|
|
市場調査レポート
商品コード
1386901
粒子線治療の世界市場:製品・サービス別、システム別、タイプ別、用途別、がんタイプ別、用途別-2028年までの予測Particle Therapy Market by Type (Proton Therapy, Heavy Ion), Products (Cyclotron, Synchrotron, Synchrocyclotron), Services, System (Single-room, Multi-room), Cancer Type (Pediatric, Prostate), Application (Treatment, Research) - Global Forecast to 2028 |
||||||
カスタマイズ可能
|
|||||||
| 粒子線治療の世界市場:製品・サービス別、システム別、タイプ別、用途別、がんタイプ別、用途別-2028年までの予測 |
|
出版日: 2023年11月09日
発行: MarketsandMarkets
ページ情報: 英文 196 Pages
納期: 即納可能
|
- 全表示
- 概要
- 目次
| 調査範囲 | |
|---|---|
| 調査対象年 | 2020年~2028年 |
| 基準年 | 2022年 |
| 予測期間 | 2023年~2028年 |
| 単位 | 金額(10億米ドル) |
| セグメント | 製品・サービス別、システム別、タイプ別、用途別、がんタイプ別、地域別 |
| 対象地域 | 北米、欧州、アジア太平洋、ラテンアメリカ、中東・アフリカ |
世界の粒子線治療の市場規模は、2023年の7億米ドルから2028年には11億米ドルに達し、予測期間中にCAGR 8.2%で成長すると予測されています。
粒子線治療市場の急成長が予測される背景には、がんの増加、技術の進歩、がんに関する個人の意識の向上、がん研究への資金提供の増加などがあります。
粒子線治療市場は、がんタイプ別では、2022年には小児がんが最も高い成長率を示しています。小児がんの増加は、遺伝的素因、乳幼児の発育要因、未熟な免疫系、環境暴露、母親要因、細胞の脆弱性などの理由によるものです。
アジア太平洋諸国での成熟しつつある人口統計プロファイルは、治療に関連する副作用を最小限に抑えることを特徴とする効率的ながん治療法の必要性を強調しています。粒子線治療市場の精密さ主導の特性とこの共鳴は、市場展望を強化する上で極めて重要な役割を立証しています。
アジア太平洋の病院では多言語による患者ケアを提供しており、粒子線治療市場のような先進治療へのアクセスも容易であることから、この地域は特に多様な患者の間で独特の魅力を放っています。このような言語による包括性と最先端の医療介入の融合は、この地域の好まれているヘルスケア目的地としての望ましさを著しく高め、それによってヘルスケア市場の競争力を強化し、粒子線治療市場の隆盛を後押ししています。
当レポートでは、世界の粒子線治療市場について調査し、製品・サービス別、システム別、タイプ別、用途別、がんタイプ別、地域別動向、および市場に参入する企業のプロファイルなどをまとめています。
目次
第1章 イントロダクション
第2章 調査手法
第3章 エグゼクティブサマリー
第4章 重要考察
第5章 市場概要
- イントロダクション
- 生態系/市場マップ
- 価格分析
- サプライチェーン分析
- 特許分析
- バリューチェーン分析
- 技術分析
- 2023年~2024年の主な会議とイベント
- 規制状況
- 償還分析
- 貿易分析
- ポーターのファイブフォース分析
- 主要な利害関係者と購入基準
- ケーススタディ分析
- 顧客のビジネスに影響を与える動向/混乱
第6章 粒子線治療市場、製品・サービス別
- イントロダクション
- 製品
- サービス
第7章 粒子線治療市場、タイプ別
- イントロダクション
- 陽子線治療
- 重粒子線治療
第8章 粒子線治療市場、システム別
- イントロダクション
- マルチルームシステム
- シングルルームシステム
第9章 粒子線治療市場、用途別
- イントロダクション
- 治療
- 研究
第10章 粒子線治療市場、がんタイプ別
- イントロダクション
- 小児がん
- 前立腺がん
- 肺がん
- 乳がん
- 頭頸部がん
- その他
第11章 粒子線治療市場、地域別
- イントロダクション
- 北米
- 欧州
- アジア太平洋
- ラテンアメリカ
- 中東・アフリカ
第12章 競合情勢
- 概要
- 主要参入企業が採用した戦略
- 市場トッププレーヤーの収益シェア分析
- 市場シェア分析
- 企業評価マトリックス
- 主要企業の競合ベンチマーキング
- スタートアップ/中小企業の評価マトリックス
- 競争シナリオと動向
第13章 企業プロファイル
- 主要参入企業
- IBA WORLDWIDE
- VARIAN MEDICAL SYSTEMS, INC.
- HITACHI, LTD.
- MEVION MEDICAL SYSTEMS
- SUMITOMO HEAVY INDUSTRIES LTD.
- PROVISION HEALTHCARE, LLC
- TOSHIBA MEDICAL SYSTEMS CORPORATION
- OPTIVUS PROTON THERAPY, INC.
- PROTOM INTERNATIONAL, INC.
- ADVANCED ONCOTHERAPY PLC
- その他の企業
- DANFYSIK A/S
- P-CURE, LTD.
- PTW FREIBURG GMBH
第14章 付録
Report Description
| Scope of the Report | |
|---|---|
| Years Considered for the Study | 2020-2028 |
| Base Year | 2022 |
| Forecast Period | 2023-2028 |
| Units Considered | Value (USD) Billion |
| Segments | Product, system, type, application, cancer-type and region |
| Regions covered | North America, Europe, Asia Pacific, Latin America and Middle East & Africa |
The global particle therapy market is projected to reach USD 1.1 billion by 2028 from USD 0.7 billion in 2023, growing at a CAGR of 8.2% during the forecast period. The projected surge in market growth for the Particle therapy market is seen due to an increase in cancers, advancements in technologies, and increased awareness among individuals regarding cancer, Increased funding for cancer research.
"The Paediatric application segment to register highest CAGR over the forecast period of 2023-2028."
Based on the cancer type, the Particle therapy market is segmented into pediatric cancer, breast cancer, lung cancer, prostate cancer, head and neck, and other cancers. Pediatric cancer has witnessed the highest growth rate In 2022. The increase in Paediatric cancers can be seen due to the following reasons genetic predisposition, developmental factors in infants, immature immune system, environmental exposures, maternal factors, and cellular vulnerability among others.
"Asia Pacific to register significant growth rate in the market during the forecast period."
For the forecasting period 2023-2028, the APAC region is expected to register a significant growth rate in the market during the forecast period. Asia Pacific comprises India, China, Japan, Australia, South Korea, and RoAPAC. The Asia-Pacific (APAC) region has witnessed a significant increase in the market growth rate for the particle therapy market. There are several drivers that contributed to this growth:
The maturing demographic profile observed in APAC countries accentuates the imperative for efficacious cancer treatment modalities characterized by minimized treatment-related adverse effects. This resonance with the precision-driven attributes of the Particle therapy market substantiates its pivotal role in enhancing the market landscape.
The incorporation of multilingual patient care offerings within APAC hospitals, combined with the accessibility to advanced treatments like the Particle therapy market, confers a unique allure to the region, particularly among a diverse patient spectrum. This fusion of linguistic inclusiveness and state-of-the-art medical interventions significantly enhances the region's desirability as a favored healthcare destination, thereby fortifying its competitive position within the healthcare market landscape and concomitantly propelling the prominence of particle therapy market.
A breakdown of the primary participants referred to for this report is provided below:
- By Company Type: Tier 1-40%, Tier 2-30%, and Tier 3- 30%
- By Designation: C-level-27%, Director-level-18%, and Others-55%
- By Region: North America-50%, Europe-20%, Asia Pacific-15%, Latin America-10%, and the Middle East & Africa-5%
Prominent players in particle therapy market are Varian Medical Systems, Inc.(US), IBA Worldwide(EU) and Hitachi (Japan) among others.
Research Coverage
- The report studies the Particle therapy market based on type, system, product, application, cancer type and region.
- The report analyzes factors (such as drivers, restraints, opportunities, and challenges) affecting the market growth.
- The report evaluates the opportunities and challenges in the market for stakeholders and provides details of the competitive landscape for market leaders.
- The report studies micro markets with respect to their growth trends, prospects, and contributions to the global Particle therapy market.
- The report forecasts the revenue of market segments with respect to five major regions.
Key Benefits of Buying the Report:
The report will help the market leaders/new entrants/smaller firms in this market with investment evaluation viability within the Particle therapy market through a thorough analysis of comprehensive data, thereby facilitating robust risk assessment and enabling well-informed investment determinations. Benefit from meticulous market segmentation encompassing application, end-user, and regional dimensions, affording tailored insights for precise segment targeting. The report also provides an all-encompassing evaluation of encapsulating pivotal trends, growth catalysts, challenges, and prospects, thereby empowering strategic decision-making with astute discernment.
The report provides insights on the following pointers:
- Analysis of key drivers (increasing prevalence of cancer patient population, Increasing government initiatives for cancer management, advancements in non-invasive treatments through radiation therapy), restraints (Dearth of skilled radiologist/oncologist, high cost of particle therapy, Complexity of imaging technology for treatments), opportunities (Expansion of key players in emerging countries, Rising healthcare expenditure across developing countries), and challenges (Availability of alternative technology) influencing the growth of the Particle therapy market
- Product Development/Innovation: Detailed insights on upcoming technologies, research & development activities, and new product & service launches in the particle therapy market.
- Market Development: Comprehensive information about lucrative markets - the report analyses the Particle therapy market across varied regions.
- Market Diversification: Exhaustive information about new products, untapped geographies, recent developments, and investments in the Particle therapy market
- Competitive Assessment: In-depth assessment of market shares, growth strategies, and service offerings of leading players like Varian Medical Systems, Inc.(US), IBA Worldwide(EU) and Hitachi (Japan) among others.
TABLE OF CONTENTS
1 INTRODUCTION
- 1.1 STUDY OBJECTIVES
- 1.2 MARKET DEFINITION
- 1.3 INCLUSIONS & EXCLUSIONS
- 1.4 MARKET SCOPE
- 1.4.1 MARKETS COVERED
- 1.4.2 REGIONS COVERED
- 1.4.3 YEARS CONSIDERED
- 1.4.4 CURRENCY CONSIDERED
- TABLE 1 EXCHANGE RATES UTILIZED FOR CONVERSION TO USD
- 1.5 STAKEHOLDERS
- 1.6 SUMMARY OF CHANGES
- 1.6.1 RECESSION IMPACT: PARTICLE THERAPY MARKET
2 RESEARCH METHODOLOGY
- 2.1 RESEARCH DATA
- FIGURE 1 RESEARCH DESIGN
- 2.1.1 SECONDARY DATA
- 2.1.1.1 Key data from secondary sources
- 2.1.2 PRIMARY DATA
- 2.1.2.1 Key primary sources
- 2.1.2.2 Key data from primary sources
- 2.1.2.3 Breakdown of primaries
- FIGURE 2 BREAKDOWN OF PRIMARY INTERVIEWS (SUPPLY-SIDE): BY COMPANY TYPE, DESIGNATION, AND REGION
- FIGURE 3 BREAKDOWN OF PRIMARY INTERVIEWS (DEMAND-SIDE): BY END USER, DESIGNATION, AND REGION
- 2.1.2.4 Key industry insights
- 2.2 MARKET SIZE ESTIMATION
- FIGURE 4 MARKET SIZE ESTIMATION: REVENUE SHARE ANALYSIS, 2022
- FIGURE 5 SUPPLY-SIDE ANALYSIS
- FIGURE 6 TOP-DOWN APPROACH
- FIGURE 7 CAGR PROJECTIONS FROM ANALYSIS OF DRIVERS, RESTRAINTS, OPPORTUNITIES, AND CHALLENGES
- FIGURE 8 CAGR PROJECTIONS
- 2.3 MARKET BREAKDOWN AND DATA TRIANGULATION
- FIGURE 9 DATA TRIANGULATION METHODOLOGY
- 2.4 RESEARCH LIMITATIONS
- 2.4.1 SCOPE-RELATED LIMITATIONS
- 2.4.2 METHODOLOGY-RELATED LIMITATIONS
- 2.5 RISK ASSESSMENT
- 2.6 STUDY ASSUMPTIONS
- 2.6.1 IMPACT OF RECESSION ON PARTICLE THERAPY MARKET
3 EXECUTIVE SUMMARY
- FIGURE 10 PARTICLE THERAPY MARKET, BY TYPE, 2023 VS. 2028 (USD MILLION)
- FIGURE 11 PARTICLE THERAPY MARKET, BY PRODUCT & SERVICE, 2023 VS. 2028 (USD MILLION)
- FIGURE 12 PARTICLE THERAPY PRODUCTS MARKET, BY TYPE, 2023 VS. 2028 (USD MILLION)
- FIGURE 13 PARTICLE THERAPY MARKET, BY SYSTEM, 2023 VS. 2028 (USD MILLION)
- FIGURE 14 PARTICLE THERAPY MARKET, BY CANCER TYPE, 2023 VS. 2028 (USD MILLION)
- FIGURE 15 PARTICLE THERAPY MARKET, BY APPLICATION, 2023 VS. 2028 (USD MILLION)
- FIGURE 16 GEOGRAPHICAL SNAPSHOT OF PARTICLE THERAPY MARKET
4 PREMIUM INSIGHTS
- 4.1 PARTICLE THERAPY MARKET OVERVIEW
- FIGURE 17 INCREASING PREVALENCE OF CANCER TO DRIVE MARKET
- 4.2 REGIONAL MIX: PARTICLE THERAPY MARKET
- FIGURE 18 ASIA PACIFIC TO REGISTER HIGHEST GROWTH RATE DURING STUDY PERIOD
- 4.3 ASIA PACIFIC: PARTICLE THERAPY MARKET, BY TYPE AND COUNTRY (2022)
- FIGURE 19 PROTON THERAPY ACCOUNTED FOR LARGEST SHARE OF ASIA PACIFIC MARKET IN 2022
- 4.4 PARTICLE THERAPY MARKET: GEOGRAPHIC GROWTH OPPORTUNITIES
- FIGURE 20 CHINA TO REGISTER HIGHEST CAGR DURING FORECAST PERIOD
- 4.5 DEVELOPED VS. EMERGING ECONOMIES: PARTICLE THERAPY MARKET
- FIGURE 21 EMERGING ECONOMIES TO REGISTER HIGHER GROWTH DURING FORECAST PERIOD
5 MARKET OVERVIEW
- 5.1 INTRODUCTION
- FIGURE 22 PARTICLE THERAPY MARKET: DRIVERS, RESTRAINTS, OPPORTUNITIES, AND CHALLENGES
- 5.1.1 DRIVERS
- 5.1.1.1 Advantages of particle therapy over photon therapy
- 5.1.1.2 Growing prevalence of cancer
- 5.1.1.3 Rising adoption of particle therapy in clinical trials
- 5.1.1.4 Increasing number of particle therapy centers worldwide
- 5.1.1.5 Technological advancements
- 5.1.2 RESTRAINTS
- 5.1.2.1 Infrastructural challenges in healthcare facilities
- 5.1.2.2 Affordability and accessibility of treatments
- 5.1.2.3 Unfavorable reimbursement policies and limited insurance coverage for particle therapy
- 5.1.3 OPPORTUNITIES
- 5.1.3.1 Growth potential of emerging economies
- 5.1.4 CHALLENGES
- 5.1.4.1 Difficulties in visualizing tumors during particle therapy procedures
- 5.1.4.2 Potential for unwanted radiation exposure
- 5.2 ECOSYSTEM/MARKET MAP
- FIGURE 23 PARTICLE THERAPY MARKET: ECOSYSTEM ANALYSIS
- 5.3 PRICING ANALYSIS
- 5.4 SUPPLY CHAIN ANALYSIS
- FIGURE 24 SUPPLY CHAIN ANALYSIS: PARTICLE THERAPY MARKET
- 5.5 PATENT ANALYSIS
- 5.5.1 PATENT PUBLICATION TRENDS FOR PARTICLE THERAPY MARKET
- FIGURE 25 TOTAL PATENTS GRANTED FROM 2012-2022
- FIGURE 26 TOP PATENT APPLICANTS IN PARTICLE THERAPY
- FIGURE 27 TOP PATENT OWNERS IN PARTICLE THERAPY
- 5.6 VALUE CHAIN ANALYSIS
- FIGURE 28 PARTICLE THERAPY MARKET: VALUE CHAIN ANALYSIS
- 5.7 TECHNOLOGY ANALYSIS
- 5.8 KEY CONFERENCES & EVENTS IN 2023-2024
- 5.9 REGULATORY LANDSCAPE
- TABLE 2 NORTH AMERICA: LIST OF REGULATORY BODIES, GOVERNMENT AGENCIES, AND OTHER ORGANIZATIONS
- TABLE 3 EUROPE: LIST OF REGULATORY BODIES, GOVERNMENT AGENCIES, AND OTHER ORGANIZATIONS
- TABLE 4 ASIA PACIFIC: LIST OF REGULATORY BODIES, GOVERNMENT AGENCIES, AND OTHER ORGANIZATIONS
- TABLE 5 REST OF THE WORLD: LIST OF REGULATORY BODIES, GOVERNMENT AGENCIES, AND OTHER ORGANIZATIONS
- TABLE 6 REGULATORY STANDARDS/APPROVALS REQUIRED FOR PARTICLE THERAPY PRODUCTS BY COUNTRY/REGION
- 5.10 REIMBURSEMENT ANALYSIS
- TABLE 7 MEDICAL REIMBURSEMENT CODES FOR PARTICLE THERAPY PROCEDURES IN US, 2022
- 5.11 TRADE ANALYSIS
- TABLE 8 INSTALLED BASE OF RADIOTHERAPY DEVICES
- 5.12 PORTER'S FIVE FORCES ANALYSIS
- TABLE 9 PARTICLE THERAPY MARKET: PORTER'S FIVE FORCES ANALYSIS
- 5.12.1 THREAT OF NEW ENTRANTS
- 5.12.2 THREAT OF SUBSTITUTES
- 5.12.3 BARGAINING POWER OF SUPPLIERS
- 5.12.4 BARGAINING POWER OF BUYERS
- 5.12.5 DEGREE OF COMPETITION
- 5.13 KEY STAKEHOLDERS & BUYING CRITERIA
- 5.13.1 KEY STAKEHOLDERS IN BUYING PROCESS
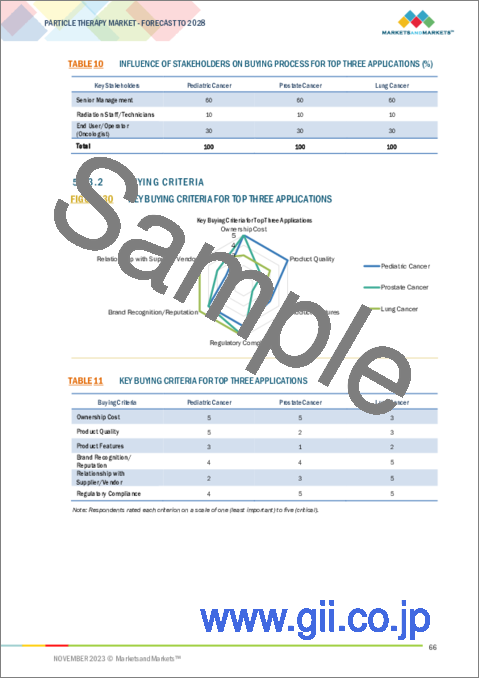
- FIGURE 29 INFLUENCE OF STAKEHOLDERS ON BUYING PROCESS FOR TOP THREE APPLICATIONS
- TABLE 10 INFLUENCE OF STAKEHOLDERS ON BUYING PROCESS FOR TOP THREE APPLICATIONS (%)
- 5.13.2 BUYING CRITERIA
- FIGURE 30 KEY BUYING CRITERIA FOR TOP THREE APPLICATIONS
- TABLE 11 KEY BUYING CRITERIA FOR TOP THREE APPLICATIONS
- 5.14 CASE STUDY ANALYSIS
- TABLE 12 CASE STUDY: ENHANCING PATIENT SAFETY-RT'S ROLE IN MINIMIZING RADIATION RISK IN AN ELDERLY PATIENT (90-YEAR-OLD FEMALE WITH A MEDICALLY INOPERABLE EARLY-STAGE NSCLC)
- 5.15 TRENDS/DISRUPTIONS IMPACTING CUSTOMER'S BUSINESS
- FIGURE 31 EMERGING TRENDS AND OPPORTUNITIES AFFECTING FUTURE REVENUE MIX
6 PARTICLE THERAPY MARKET, BY PRODUCT & SERVICE
- 6.1 INTRODUCTION
- TABLE 13 PARTICLE THERAPY MARKET, BY PRODUCT & SERVICE, 2021-2028 (USD MILLION)
- 6.2 PRODUCTS
- TABLE 14 PARTICLE THERAPY PRODUCTS MARKET, BY TYPE, 2021-2028 (USD MILLION)
- TABLE 15 PARTICLE THERAPY PRODUCTS MARKET, BY REGION, 2021-2028 (USD MILLION)
- 6.2.1 CYCLOTRONS
- 6.2.1.1 Advantages offered by cyclotrons over other accelerators to drive market
- TABLE 16 CYCLOTRONS MARKET, BY REGION, 2021-2028 (USD MILLION)
- TABLE 17 NORTH AMERICA: CYCLOTRONS MARKET, BY COUNTRY, 2021-2028 (USD MILLION)
- TABLE 18 EUROPE: CYCLOTRONS MARKET, BY COUNTRY, 2021-2028 (USD MILLION)
- TABLE 19 ASIA PACIFIC: CYCLOTRONS MARKET, BY COUNTRY, 2021-2028 (USD MILLION)
- TABLE 20 LATIN AMERICA: CYCLOTRONS MARKET, BY COUNTRY, 2021-2028 (USD MILLION)
- 6.2.2 SYNCHROTRONS
- 6.2.2.1 Increasing investments for development of synchrotron facilities to aid market growth
- TABLE 21 SYNCHROTRONS MARKET, BY REGION, 2021-2028 (USD MILLION)
- TABLE 22 NORTH AMERICA: SYNCHROTRONS MARKET, BY COUNTRY, 2021-2028 (USD MILLION)
- TABLE 23 EUROPE: SYNCHROTRONS MARKET, BY COUNTRY, 2021-2028 (USD MILLION)
- TABLE 24 ASIA PACIFIC: SYNCHROTRONS MARKET, BY COUNTRY, 2021-2028 (USD MILLION)
- TABLE 25 LATIN AMERICA: SYNCHROTRONS MARKET, BY COUNTRY, 2021-2028 (USD MILLION)
- 6.2.3 SYNCHROCYCLOTRONS
- 6.2.3.1 High space requirements to limit adoption
- TABLE 26 SYNCHROCYCLOTRONS MARKET, BY REGION, 2021-2028 (USD MILLION)
- TABLE 27 NORTH AMERICA: SYNCHROCYCLOTRONS MARKET, BY COUNTRY, 2021-2028 (USD MILLION)
- TABLE 28 EUROPE: SYNCHROCYCLOTRONS MARKET, BY COUNTRY, 2021-2028 (USD MILLION)
- TABLE 29 ASIA PACIFIC: SYNCHROCYCLOTRONS MARKET, BY COUNTRY, 2021-2028 (USD MILLION)
- TABLE 30 LATIN AMERICA: SYNCHROCYCLOTRONS MARKET, BY COUNTRY, 2021-2028 (USD MILLION)
- 6.3 SERVICES
- 6.3.1 AVAILABILITY OF WIDE RANGE OF SERVICES TO PROPEL MARKET
- TABLE 31 PARTICLE THERAPY SERVICES MARKET, BY REGION, 2021-2028 (USD MILLION)
- TABLE 32 NORTH AMERICA: PARTICLE THERAPY SERVICES MARKET, BY COUNTRY, 2021-2028 (USD MILLION)
- TABLE 33 EUROPE: PARTICLE THERAPY SERVICES MARKET, BY COUNTRY, 2021-2028 (USD MILLION)
- TABLE 34 ASIA PACIFIC: PARTICLE THERAPY SERVICES MARKET, BY COUNTRY, 2021-2028 (USD MILLION)
- TABLE 35 LATIN AMERICA: PARTICLE THERAPY SERVICES MARKET, BY COUNTRY, 2021-2028 (USD MILLION)
7 PARTICLE THERAPY MARKET, BY TYPE
- 7.1 INTRODUCTION
- TABLE 36 PARTICLE THERAPY MARKET, BY TYPE, 2021-2028 (USD MILLION)
- 7.2 PROTON THERAPY
- 7.2.1 GROWING ADOPTION AS PRIMARY TREATMENT FOR VARIOUS CANCER TYPES TO DRIVE MARKET
- TABLE 37 PROTON THERAPY MARKET, BY REGION, 2021-2028 (USD MILLION)
- 7.3 HEAVY ION THERAPY
- 7.3.1 SLOWER GROWTH ATTRIBUTED TO EXPERIMENTAL PHASE ACROSS MANY REGIONS
- TABLE 38 HEAVY ION THERAPY MARKET, BY REGION, 2021-2028 (USD MILLION)
8 PARTICLE THERAPY MARKET, BY SYSTEM
- 8.1 INTRODUCTION
- TABLE 39 PARTICLE THERAPY MARKET, BY SYSTEM, 2021-2028 (USD MILLION)
- 8.2 MULTI-ROOM SYSTEMS
- 8.2.1 LOWER SHIELDING REQUIREMENTS AND FASTER BEAM-SWITCHING CAPABILITIES TO DRIVE INTEREST AMONG END USERS
- TABLE 40 PARTICLE THERAPY MARKET FOR MULTI-ROOM SYSTEMS, BY REGION, 2021-2028 (USD MILLION)
- 8.3 SINGLE-ROOM SYSTEMS
- 8.3.1 COMPACT STRUCTURE AND REDUCED CAPITAL COST TO PROPEL GROWTH
- TABLE 41 PARTICLE THERAPY MARKET FOR SINGLE-ROOM SYSTEMS, BY REGION, 2021-2028 (USD MILLION)
9 PARTICLE THERAPY MARKET, BY APPLICATION
- 9.1 INTRODUCTION
- TABLE 42 PARTICLE THERAPY MARKET, BY APPLICATION, 2021-2028 (USD MILLION)
- 9.2 TREATMENT APPLICATIONS
- 9.2.1 INTRODUCTION OF SMALL FOOTPRINT SINGLE-ROOM PARTICLE THERAPY CENTERS TO SUPPORT MARKET GROWTH
- TABLE 43 PARTICLE THERAPY MARKET FOR TREATMENT APPLICATIONS, BY REGION, 2021-2028 (USD MILLION)
- 9.3 RESEARCH APPLICATIONS
- 9.3.1 INCREASING AVAILABILITY OF FUNDING FOR PARTICLE THERAPY-RELATED RESEARCH PROJECTS TO PROVIDE GROWTH OPPORTUNITIES
- TABLE 44 PARTICLE THERAPY MARKET FOR RESEARCH APPLICATIONS, BY REGION, 2021-2028 (USD MILLION)
10 PARTICLE THERAPY MARKET, BY CANCER TYPE
- 10.1 INTRODUCTION
- TABLE 45 PARTICLE THERAPY MARKET, BY CANCER TYPE, 2021-2028 (USD MILLION)
- 10.2 PEDIATRIC CANCER
- 10.2.1 INCREASING CASES OF PEDIATRIC CANCER TO PROPEL MARKET
- TABLE 46 PARTICLE THERAPY MARKET FOR PEDIATRIC CANCER, BY REGION, 2021-2028 (USD MILLION)
- 10.3 PROSTATE CANCER
- 10.3.1 ADVANTAGES OFFERED BY PARTICLE THERAPY FOR TREATMENT OF PROSTATE CANCER TO DRIVE MARKET
- TABLE 47 PARTICLE THERAPY MARKET FOR PROSTATE CANCER, BY REGION, 2021-2028 (USD MILLION)
- 10.4 LUNG CANCER
- 10.4.1 GROWING CLINICAL TESTING OF PROTON THERAPY FOR LUNG CANCER TO DRIVE MARKET
- TABLE 48 PARTICLE THERAPY MARKET FOR LUNG CANCER, BY REGION, 2021-2028 (USD MILLION)
- 10.5 BREAST CANCER
- 10.5.1 LIMITED RADIATION EXPOSURE WITH REDUCED SIDE-EFFECTS TO INCREASE ADOPTION OF PARTICLE THERAPY
- TABLE 49 PARTICLE THERAPY MARKET FOR BREAST CANCER, BY REGION, 2021-2028 (USD MILLION)
- 10.6 HEAD & NECK CANCER
- 10.6.1 USE OF PENCIL-BEAM SCANNING FOR PROTON THERAPY TO SUPPORT MARKET GROWTH
- TABLE 50 PARTICLE THERAPY MARKET FOR HEAD & NECK CANCER, BY REGION, 2021-2028 (USD MILLION)
- 10.7 OTHER CANCERS
- TABLE 51 PARTICLE THERAPY MARKET FOR OTHER CANCERS, BY REGION, 2021-2028 (USD MILLION)
11 PARTICLE THERAPY MARKET, BY REGION
- 11.1 INTRODUCTION
- TABLE 52 PARTICLE THERAPY MARKET, BY REGION, 2021-2028 (USD MILLION)
- 11.2 NORTH AMERICA
- 11.2.1 NORTH AMERICA: RECESSION IMPACT
- FIGURE 32 NORTH AMERICA: PARTICLE THERAPY MARKET SNAPSHOT
- TABLE 53 NORTH AMERICA: PARTICLE THERAPY MARKET, BY COUNTRY, 2021-2028 (USD MILLION)
- TABLE 54 NORTH AMERICA: PARTICLE THERAPY MARKET, BY TYPE, 2021-2028 (USD MILLION)
- TABLE 55 NORTH AMERICA: PARTICLE THERAPY MARKET, BY PRODUCT & SERVICE, 2021-2028 (USD MILLION)
- TABLE 56 NORTH AMERICA: PARTICLE THERAPY PRODUCTS MARKET, BY TYPE, 2021-2028 (USD MILLION)
- TABLE 57 NORTH AMERICA: PARTICLE THERAPY MARKET, BY SYSTEM, 2021-2028 (USD MILLION)
- TABLE 58 NORTH AMERICA: PARTICLE THERAPY MARKET, BY CANCER TYPE, 2021-2028 (USD MILLION)
- TABLE 59 NORTH AMERICA: PARTICLE THERAPY MARKET, BY APPLICATION, 2021-2028 (USD MILLION)
- 11.2.2 US
- 11.2.2.1 Growing number of particle therapy centers to drive market growth
- TABLE 60 US: MACROECONOMIC INDICATORS
- TABLE 61 US: PARTICLE THERAPY MARKET, BY PRODUCT & SERVICE, 2021-2028 (USD MILLION)
- TABLE 62 US: PARTICLE THERAPY PRODUCTS MARKET, BY TYPE, 2021-2028 (USD MILLION)
- 11.2.3 CANADA
- 11.2.3.1 Government initiative to include particle therapy as treatment method to boost market
- TABLE 63 CANADA: MACROECONOMIC INDICATORS
- TABLE 64 CANADA: PARTICLE THERAPY MARKET, BY PRODUCT & SERVICE, 2021-2028 (USD MILLION)
- TABLE 65 CANADA: PARTICLE THERAPY PRODUCTS MARKET, BY TYPE, 2021-2028 (USD MILLION)
- 11.3 EUROPE
- 11.3.1 EUROPE: RECESSION IMPACT
- TABLE 66 EUROPE: PARTICLE THERAPY MARKET, BY COUNTRY, 2021-2028 (USD MILLION)
- TABLE 67 EUROPE: PARTICLE THERAPY MARKET, BY TYPE, 2021-2028 (USD MILLION)
- TABLE 68 EUROPE: PARTICLE THERAPY MARKET, BY PRODUCT & SERVICE, 2021-2028 (USD MILLION)
- TABLE 69 EUROPE: PARTICLE THERAPY PRODUCTS MARKET, BY TYPE, 2021-2028 (USD MILLION)
- TABLE 70 EUROPE: PARTICLE THERAPY MARKET, BY SYSTEM, 2021-2028 (USD MILLION)
- TABLE 71 EUROPE: PARTICLE THERAPY MARKET, BY CANCER TYPE, 2021-2028 (USD MILLION)
- TABLE 72 EUROPE: PARTICLE THERAPY MARKET, BY APPLICATION, 2021-2028 (USD MILLION)
- 11.3.2 GERMANY
- 11.3.2.1 Increasing research on proton and heavy ion therapy to drive market growth
- TABLE 73 GERMANY: MACROECONOMIC INDICATORS
- TABLE 74 GERMANY: PARTICLE THERAPY MARKET, BY PRODUCT & SERVICE, 2021-2028 (USD MILLION)
- TABLE 75 GERMANY: PARTICLE THERAPY PRODUCTS MARKET, BY TYPE, 2021-2028 (USD MILLION)
- 11.3.3 FRANCE
- 11.3.3.1 Increasing healthcare expenditure to support particle therapy product adoption
- TABLE 76 FRANCE: MACROECONOMIC INDICATORS
- TABLE 77 FRANCE: PARTICLE THERAPY MARKET, BY PRODUCT & SERVICE, 2021-2028 (USD MILLION)
- TABLE 78 FRANCE: PARTICLE THERAPY PRODUCTS MARKET, BY TYPE, 2021-2028 (USD MILLION)
- 11.3.4 UK
- 11.3.4.1 Increasing acceptance of particle therapy techniques in cancer treatment to support market growth
- TABLE 79 UK: MACROECONOMIC INDICATORS
- TABLE 80 UK: PARTICLE THERAPY MARKET, BY PRODUCT & SERVICE, 2021-2028 (USD MILLION)
- TABLE 81 UK: PARTICLE THERAPY PRODUCTS MARKET, BY TYPE, 2021-2028 (USD MILLION)
- 11.3.5 ITALY
- 11.3.5.1 Increasing awareness activities to support market growth
- TABLE 82 ITALY: MACROECONOMIC INDICATORS
- TABLE 83 ITALY: PARTICLE THERAPY MARKET, BY PRODUCT & SERVICE, 2021-2028 (USD MILLION)
- TABLE 84 ITALY: PARTICLE THERAPY PRODUCTS MARKET, BY TYPE, 2021-2028 (USD MILLION)
- 11.3.6 SPAIN
- 11.3.6.1 Increasing number of conferences and government funding for cancer research to drive market
- TABLE 85 SPAIN: MACROECONOMIC INDICATORS
- TABLE 86 SPAIN: PARTICLE THERAPY MARKET, BY PRODUCT & SERVICE, 2021-2028 (USD MILLION)
- TABLE 87 SPAIN: PARTICLE THERAPY PRODUCTS MARKET, BY TYPE, 2021-2028 (USD MILLION)
- 11.3.7 REST OF EUROPE
- TABLE 88 REST OF EUROPE: PARTICLE THERAPY MARKET, BY PRODUCT & SERVICE, 2021-2028 (USD MILLION)
- TABLE 89 REST OF EUROPE: PARTICLE THERAPY PRODUCTS MARKET, BY TYPE, 2021-2028 (USD MILLION)
- 11.4 ASIA PACIFIC
- 11.4.1 ASIA PACIFIC: RECESSION IMPACT
- FIGURE 33 ASIA PACIFIC: PARTICLE THERAPY MARKET SNAPSHOT
- TABLE 90 ASIA PACIFIC: PARTICLE THERAPY MARKET, BY COUNTRY, 2021-2028 (USD MILLION)
- TABLE 91 ASIA PACIFIC: PARTICLE THERAPY MARKET, BY TYPE, 2021-2028 (USD MILLION)
- TABLE 92 ASIA PACIFIC: PARTICLE THERAPY MARKET, BY PRODUCT & SERVICE, 2021-2028 (USD MILLION)
- TABLE 93 ASIA PACIFIC: PARTICLE THERAPY PRODUCTS MARKET, BY TYPE, 2021-2028 (USD MILLION)
- TABLE 94 ASIA PACIFIC: PARTICLE THERAPY MARKET, BY SYSTEM, 2021-2028 (USD MILLION)
- TABLE 95 ASIA PACIFIC: PARTICLE THERAPY MARKET, BY CANCER TYPE, 2021-2028 (USD MILLION)
- TABLE 96 ASIA PACIFIC: PARTICLE THERAPY MARKET, BY APPLICATION, 2021-2028 (USD MILLION)
- 11.4.2 JAPAN
- 11.4.2.1 Increasing use of heavy ion therapy for cancer treatment to propel market
- TABLE 97 JAPAN: MACROECONOMIC INDICATORS
- TABLE 98 JAPAN: PARTICLE THERAPY MARKET, BY PRODUCT & SERVICE, 2021-2028 (USD MILLION)
- TABLE 99 JAPAN: PARTICLE THERAPY PRODUCTS MARKET, BY TYPE, 2021-2028 (USD MILLION)
- 11.4.3 CHINA
- 11.4.3.1 High cancer burden to drive market
- TABLE 100 CHINA: MACROECONOMIC INDICATORS
- TABLE 101 CHINA: PARTICLE THERAPY MARKET, BY PRODUCT & SERVICE, 2021-2028 (USD MILLION)
- TABLE 102 CHINA: PARTICLE THERAPY PRODUCTS MARKET, BY TYPE, 2021-2028 (USD MILLION)
- 11.4.4 INDIA
- 11.4.4.1 Improving research capabilities to support market growth
- TABLE 103 INDIA: MACROECONOMIC INDICATORS
- TABLE 104 INDIA: PARTICLE THERAPY MARKET, BY PRODUCT & SERVICE, 2021-2028 (USD MILLION)
- TABLE 105 INDIA: PARTICLE THERAPY PRODUCTS MARKET, BY TYPE, 2021-2028 (USD MILLION)
- 11.4.5 AUSTRALIA
- 11.4.5.1 Healthcare funding availability to boost market growth
- TABLE 106 AUSTRALIA: MACROECONOMIC INDICATORS
- TABLE 107 AUSTRALIA: PARTICLE THERAPY MARKET, BY PRODUCT & SERVICE, 2021-2028 (USD MILLION)
- TABLE 108 AUSTRALIA: PARTICLE THERAPY PRODUCTS MARKET, BY TYPE, 2021-2028 (USD MILLION)
- 11.4.6 SOUTH KOREA
- 11.4.6.1 Rising prevalence of cancer to drive market
- TABLE 109 SOUTH KOREA: MACROECONOMIC INDICATORS
- TABLE 110 SOUTH KOREA: PARTICLE THERAPY MARKET, BY PRODUCT & SERVICE, 2021-2028 (USD MILLION)
- TABLE 111 SOUTH KOREA: PARTICLE THERAPY PRODUCTS MARKET, BY TYPE, 2021-2028 (USD MILLION)
- 11.4.7 REST OF ASIA PACIFIC
- TABLE 112 REST OF ASIA PACIFIC: PARTICLE THERAPY MARKET, BY PRODUCT & SERVICE, 2021-2028 (USD MILLION)
- TABLE 113 REST OF ASIA PACIFIC: PARTICLE THERAPY PRODUCTS MARKET, BY TYPE, 2021-2028 (USD MILLION)
- 11.5 LATIN AMERICA
- 11.5.1 LATIN AMERICA: RECESSION IMPACT
- TABLE 114 LATIN AMERICA: PARTICLE THERAPY MARKET, BY COUNTRY, 2021-2028 (USD MILLION)
- TABLE 115 LATIN AMERICA: PARTICLE THERAPY MARKET, BY TYPE, 2021-2028 (USD MILLION)
- TABLE 116 LATIN AMERICA: PARTICLE THERAPY MARKET, BY PRODUCT & SERVICE, 2021-2028 (USD MILLION)
- TABLE 117 LATIN AMERICA: PARTICLE THERAPY PRODUCTS MARKET, BY TYPE, 2021-2028 (USD MILLION)
- TABLE 118 LATIN AMERICA: PARTICLE THERAPY MARKET, BY SYSTEM, 2021-2028 (USD MILLION)
- TABLE 119 LATIN AMERICA: PARTICLE THERAPY MARKET, BY CANCER TYPE, 2021-2028 (USD MILLION)
- TABLE 120 LATIN AMERICA: PARTICLE THERAPY MARKET, BY APPLICATION, 2021-2028 (USD MILLION)
- 11.5.2 BRAZIL
- 11.5.2.1 Government initiates to propel market growth
- TABLE 121 BRAZIL: MACROECONOMIC INDICATORS
- TABLE 122 BRAZIL: PARTICLE THERAPY MARKET, BY PRODUCT & SERVICE, 2021-2028 (USD MILLION)
- TABLE 123 BRAZIL: PARTICLE THERAPY PRODUCTS MARKET, BY TYPE, 2021-2028 (USD MILLION)
- 11.5.3 MEXICO
- 11.5.3.1 Growing adoption of advanced medical technologies to drive market
- TABLE 124 MEXICO: MACROECONOMIC INDICATORS
- TABLE 125 MEXICO: PARTICLE THERAPY MARKET, BY PRODUCT & SERVICE, 2021-2028 (USD MILLION)
- TABLE 126 MEXICO: PARTICLE THERAPY PRODUCTS MARKET, BY TYPE, 2021-2028 (USD MILLION)
- 11.5.4 REST OF LATIN AMERICA
- TABLE 127 REST OF LATIN AMERICA: PARTICLE THERAPY MARKET, BY PRODUCT & SERVICE, 2021-2028 (USD MILLION)
- TABLE 128 REST OF LATIN AMERICA: PARTICLE THERAPY PRODUCTS MARKET, BY TYPE, 2021-2028 (USD MILLION)
- 11.6 MIDDLE EAST & AFRICA
- 11.6.1 IMPROVING HEALTHCARE INFRASTRUCTURE AND INCREASING PUBLIC-PRIVATE INVESTMENTS TO DRIVE MARKET
- 11.6.2 MIDDLE EAST & AFRICA: RECESSION IMPACT
- TABLE 129 MIDDLE EAST & AFRICA: PARTICLE THERAPY MARKET, BY TYPE, 2021-2028 (USD MILLION)
- TABLE 130 MIDDLE EAST & AFRICA: PARTICLE THERAPY MARKET, BY PRODUCT & SERVICE, 2021-2028 (USD MILLION)
- TABLE 131 MIDDLE EAST & AFRICA: PARTICLE THERAPY PRODUCTS MARKET, BY TYPE, 2021-2028 (USD MILLION)
- TABLE 132 MIDDLE EAST & AFRICA: PARTICLE THERAPY MARKET, BY SYSTEM, 2021-2028 (USD MILLION)
- TABLE 133 MIDDLE EAST & AFRICA: PARTICLE THERAPY MARKET, BY CANCER TYPE, 2021-2028 (USD MILLION)
- TABLE 134 MIDDLE EAST & AFRICA: PARTICLE THERAPY MARKET, BY APPLICATION, 2021-2028 (USD MILLION)
12 COMPETITIVE LANDSCAPE
- 12.1 OVERVIEW
- 12.2 STRATEGIES ADOPTED BY KEY PLAYERS
- FIGURE 34 KEY DEVELOPMENTS IN PARTICLE THERAPY MARKET
- 12.3 REVENUE SHARE ANALYSIS OF TOP MARKET PLAYERS
- FIGURE 35 REVENUE ANALYSIS OF TOP PLAYERS IN PARTICLE THERAPY MARKET
- 12.4 MARKET SHARE ANALYSIS
- FIGURE 36 MARKET SHARE ANALYSIS, BY KEY PLAYER, 2022
- 12.5 COMPANY EVALUATION MATRIX
- 12.5.1 STARS
- 12.5.2 EMERGING LEADERS
- 12.5.3 PERVASIVE PLAYERS
- 12.5.4 PARTICIPANTS
- FIGURE 37 PARTICLE THERAPY MARKET: COMPANY EVALUATION MATRIX, 2022
- 12.6 COMPETITIVE BENCHMARKING FOR KEY PLAYERS
- 12.6.1 PARTICLE THERAPY MARKET: COMPANY FOOTPRINT
- TABLE 135 OVERALL COMPANY FOOTPRINT (10 COMPANIES)
- TABLE 136 TYPE FOOTPRINT (10 COMPANIES)
- TABLE 137 PRODUCT & SERVICE FOOTPRINT (10 COMPANIES)
- TABLE 138 SYSTEM FOOTPRINT (10 COMPANIES)
- TABLE 139 CANCER TYPE FOOTPRINT (10 COMPANIES)
- TABLE 140 APPLICATION FOOTPRINT (10 COMPANIES)
- TABLE 141 REGIONAL FOOTPRINT (10 COMPANIES)
- 12.7 STARTUP/SME EVALUATION MATRIX
- 12.7.1 PROGRESSIVE COMPANIES
- 12.7.2 RESPONSIVE COMPANIES
- 12.7.3 DYNAMIC COMPANIES
- 12.7.4 STARTING BLOCKS
- FIGURE 38 PARTICLE THERAPY MARKET: STARTUP/SME EVALUATION MATRIX, 2022
- 12.7.5 COMPETITIVE BENCHMARKING FOR STARTUPS/SMES
- TABLE 142 OVERALL COMPANY FOOTPRINT
- TABLE 143 TYPE FOOTPRINT
- TABLE 144 PRODUCT & SERVICE FOOTPRINT
- TABLE 145 SYSTEM FOOTPRINT
- TABLE 146 CANCER TYPE FOOTPRINT
- TABLE 147 APPLICATION FOOTPRINT
- TABLE 148 REGIONAL FOOTPRINT
- 12.8 COMPETITIVE SCENARIOS & TRENDS
- 12.8.1 PRODUCT LAUNCHES & APPROVALS
- TABLE 149 PARTICLE THERAPY MARKET: PRODUCT LAUNCHES & APPROVALS (JANUARY 2020-AUGUST 2023)
- 12.8.2 DEALS
- TABLE 150 PARTICLE THERAPY MARKET: DEALS (JANUARY 2020-AUGUST 2023)
- 12.8.3 OTHER DEVELOPMENTS
- TABLE 151 PARTICLE THERAPY MARKET: OTHER DEVELOPMENTS (JANUARY 2020-AUGUST 2023)
13 COMPANY PROFILES
- 13.1 KEY PLAYERS
- (Business Overview, Products/Services/Solutions Offered, MnM View, Key Strengths and Right to Win, Strategic Choices Made, Weaknesses and Competitive Threats, Recent Developments)**
- 13.1.1 IBA WORLDWIDE
- TABLE 152 IBA WORLDWIDE: BUSINESS OVERVIEW
- FIGURE 39 IBA WORLDWIDE: COMPANY SNAPSHOT (2022)
- 13.1.2 VARIAN MEDICAL SYSTEMS, INC.
- TABLE 153 VARIAN MEDICAL SYSTEMS, INC.: BUSINESS OVERVIEW
- FIGURE 40 VARIAN MEDICAL SYSTEMS, INC.: COMPANY SNAPSHOT (2022)
- 13.1.3 HITACHI, LTD.
- TABLE 154 HITACHI, LTD.: BUSINESS OVERVIEW
- FIGURE 41 HITACHI, LTD.: COMPANY SNAPSHOT (2021)
- 13.1.4 MEVION MEDICAL SYSTEMS
- TABLE 155 MEVION MEDICAL SYSTEMS: BUSINESS OVERVIEW
- 13.1.5 SUMITOMO HEAVY INDUSTRIES LTD.
- TABLE 156 SUMITOMO HEAVY INDUSTRIES LTD.: BUSINESS OVERVIEW
- FIGURE 42 SUMITOMO HEAVY INDUSTRIES LTD.: COMPANY SNAPSHOT (2021)
- 13.1.6 PROVISION HEALTHCARE, LLC
- TABLE 157 PROVISION HEALTHCARE, LLC: BUSINESS OVERVIEW
- 13.1.7 TOSHIBA MEDICAL SYSTEMS CORPORATION
- TABLE 158 TOSHIBA MEDICAL SYSTEMS CORPORATION: BUSINESS OVERVIEW
- FIGURE 43 TOSHIBA MEDICAL SYSTEMS CORPORATION: COMPANY SNAPSHOT (2022)
- 13.1.8 OPTIVUS PROTON THERAPY, INC.
- TABLE 159 OPTIVUS PROTON THERAPY, INC.: BUSINESS OVERVIEW
- 13.1.9 PROTOM INTERNATIONAL, INC.
- TABLE 160 PROTOM INTERNATIONAL, INC.: BUSINESS OVERVIEW
- 13.1.10 ADVANCED ONCOTHERAPY PLC
- TABLE 161 ADVANCED ONCOTHERAPY PLC: BUSINESS OVERVIEW
- 13.2 OTHER PLAYERS
- 13.2.1 DANFYSIK A/S
- TABLE 162 DANFYSIK A/S: BUSINESS OVERVIEW
- 13.2.2 P-CURE, LTD.
- TABLE 163 P-CURE, LTD.: BUSINESS OVERVIEW
- 13.2.3 PTW FREIBURG GMBH
- TABLE 164 PTW FREIBURG GMBH: BUSINESS OVERVIEW
- *Business Overview, Products/Services/Solutions Offered, MnM View, Key Strengths and Right to Win, Strategic Choices Made, Weaknesses and Competitive Threats, Recent Developments might not be captured in case of unlisted companies.
14 APPENDIX
- 14.1 DISCUSSION GUIDE
- 14.2 KNOWLEDGESTORE: MARKETSANDMARKETS' SUBSCRIPTION PORTAL
- 14.3 CUSTOMIZATION OPTIONS
- 14.4 RELATED REPORTS
- 14.5 AUTHOR DETAILS