22%, Latin America-6%, and the Middle East & Africa-2%
Prominent players in the microcatheter market are Terumo Corporation (Tokyo, Japan), Boston Scientific Corporation (Massachusetts, US), and Cook Medical (Indiana, US), among others.
Research Coverage
This report studies the microcatheter market based on product, type, application, end-user, and region. It also covers the factors affecting market growth, analyzes the various opportunities and challenges in the market, and provides details of the competitive landscape for market leaders. Furthermore, the report analyzes micro markets with respect to their individual growth trends and forecasts the revenue of the market segments with respect to five main regions (and the respective countries in these regions). The scope of the report covers detailed information regarding the major factors, such as drivers, restraints, challenges, and opportunities, influencing the growth of the microcatheters market.
A detailed analysis of the key industry players has been done to provide insights into their business overview, solutions, services; key strategies; Contracts, partnerships, and agreements. new product & service launches, mergers and acquisitions, and recent developments associated with the microcatheters market. Competitive analysis of upcoming startups in the microcatheters market ecosystem is covered in this report.
Key Benefits of Buying the Report:
The report will help the market leaders/new entrants in this market with information on the closest approximations of the revenue numbers for the overall microcatheters market and the subsegments. This report will help stakeholders understand the competitive landscape and gain more insights to position their businesses better and plan suitable go-to-market strategies. The report also helps stakeholders understand the pulse of the market and provides them with information on key market drivers, restraints, challenges, and opportunities.
The report provides insights on the following pointers:
- Analysis of key drivers (increasing prevalence of chronic diseases, growing aging population, advancements in minimally invasive techniques), restraints (stringent regulatory requirements, high cost of microcatheters, limited availability in developing regions), opportunities (technological advancements, increasing adoption of minimally invasive procedures), and challenges (cost pressures, product complexity and variability) influencing the growth of the microcatheters market
- Product Development/Innovation: Detailed insights on upcoming technologies, research & development activities, and new product & service launches in the microcatheters market.
- Market Development: Comprehensive information about lucrative markets - the report analyses the microcatheters market across varied regions.
- Market Diversification: Exhaustive information about new products, untapped geographies, recent developments, and investments in the microcatheters market
- Competitive Assessment: In-depth assessment of market shares, growth strategies, and service offerings of leading players like Terumo Corporation (Tokyo, Japan), Boston Scientific Corporation (Massachusetts, US), and Cook Medical (Indiana, US), among others.
TABLE OF CONTENTS
1 INTRODUCTION
- 1.1 STUDY OBJECTIVES
- 1.2 MARKET DEFINITION
- 1.3 MARKET SCOPE
- 1.3.1 MARKETS COVERED
- 1.3.2 REGIONS COVERED
- 1.3.3 INCLUSIONS AND EXCLUSIONS
- 1.3.4 YEARS CONSIDERED
- 1.4 CURRENCY CONSIDERED
- 1.5 RESEARCH LIMITATIONS
- 1.6 MARKET STAKEHOLDERS
- 1.7 SUMMARY OF CHANGES
2 RESEARCH METHODOLOGY
- 2.1 RESEARCH DATA
- FIGURE 1 RESEARCH DESIGN: MICROCATHETERS MARKET
- FIGURE 2 RESEARCH DESIGN
- 2.1.1 SECONDARY DATA
- FIGURE 3 KEY DATA FROM SECONDARY SOURCES
- 2.1.2 PRIMARY DATA
- 2.1.2.1 Primary sources
- 2.1.2.2 Key industry insights
- 2.1.2.3 Breakdown of primaries
- FIGURE 4 BREAKDOWN OF PRIMARY INTERVIEWS: SUPPLY-SIDE AND DEMAND-SIDE PARTICIPANTS
- FIGURE 5 BREAKDOWN OF PRIMARY INTERVIEWS: BY COMPANY TYPE, DESIGNATION, AND REGION
- 2.2 MARKET SIZE ESTIMATION
- FIGURE 6 RESEARCH METHODOLOGY: HYPOTHESIS BUILDING
- 2.2.1 BOTTOM-UP APPROACH
- 2.2.1.1 Approach 1: Company revenue estimation approach
- FIGURE 7 MICROCATHETERS MARKET: COMPANY REVENUE ESTIMATION APPROACH
- 2.2.1.2 Approach 2: Customer-based market estimation approach
- FIGURE 8 MICROCATHETERS MARKET: CUSTOMER-BASED MARKET SIZE ESTIMATION APPROACH
- 2.2.1.3 Approach 3: Top-down approach
- FIGURE 9 MICROCATHETERS MARKET: TOP-DOWN APPROACH
- 2.2.1.4 Approach 4: Primary interviews
- 2.3 MARKET BREAKDOWN AND DATA TRIANGULATION
- FIGURE 10 DATA TRIANGULATION METHODOLOGY
- 2.4 MARKET SHARE ASSESSMENT
- 2.5 STUDY ASSUMPTIONS
- 2.6 GROWTH RATE ASSUMPTIONS
- 2.7 RISK ASSESSMENT
- TABLE 1 MICROCATHETERS MARKET: RISK ASSESSMENT
3 EXECUTIVE SUMMARY
- FIGURE 11 MICROCATHETERS MARKET, BY PRODUCT, 2023 VS. 2028 (USD MILLION)
- FIGURE 12 MICROCATHETERS MARKET, BY APPLICATION, 2023 VS. 2028 (USD MILLION)
- FIGURE 13 MICROCATHETERS MARKET, BY PRODUCT DESIGN, 2023 VS. 2028 (USD MILLION)
- FIGURE 14 MICROCATHETERS MARKET, BY END USER, 2023 VS. 2028 (USD MILLION)
- FIGURE 15 GEOGRAPHICAL SNAPSHOT OF MICROCATHETERS MARKET
4 PREMIUM INSIGHTS
- 4.1 MICROCATHETERS MARKET OVERVIEW
- FIGURE 16 INCREASING GERIATRIC POPULATION AND GROWING R&D INVESTMENTS BY GOVERNMENT TO DRIVE MARKET
- 4.2 MICROCATHETERS MARKET, BY TYPE, 2023 VS. 2028 (USD MILLION)
- FIGURE 17 DELIVERY MICROCATHETERS TO DOMINATE MICROCATHETERS MARKET IN 2028
- 4.3 MICROCATHETERS MARKET, BY REGION AND END USER, 2022 (USD MILLION)
- FIGURE 18 HOSPITALS, SURGICAL CENTERS, AND SPECIALTY CLINICS SEGMENT DOMINATED MICROCATHETERS MARKET IN 2022
- 4.4 GEOGRAPHICAL SNAPSHOT OF MICROCATHETERS MARKET
- FIGURE 19 INDIA TO BE FASTEST GROWING COUNTRY IN GLOBAL MICROCATHETERS MARKET DURING STUDY PERIOD
5 MARKET OVERVIEW
- 5.1 INTRODUCTION
- 5.2 MARKET DYNAMICS
- FIGURE 20 MICROCATHETERS MARKET: DRIVERS, RESTRAINTS, OPPORTUNITIES, AND CHALLENGES
- 5.2.1 DRIVERS
- 5.2.1.1 Rising target patient population suffering from chronic diseases globally
- FIGURE 21 NUMBER OF NEW CANCER CASES (GLOBAL), BY CANCER SITE (2018 VS. 2020)
- 5.2.1.2 Advancements in microcatheter technologies
- 5.2.1.3 Increase in government initiatives, healthcare funding, and health-related services
- TABLE 2 NATIONAL AVERAGE MEDICARE REIMBURSEMENT CPT CODES, 2022
- 5.2.1.4 Increased preference for minimally invasive surgeries over traditional open surgeries
- 5.2.2 RESTRAINTS
- 5.2.2.1 Stringent regulatory guidelines in mature markets
- 5.2.2.2 High cost of microcatheters and budget constraints in healthcare facilities
- 5.2.3 OPPORTUNITIES
- 5.2.3.1 Expansion of key players in emerging economies
- 5.2.3.2 Rising healthcare expenditure across emerging economies
- FIGURE 22 GOVERNMENT HEALTH EXPENDITURE (% OF GDP)
- FIGURE 23 GOVERNMENT HEALTH EXPENDITURE [AS % OF GENERAL GOVERNMENT EXPENDITURE]
- 5.2.4 CHALLENGES
- 5.2.4.1 Availability of alternative technologies for catheterization
- 5.3 KEY STAKEHOLDERS AND BUYING CRITERIA
- 5.3.1 KEY STAKEHOLDERS IN BUYING PROCESS
- FIGURE 24 INFLUENCE OF STAKEHOLDERS ON BUYING PROCESS FOR MICROCATHETERS MARKET
- TABLE 3 INFLUENCE OF STAKEHOLDERS ON BUYING PROCESS FOR KEY PRODUCT SEGMENTS (%)
- 5.4 PORTER'S FIVE FORCES ANALYSIS
- TABLE 4 MICROCATHETERS MARKET: PORTER'S FIVE FORCES ANALYSIS
- 5.4.1 THREAT OF NEW ENTRANTS
- 5.4.2 THREAT OF SUBSTITUTES
- 5.4.3 BARGAINING POWER OF SUPPLIERS
- 5.4.4 BARGAINING POWER OF BUYERS
- 5.4.5 INTENSITY OF COMPETITIVE RIVALRY
- 5.5 REGULATORY ANALYSIS
- 5.5.1 US
- TABLE 5 US: MEDICAL DEVICE REGULATORY APPROVAL PROCESS
- 5.5.2 EUROPE
- 5.5.3 JAPAN
- TABLE 6 JAPAN: MEDICAL DEVICE CLASSIFICATION UNDER PMDA
- 5.6 PRICING ANALYSIS
- TABLE 7 PRICING ANALYSIS: MICROCATHETERS MARKET, 2023 (USD)
- 5.7 REIMBURSEMENT SCENARIO ANALYSIS
- TABLE 8 CPT CODES FOR MAJOR MICROCATHETERS
- 5.8 ECOSYSTEM MAPPING
- 5.9 VALUE CHAIN ANALYSIS
- 5.9.1 RESEARCH & DEVELOPMENT
- 5.9.2 PROCUREMENT AND PRODUCT DEVELOPMENT
- 5.9.3 MARKETING, SALES AND DISTRIBUTION, AND POST-SALES SERVICES
- FIGURE 25 VALUE CHAIN ANALYSIS: MICROCATHETERS MARKET
- 5.10 SUPPLY CHAIN ANALYSIS
- 5.10.1 PROMINENT COMPANIES
- 5.10.2 SMALL & MEDIUM-SIZED COMPANIES
- 5.10.3 END USERS
- FIGURE 26 SUPPLY CHAIN ANALYSIS: MICROCATHETERS MARKET
- 5.11 PATENT ANALYSIS
- TABLE 9 IMPORT DATA FOR MICROCATHETERS (HS CODE 9018), BY COUNTRY, 2018-2022 (USD THOUSAND)
- TABLE 10 EXPORT DATA FOR MICROCATHETERS (HS CODE 9018), BY COUNTRY, 2018-2022 (USD THOUSAND)
- 5.12 TECHNOLOGY ANALYSIS
- 5.13 CASE STUDY ANALYSIS
- TABLE 11 CASE STUDY: EMBOLIZATION FOR CHEST WALL HEMORRHAGE-DIREXION MICROCATHETER STORY
- 5.14 KEY CONFERENCES AND EVENTS IN 2023-2024
- TABLE 12 DETAILED LIST OF KEY CONFERENCES AND EVENTS IN 2023-2024
- 5.15 TRENDS/DISRUPTIONS IMPACTING CUSTOMERS' BUSINESSES
- FIGURE 27 EMERGING TRENDS AND OPPORTUNITIES AFFECTING FUTURE REVENUE MIX
- 5.16 IMPACT OF ECONOMIC RECESSION ON MICROCATHETERS MARKET
6 MICROCATHETERS MARKET, BY PRODUCT
- 6.1 INTRODUCTION
- TABLE 13 MICROCATHETERS MARKET, BY PRODUCT, 2020-2028 (USD MILLION)
- TABLE 14 MICROCATHETERS MARKET, BY APPLICATION, 2020-2028 (USD MILLION)
- TABLE 15 MICROCATHETERS MARKET, BY END USER, 2020-2028 (USD MILLION)
- TABLE 16 MICROCATHETERS MARKET, BY PRODUCT DESIGN, 2020-2028 (USD MILLION)
- 6.2 DELIVERY MICROCATHETERS
- 6.2.1 INCREASING NUMBER OF TARGET INTERVENTIONAL PROCEDURES TO DRIVE MARKET
- TABLE 17 MICROCATHETERS MARKET FOR DELIVERY MICROCATHETERS, BY REGION, 2020-2028 (USD MILLION)
- TABLE 18 MICROCATHETERS MARKET FOR DELIVERY MICROCATHETERS, BY PRODUCT DESIGN, 2020-2028 (USD MILLION)
- TABLE 19 MICROCATHETERS MARKET FOR DELIVERY MICROCATHETERS, BY APPLICATION, 2020-2028 (USD MILLION)
- TABLE 20 MICROCATHETERS MARKET FOR DELIVERY MICROCATHETERS, BY END USER, 2020-2028 (USD MILLION)
- 6.3 ASPIRATION MICROCATHETERS
- 6.3.1 GROWING BURDEN OF CARDIOVASCULAR DISEASES AND RELATED MEDICAL CONDITIONS TO DRIVE MARKET
- TABLE 21 MICROCATHETERS MARKET FOR ASPIRATION MICROCATHETERS, BY REGION, 2020-2028 (USD MILLION)
- TABLE 22 MICROCATHETERS MARKET FOR ASPIRATION MICROCATHETERS, BY PRODUCT DESIGN, 2020-2028 (USD MILLION)
- TABLE 23 MICROCATHETERS MARKET FOR ASPIRATION MICROCATHETERS, BY APPLICATION, 2020-2028 (USD MILLION)
- TABLE 24 MICROCATHETERS MARKET FOR ASPIRATION MICROCATHETERS, BY END USER, 2020-2028 (USD MILLION)
- 6.4 DIAGNOSTICS MICROCATHETERS
- 6.4.1 LARGE TARGET PATIENT POPULATION FOR VASCULAR DISEASES TO DRIVE MARKET
- TABLE 25 MICROCATHETERS MARKET FOR DIAGNOSTIC MICROCATHETERS, BY REGION, 2020-2028 (USD MILLION)
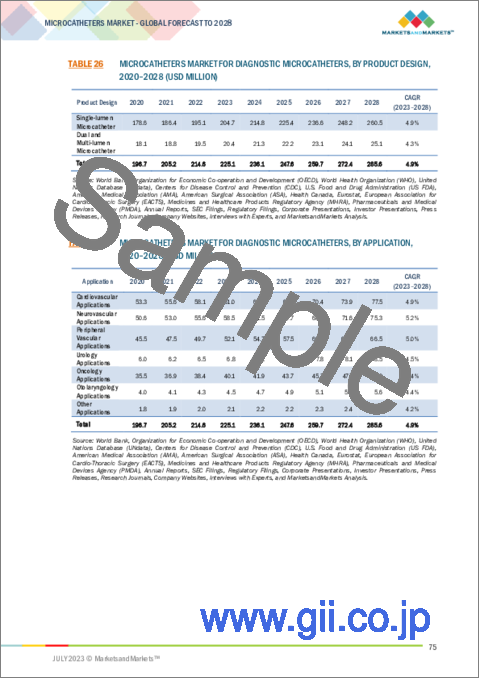
- TABLE 26 MICROCATHETERS MARKET FOR DIAGNOSTIC MICROCATHETERS, BY PRODUCT DESIGN, 2020-2028 (USD MILLION)
- TABLE 27 MICROCATHETERS MARKET FOR DIAGNOSTIC MICROCATHETERS, BY APPLICATION, 2020-2028 (USD MILLION)
- TABLE 28 MICROCATHETERS MARKET FOR DIAGNOSTIC MICROCATHETERS, BY END USER, 2020-2028 (USD MILLION)
- 6.5 STEERABLE MICROCATHETERS
- 6.5.1 GROWING NUMBER OF IMAGE-GUIDED AND MINIMALLY INVASIVE MEDICAL PROCEDURES TO DRIVE MARKET
- TABLE 29 MICROCATHETERS MARKET FOR STEERABLE MICROCATHETERS, BY REGION, 2020-2028 (USD MILLION)
- TABLE 30 MICROCATHETERS MARKET FOR STEERABLE MICROCATHETERS, BY PRODUCT DESIGN, 2020-2028 (USD MILLION)
- TABLE 31 MICROCATHETERS MARKET FOR STEERABLE MICROCATHETERS, BY APPLICATION, 2020-2028 (USD MILLION)
- TABLE 32 MICROCATHETERS MARKET FOR STEERABLE MICROCATHETERS, BY END USER, 2020-2028 (USD MILLION)
7 MICROCATHETERS MARKET, BY PRODUCT DESIGN
- 7.1 INTRODUCTION
- TABLE 33 MICROCATHETERS MARKET, BY PRODUCT DESIGN, 2020-2028 (USD MILLION)
- 7.2 SINGLE-LUMEN MICROCATHETERS
- 7.2.1 INCREASING NUMBER OF TARGET MEDICAL PROCEDURES AND OPERATIONAL ADVANTAGES TO DRIVE MARKET
- TABLE 34 MICROCATHETERS MARKET FOR SINGLE-LUMEN MICROCATHETERS, BY REGION, 2020-2028 (USD MILLION)
- 7.3 DUAL AND MULTI-LUMEN MICROCATHETERS
- 7.3.1 IMPROVED SURGICAL PRECISION AND INCREASED ADOPTION IN COMPLEX VASCULAR PROCEDURES TO DRIVE MARKET
- TABLE 35 MICROCATHETERS MARKET FOR DUAL AND MULTI-LUMEN MICROCATHETERS, BY REGION, 2020-2028 (USD MILLION)
8 MICROCATHETERS MARKET, BY APPLICATION
- 8.1 INTRODUCTION
- TABLE 36 MICROCATHETERS MARKET, BY APPLICATION, 2020-2028 (USD MILLION)
- 8.2 CARDIOVASCULAR APPLICATIONS
- 8.2.1 EFFECTIVE DEVICE PLACEMENT DURING MINIMALLY INVASIVE PROCEDURES AND CRITICAL CARDIAC SURGERIES TO DRIVE MARKET
- TABLE 37 MICROCATHETERS MARKET FOR CARDIOVASCULAR APPLICATIONS, BY REGION, 2020-2028 (USD MILLION)
- 8.3 NEUROVASCULAR APPLICATIONS
- 8.3.1 INCREASING INCIDENCE OF TARGET NEUROLOGICAL CONDITIONS TO DRIVE MARKET
- TABLE 38 MICROCATHETERS MARKET FOR NEUROVASCULAR APPLICATIONS, BY REGION, 2020-2028 (USD MILLION)
- 8.4 PERIPHERAL VASCULAR APPLICATIONS
- 8.4.1 RISING NUMBER OF DIAGNOSTIC AND INTERVENTIONAL PROCEDURES TO DRIVE MARKET
- TABLE 39 MICROCATHETERS MARKET FOR PERIPHERAL VASCULAR APPLICATIONS, BY REGION, 2020-2028 (USD MILLION)
- 8.5 UROLOGY APPLICATIONS
- 8.5.1 INCREASING PREVALENCE OF UROLOGICAL DISORDERS AND ADVANCEMENTS IN MINIMALLY INVASIVE TECHNIQUES TO DRIVE MARKET
- TABLE 40 MICROCATHETERS MARKET FOR UROLOGY APPLICATIONS, BY REGION, 2020-2028 (USD MILLION)
- 8.6 ONCOLOGY APPLICATIONS
- 8.6.1 INCREASING CLINICAL RESEARCH ON MINIMALLY INVASIVE CANCER TREATMENT TO DRIVE MARKET
- TABLE 41 MICROCATHETERS MARKET FOR ONCOLOGY APPLICATIONS, BY REGION, 2020-2028 (USD MILLION)
- 8.7 OTOLARYNGOLOGY APPLICATIONS
- 8.7.1 INCREASED NEED FOR DELICATE ACCESS AND PRECISE NAVIGATION IN DIAGNOSIS TO DRIVE MARKET
- TABLE 42 MICROCATHETERS MARKET FOR OTOLARYNGOLOGY APPLICATIONS, BY REGION, 2020-2028 (USD MILLION)
- 8.8 OTHER APPLICATIONS
- TABLE 43 MICROCATHETERS MARKET FOR OTHER APPLICATIONS, BY REGION, 2020-2028 (USD MILLION)
9 MICROCATHETERS MARKET, BY END USER
- 9.1 INTRODUCTION
- TABLE 44 MICROCATHETERS MARKET, BY END USER, 2020-2028 (USD MILLION)
- 9.2 HOSPITALS, SURGICAL CENTERS, AND SPECIALTY CLINICS
- 9.2.1 LARGE NUMBER OF SURGICAL AND DIAGNOSTIC PROCEDURES TO DRIVE MARKET
- TABLE 45 MICROCATHETERS MARKET FOR HOSPITALS, SURGICAL CENTERS, AND SPECIALTY CLINICS, BY REGION, 2020-2028 (USD MILLION)
- 9.3 AMBULATORY CARE CENTERS
- 9.3.1 GROWING PATIENT SUBMISSIONS FOR DIAGNOSTIC PROCEDURES IN EMERGING ECONOMIES TO DRIVE MARKET
- TABLE 46 MICROCATHETERS MARKET FOR AMBULATORY CARE CENTERS, BY REGION, 2020-2028 (USD MILLION)
10 MICROCATHETERS MARKET, BY REGION
- 10.1 INTRODUCTION
- TABLE 47 MICROCATHETERS MARKET, BY REGION, 2020-2028 (USD MILLION)
- 10.2 NORTH AMERICA
- FIGURE 28 NORTH AMERICA: MICROCATHETERS MARKET SNAPSHOT
- 10.2.1 RECESSION IMPACT: NORTH AMERICA
- TABLE 48 NORTH AMERICA: MICROCATHETERS MARKET, BY COUNTRY, 2020-2028 (USD MILLION)
- TABLE 49 NORTH AMERICA: MICROCATHETERS MARKET, BY PRODUCT, 2020-2028 (USD MILLION)
- TABLE 50 NORTH AMERICA: MICROCATHETERS MARKET, BY PRODUCT DESIGN, 2020-2028 (USD MILLION)
- TABLE 51 NORTH AMERICA: MICROCATHETERS MARKET, BY APPLICATION, 2020-2028 (USD MILLION)
- TABLE 52 NORTH AMERICA: MICROCATHETERS MARKET, BY END USER, 2020-2028 (USD MILLION)
- 10.2.2 US
- 10.2.2.1 US to dominate North American microcatheters market during forecast period
- TABLE 53 US: MICROCATHETERS MARKET, BY PRODUCT, 2020-2028 (USD MILLION)
- 10.2.3 CANADA
- 10.2.3.1 Increasing prevalence of target diseases and high demand for timely diagnosis to drive market
- TABLE 54 CANADA: MICROCATHETERS MARKET, BY PRODUCT, 2020-2028 (USD MILLION)
- 10.3 EUROPE
- 10.4 RECESSION IMPACT: EUROPE
- TABLE 55 EUROPE: MICROCATHETERS MARKET, BY COUNTRY, 2020-2028 (USD MILLION)
- TABLE 56 EUROPE: MICROCATHETERS MARKET, BY PRODUCT, 2020-2028 (USD MILLION)
- TABLE 57 EUROPE: MICROCATHETERS MARKET, BY PRODUCT DESIGN, 2020-2028 (USD MILLION)
- TABLE 58 EUROPE: MICROCATHETERS MARKET, BY APPLICATION, 2020-2028 (USD MILLION)
- TABLE 59 EUROPE: MICROCATHETERS MARKET, BY END USER, 2020-2028 (USD MILLION)
- 10.4.1 GERMANY
- 10.4.1.1 Increasing aging population and rising life expectancy to drive market
- TABLE 60 GERMANY: MICROCATHETERS MARKET, BY PRODUCT, 2020-2028 (USD MILLION)
- 10.4.2 FRANCE
- 10.4.2.1 Significant evolution of healthcare sector and growth in geriatric population to drive market
- TABLE 61 FRANCE: MICROCATHETERS MARKET, BY PRODUCT, 2020-2028 (USD MILLION)
- 10.4.3 UK
- 10.4.3.1 High burden of chronic diseases and large volumes of surgeries to drive market
- TABLE 62 UK: MICROCATHETERS MARKET, BY PRODUCT, 2020-2028 (USD MILLION)
- 10.4.4 ITALY
- 10.4.4.1 Increasing geriatric population to drive market
- TABLE 63 ITALY: MICROCATHETERS MARKET, BY PRODUCT, 2020-2028 (USD MILLION)
- 10.4.5 SPAIN
- 10.4.5.1 Increasing technological developments in healthcare sector to drive market
- TABLE 64 SPAIN: MICROCATHETERS MARKET, BY PRODUCT, 2020-2028 (USD MILLION)
- 10.4.6 REST OF EUROPE
- TABLE 65 REST OF EUROPE: MICROCATHETERS MARKET, BY PRODUCT, 2020-2028 (USD MILLION)
- 10.5 ASIA PACIFIC
- 10.6 RECESSION IMPACT: ASIA PACIFIC
- FIGURE 29 ASIA PACIFIC: MICROCATHETERS MARKET SNAPSHOT
- TABLE 66 ASIA PACIFIC: MICROCATHETERS MARKET, BY COUNTRY, 2020-2028 (USD MILLION)
- TABLE 67 ASIA PACIFIC: MICROCATHETERS MARKET, BY PRODUCT, 2020-2028 (USD MILLION)
- TABLE 68 ASIA PACIFIC: MICROCATHETERS MARKET, BY PRODUCT DESIGN, 2020-2028 (USD MILLION)
- TABLE 69 ASIA PACIFIC: MICROCATHETERS MARKET, BY APPLICATION, 2020-2028 (USD MILLION)
- TABLE 70 ASIA PACIFIC: MICROCATHETERS MARKET, BY END USER, 2020-2028 (USD MILLION)
- 10.6.1 JAPAN
- 10.6.1.1 Increasing geriatric population and surgical procedures to drive market
- TABLE 71 JAPAN: MICROCATHETERS MARKET, BY PRODUCT, 2020-2028 (USD MILLION)
- 10.6.2 CHINA
- 10.6.2.1 Growing prevalence of chronic disorders and developing healthcare infrastructure to drive market
- TABLE 72 CHINA: MICROCATHETERS MARKET, BY PRODUCT, 2020-2028 (USD MILLION)
- 10.6.3 INDIA
- 10.6.3.1 Increasing target patient population with rising availability of advanced surgical treatments to drive market
- TABLE 73 INDIA: MICROCATHETERS MARKET, BY PRODUCT, 2020-2028 (USD MILLION)
- 10.6.4 AUSTRALIA
- 10.6.4.1 Developing healthcare sector and increasing availability of advanced surgical treatments to drive market
- TABLE 74 AUSTRALIA: MICROCATHETERS MARKET, BY PRODUCT, 2020-2028 (USD MILLION)
- 10.6.5 SOUTH KOREA
- 10.6.5.1 Growing target patient population to drive market
- TABLE 75 SOUTH KOREA: MICROCATHETERS MARKET, BY PRODUCT, 2020-2028 (USD MILLION)
- 10.6.6 REST OF ASIA PACIFIC
- TABLE 76 REST OF ASIA PACIFIC: MICROCATHETERS MARKET, BY PRODUCT, 2020-2028 (USD MILLION)
- 10.7 LATIN AMERICA
- 10.8 RECESSION IMPACT: LATIN AMERICA
- TABLE 77 LATIN AMERICA: MICROCATHETERS MARKET, BY COUNTRY, 2020-2028 (USD MILLION)
- TABLE 78 LATIN AMERICA: MICROCATHETERS MARKET, BY PRODUCT, 2020-2028 (USD MILLION)
- TABLE 79 LATIN AMERICA: MICROCATHETERS MARKET, BY PRODUCT DESIGN, 2020-2028 (USD MILLION)
- TABLE 80 LATIN AMERICA: MICROCATHETERS MARKET, BY APPLICATION, 2020-2028 (USD MILLION)
- TABLE 81 LATIN AMERICA: MICROCATHETERS MARKET, BY END USER, 2020-2028 (USD MILLION)
- 10.8.1 BRAZIL
- 10.8.1.1 Developing healthcare sector and increasing availability of advanced surgical treatments to drive market
- TABLE 82 BRAZIL: MICROCATHETERS MARKET, BY PRODUCT, 2020-2028 (USD MILLION)
- 10.8.2 MEXICO
- 10.8.2.1 Favorable government initiatives and rapidly growing healthcare sector to drive market
- TABLE 83 MEXICO: MICROCATHETERS MARKET, BY PRODUCT, 2020-2028 (USD MILLION)
- 10.9 MIDDLE EAST & AFRICA
- 10.9.1 INCREASING FOCUS OF MARKET PLAYERS AND ONGOING ECONOMIC GROWTH TO DRIVE MARKET
- 10.10 RECESSION IMPACT: MIDDLE EAST & AFRICA
- TABLE 84 MIDDLE EAST & AFRICA: MICROCATHETERS MARKET, BY PRODUCT, 2020-2028 (USD MILLION)
- TABLE 85 MIDDLE EAST & AFRICA: MICROCATHETERS MARKET, BY PRODUCT DESIGN, 2020-2028 (USD MILLION)
- TABLE 86 MIDDLE EAST & AFRICA: MICROCATHETERS MARKET, BY APPLICATION, 2020-2028 (USD MILLION)
- TABLE 87 MIDDLE EAST & AFRICA: MICROCATHETERS MARKET, BY END USER, 2020-2028 (USD MILLION)
11 COMPETITIVE LANDSCAPE
- 11.1 OVERVIEW
- 11.2 KEY STRATEGIES ADOPTED BY MAJOR PLAYERS
- 11.2.1 OVERVIEW OF STRATEGIES ADOPTED BY KEY PLAYERS IN MICROCATHETERS MARKET
- TABLE 88 OVERVIEW OF MAJOR STRATEGIES ADOPTED BY KEY PLAYERS IN MICROCATHETERS MARKET
- 11.3 REVENUE SHARE ANALYSIS OF KEY MARKET PLAYERS
- FIGURE 30 REVENUE SHARE ANALYSIS OF KEY PLAYERS IN MICROCATHETERS MARKET
- 11.4 MARKET RANKING ANALYSIS
- FIGURE 31 MICROCATHETERS MARKET RANKING, BY KEY PLAYER (2022)
- 11.5 COMPANY EVALUATION MATRIX FOR KEY PLAYERS (2022)
- 11.5.1 STARS
- 11.5.2 EMERGING LEADERS
- 11.5.3 PERVASIVE PLAYERS
- 11.5.4 PARTICIPANTS
- FIGURE 32 MICROCATHETERS MARKET: COMPANY EVALUATION MATRIX FOR KEY PLAYERS, 2022
- 11.6 COMPANY EVALUATION MATRIX FOR START-UPS/SMES (2022)
- 11.6.1 PROGRESSIVE COMPANIES
- 11.6.2 STARTING BLOCKS
- 11.6.3 RESPONSIVE COMPANIES
- 11.6.4 DYNAMIC COMPANIES
- FIGURE 33 MICROCATHETERS MARKET: COMPANY EVALUATION MATRIX FOR START-UPS/ SMES, 2022
- 11.7 COMPANY FOOTPRINT
- TABLE 89 PRODUCT AND REGIONAL FOOTPRINT ANALYSIS OF TOP PLAYERS IN MICROCATHETERS MARKET
- TABLE 90 COMPANY PRODUCT FOOTPRINT
- TABLE 91 COMPANY GEOGRAPHICAL FOOTPRINT
- 11.8 COMPETITIVE SCENARIOS AND TRENDS
- TABLE 92 KEY PRODUCT LAUNCHES
- TABLE 93 KEY DEALS
- TABLE 94 OTHER KEY DEVELOPMENTS
12 COMPANY PROFILES
- 12.1 KEY PLAYERS
- (Business Overview, Products Offered, Recent Developments, and MnM View (Key strengths/Right to Win, Strategic Choices Made, and Weaknesses and Competitive Threats))**
- 12.1.1 MEDTRONIC
- TABLE 95 MEDTRONIC: COMPANY OVERVIEW
- FIGURE 34 MEDTRONIC: COMPANY SNAPSHOT (2022)
- 12.1.2 BOSTON SCIENTIFIC CORPORATION
- TABLE 96 BOSTON SCIENTIFIC CORPORATION: COMPANY OVERVIEW
- FIGURE 35 BOSTON SCIENTIFIC CORPORATION: COMPANY SNAPSHOT (2022)
- 12.1.3 TERUMO MEDICAL CORPORATION
- TABLE 97 TERUMO MEDICAL CORPORATION: COMPANY OVERVIEW
- FIGURE 36 TERUMO MEDICAL CORPORATION: COMPANY SNAPSHOT (2022)
- 12.1.4 TELEFLEX INCORPORATED
- TABLE 98 TELEFLEX INCORPORATED: COMPANY OVERVIEW
- FIGURE 37 TELEFLEX INCORPORATED: COMPANY SNAPSHOT (2022)
- 12.1.5 MERIT MEDICAL SYSTEMS, INC.
- TABLE 99 MERIT MEDICAL SYSTEMS, INC.: COMPANY OVERVIEW
- FIGURE 38 MERIT MEDICAL SYSTEMS, INC.: COMPANY SNAPSHOT (2022)
- 12.1.6 ASAHI INTECC CO., LTD.
- TABLE 100 ASAHI INTECC CO., LTD.: COMPANY OVERVIEW
- FIGURE 39 ASAHI INTECC CO., LTD.: COMPANY SNAPSHOT (2022)
- 12.1.7 STRYKER
- TABLE 101 STRYKER: COMPANY OVERVIEW
- FIGURE 40 STRYKER: COMPANY SNAPSHOT (2022)
- 12.1.8 SURMODICS, INC.
- TABLE 102 SURMODICS, INC.: COMPANY OVERVIEW
- FIGURE 41 SURMODICS, INC.: COMPANY SNAPSHOT (2022)
- 12.1.9 CARDINAL HEALTH, INC.
- TABLE 103 CARDINAL HEALTH, INC.: COMPANY OVERVIEW
- FIGURE 42 CARDINAL HEALTH, INC.: COMPANY SNAPSHOT (2022)
- 12.1.10 JOHNSON & JOHNSON
- TABLE 104 JOHNSON & JOHNSON: COMPANY OVERVIEW
- FIGURE 43 JOHNSON & JOHNSON: COMPANY SNAPSHOT (2022)
- 12.1.11 GUERBET LLC
- TABLE 105 GUERBET LLC: COMPANY OVERVIEW
- FIGURE 44 GUERBET LLC: COMPANY SNAPSHOT (2022)
- 12.1.12 LEPU MEDICAL TECHNOLOGY (BEIJING) CO., LTD
- TABLE 106 LEPU MEDICAL TECHNOLOGY (BEIJING) CO., LTD.: COMPANY OVERVIEW
- FIGURE 45 LEPU MEDICAL TECHNOLOGY (BEIJING) CO., LTD.: COMPANY SNAPSHOT (2022)
- 12.1.13 BECTON, DICKINSON AND COMPANY
- TABLE 107 BECTON, DICKINSON AND COMPANY: COMPANY OVERVIEW
- FIGURE 46 BECTON, DICKINSON AND COMPANY: COMPANY SNAPSHOT (2022)
- 12.1.14 PENUMBRA, INC.
- TABLE 108 PENUMBRA, INC.: COMPANY OVERVIEW
- FIGURE 47 PENUMBRA, INC.: COMPANY SNAPSHOT (2022)
- 12.1.15 KANEKA CORPORATION
- TABLE 109 KANEKA CORPORATION: COMPANY OVERVIEW
- FIGURE 48 KANEKA CORPORATION: COMPANY SNAPSHOT (2022)
- 12.1.16 ANGIODYNAMICS, INC.
- TABLE 110 ANGIODYNAMICS, INC.: COMPANY OVERVIEW
- FIGURE 49 ANGIODYNAMICS, INC.: COMPANY SNAPSHOT (2022)
- 12.1.17 BIOCARDIA INC.
- TABLE 111 BIOCARDIA INC.: COMPANY OVERVIEW
- 12.2 OTHER PLAYERS
- 12.2.1 MILLAR, INC.
- 12.2.2 BAYLIS MEDICAL COMPANY, INC.
- 12.2.3 EMBOLX, INC.
- 12.2.4 ACANDIS GMBH
- 12.2.5 ACROSTAK
- 12.2.6 COOK MEDICAL
- 12.2.7 REFLOW MEDICAL, INC.
- 12.2.8 TZ MEDICAL, INC.
- 12.2.9 ORBUSNEICH MEDICAL
- 12.2.10 SPARTAN MICRO, INC.
- 12.2.11 TRANSIT SCIENTIFIC
- 12.2.12 TOKAI MEDICAL PRODUCTS
- 12.2.13 LIBATAPE PHARMACEUTICAL CO., LTD
- *Details on Business Overview, Products Offered, Recent Developments, and MnM View (Key strengths/Right to Win, Strategic Choices Made, and Weaknesses and Competitive Threats) might not be captured in case of unlisted companies.
13 APPENDIX
- 13.1 DISCUSSION GUIDE
- 13.2 KNOWLEDGESTORE: MARKETSANDMARKETS' SUBSCRIPTION PORTAL
- 13.3 CUSTOMIZATION OPTIONS
- 13.4 RELATED REPORTS
- 13.5 AUTHOR DETAILS