|
|
市場調査レポート
商品コード
1372817
ATRタンパク質阻害剤の世界市場:臨床試験と市場機会の洞察(2024年)Global ATR Protein Inhibitors Clinical Trials & Market Opportunity Insight 2024 |
||||||
|
|||||||
| ATRタンパク質阻害剤の世界市場:臨床試験と市場機会の洞察(2024年) |
|
出版日: 2023年11月01日
発行: KuicK Research
ページ情報: 英文 88 Pages
納期: 即日から翌営業日
|
- 全表示
- 概要
- 図表
- 目次
ATRキナーゼの阻害は、進化を続ける癌薬学研究において有望な手段として浮上しています。ATRはセリン/スレオニンプロテインキナーゼであり、DNA損傷に対する細胞応答において重要な役割を果たすことにより、ゲノムの完全性を守る役割を果たします。ATRの調節異常は様々な疾患、特に癌に関連しており、ATRを阻害することは癌細胞の脆弱性を利用し、既存の治療法の有効性を高める新たな機会を提供します。現在のところ、ATRタンパク質阻害剤は規制当局の承認を得ていないが、開発パイプラインにあるいくつかの治験候補薬が第II相試験に入ったことから、これらの新規阻害剤は今後さらに注目を集めると予想されます。
ATR阻害の主な焦点は腫瘍学の領域です。ATRタンパク質阻害剤は、化学療法や放射線療法などDNAに損傷を与える癌治療の効果を高めることが期待されており、卵巣癌、胆管癌、乳癌、小細胞癌、高悪性度神経内分泌癌などの進行固形癌や一部の血液悪性腫瘍など、さまざまな癌種における併用療法の候補となっています。これらの癌で研究されているATR阻害候補薬には、ATG-018、Elimusertib(BAY1895344)、Camonsertib(RP-3500)、Ceralasertib(AZD6738)があり、それぞれAntengene、Bayer、Repare Therapeutics、AstraZenecaによって開発されています。これらの候補薬は現在、臨床評価のさまざまな段階にあります。
ATRタンパク質阻害剤は、他の癌治療と比較していくつかの利点があります。上述したように、ATRタンパク質阻害剤は従来の治療法に対して癌細胞を感作し、必要な投与量を減らし、副作用を軽減する可能性があります。従来の治療法は強力であるが、いくつかの限界も抱えています。ATR阻害は、癌細胞が治療に対して発現する耐性メカニズムを克服するのに役立ち、その結果、患者の転帰を改善します。さらにATRタンパク質阻害剤は、患者の特定の遺伝的脆弱性を標的とすることで、より個別化された癌治療アプローチを可能にします。神経変性疾患やウイルス感染症の領域では、ATR阻害は疾患の進行を遅らせたり予防したりし、罹患者のQOLを改善することが期待されています。
ATRタンパク質阻害剤を取り巻く環境は、既存の治療法に対する優位性から楽観視されていますが、患者の分類基準の改良や潜在的な副作用の理解を深める必要性など、課題も存在します。しかし、ATRタンパク質阻害剤の将来的な可能性は大きいです。ATRタンパク質阻害剤が有望視され続けるにつれ、併用療法への組み入れがより一般的になり、癌治療レジメンを再構築する可能性が期待されます。さらに、分子プロファイリングと遺伝子検査の進歩により、より的を絞ったATR阻害が可能になるかもしれません。
当レポートは、世界のATRタンパク質阻害剤市場について調査し、市場の概要とともに、薬剤動向、臨床試験動向、地域別動向、および市場に参入する企業の競合情勢などを提供しています。
目次
第1章 ATRタンパク質阻害剤のイントロダクション
第2章 ATRタンパク質阻害剤:適応症別の研究および臨床試験の概要
- 固形癌
- 血液癌
第3章 世界のATRタンパク質阻害剤市場の展望
- 現在の市場動向と発展
- 将来の成長の道筋
第4章 ATRタンパク質阻害剤の開発動向、地域別
- 米国
- EU
- 英国
- カナダ
- 中国
- 日本
- オーストラリア
第5章 世界のATRタンパク質阻害剤の臨床試験の概要
- 国別
- 適応別
- 相別
- 治療クラス別
第6章 企業、適応症、相別の世界のATRタンパク質阻害剤の臨床パイプライン
- 研究
- 前臨床
- 第I相
- 第I/II相
- 第II相
- 第III相
第7章 世界のATRタンパク質阻害剤市場の促進要因と課題
- 促進要因と機会
- 課題と抑制要因
第8章 競合情勢
- Antengene Corporation
- Aprea
- AstraZeneca
- Bayer
- Beijing Tide Pharmaceutical
- Biocity Biopharmaceutics
- Chipscreen Biosciences
- IMPACT Therapeutics
- Laevoroc Neuro-Oncology
- Repare Therapeutics
- Shanghai De Novo Pharmatech
- Shanghai Junshi Biosciences
- ShangPharma
- Vertex Pharmaceuticals
List of Figures
- Figure 1-1: ATR Mediated DNA Damage Repair Signaling
- Figure 1-2: Benefits of ATR Inhibitors
- Figure 1-3: ATR inhibitors in Clinical Trials
- Figure 1-4: ATR Inhibitors - History & Evolution
- Figure 2-1: LATIFY Phase III Study - Initiation & Completion Years
- Figure 2-2: ATRiBRAVE Phase II Study - Initiation & Completion Years
- Figure 2-3: DASH Phase II Study - Initiation & Completion Years
- Figure 2-4: MATRiX Phase II Study - Initiation & Completion Years
- Figure 2-5: NCI-2021-10751 Phase I/II Study - Initiation & Completion Years
- Figure 2-6: ACE-CL-110 Phase I Study - Initiation & Completion Years
- Figure 3-1: ATR inhibitors - Future Growth Avenues
- Figure 5-1: Global - ATR Protein Inhibitors Clinical Trials by Country (Numbers), 2023 & 2024
- Figure 5-2: Global - ATR Protein Inhibitors Clinical Trials by Indication (Numbers), 2023 & 2024
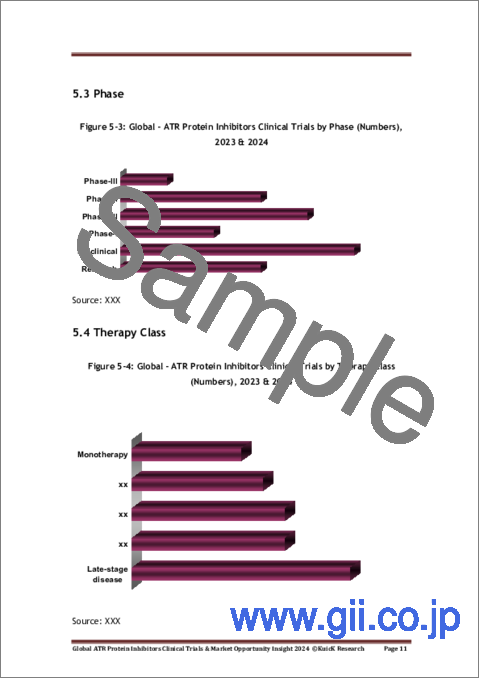
- Figure 5-3: Global - ATR Protein Inhibitors Clinical Trials by Phase (Numbers), 2023 & 2024
- Figure 5-4: Global - ATR Protein Inhibitors Clinical Trials by Therapy Class (Numbers), 2023 & 2024
- Figure 7-1: ATR Inhibitors Market Drivers
- Figure 7-2: ATR Inhibitors Market Challenges
List of Tables
- Table 4-1: US - National Cancer Institute Sponsored Clinical Trials for ATR Inhibitors
- Table 4-2: EU - Ongoing Clinical Trials for ATR Inhibitors
- Table 4-3: UK - Ongoing Clinical Trials for ATR Inhibitors
- Table 4-4: Canada - Ongoing Clinical Trials for ATR Inhibitors
- Table 4-5: China - Ongoing Clinical Trials for ATR Inhibitors
- Table 4-6: Japan - Ongoing Clinical Trials for ATR Inhibitor M1774
- Table 4-7: Australia - Ongoing Clinical Trials for ATR Inhibitors
“Global ATR Protein Inhibitors Clinical Trials & Market Opportunity Insight 2024” Report Highlights:
- ATR Protein Inhibitors In Clinical Trials: > 15 Drugs
- China Dominates ATR Protein Inhibitors Clinical Trials: > 60% Trials
- Highest Clinical Phase: Phase-III
- Global ATR Protein Inhibitors Clinical Pipeline By Company, Indication & Phase
- ATR Inhibitors Development Trends by Country
- Competitive Landscape
Inhibition of the Ataxia telangiectasia and Rad3 related kinase, or ATR kinase, has emerged as a promising avenue in the ever evolving landscape of cancer pharmaceutical research. ATR is a serine/threonine-protein kinase that acts as a guardian of genomic integrity by playing a critical role in the cellular response to DNA damage. Dysregulation of ATR has been linked to various diseases, particularly cancer, and inhibiting ATR presents a novel opportunity to exploit the vulnerabilities of cancer cells and enhance the efficacy of existing therapies. No ATR inhibitors have received regulatory approval as of yet; however, with several investigational candidates in the development pipeline now entering phase II trials, these novel inhibitors are anticipated to gain more attention in the years to come.
The primary focus of ATR inhibition is in the realm of oncology. ATR inhibitors have shown promise in enhancing the effectiveness of DNA-damaging cancer therapies such as chemotherapy and radiation, making hem potential candidates for combination therapies in various cancer types such as ovarian cancer, bile duct carcinoma, breast carcinoma, small cell cancer and high grade neuroendocrine cancers among other advanced solid tumors, as well as some hematological malignancies. Some ATR-inhibiting candidates being investigated in these cancers are ATG-018, Elimusertib (BAY 1895344), Camonsertib (RP-3500), and Ceralasertib (AZD6738), which have been developed by Antengene, Bayer, Repare Therapeutics, and AstraZeneca, respectively. These candidates are now in various phases of clinical evaluation.
Recent research, however, has expanded the scope of ATR inhibitors to neurodegenerative diseases. ATR kinase has implications in maintaining neuronal genomic activity, making it a potential target for conditions like Parkinson's disease and Alzheimer's disease. Further studies are linking ATR with microbial infections, especially those caused by viruses. Along with ATM, or Ataxia-telangiectasia mutated kinase, ATR is exploited by viruses to facilitate viral replication and increase expression of viral proteins. In the case of HIV-1 and COVID-19, both of which have high infection and mortality rates, ATR inhibition has represented another promising prospect for the development of novel therapeutics to manage these diseases.
As a result, the pharmaceutical industry has invested significantly in the development of ATR inhibition, and several clinical trials are being conducted now to evaluate the efficacy of these candidates. VE-821, developed by Vertex Pharmaceuticals, was the first specific ATR inhibitor to be developed, which served as the blueprint for the development of VE-822, now licensed to Merck as Berzosertib. The drug further served as the foundation for M1774, a potent ATR inhibitor being studied by Merck in various solid cancers. Both Berzosertib and M1774 have demonstrated encouraging results in early phase clinical trials in combination with chemotherapy. Administration of these ATR inhibitors enhanced the sensitivity of cancer cells to DNA-damaging chemotherapies, which makes the elimination of cancer cells faster.
ATR inhibitors pose several benefits over other cancer therapies. As described above, ATR inhibitors sensitize cancer cells to conventional therapies, potentially reducing the required dosage and mitigating side effects. Conventional therapies are potent but also face several limitations, majorly resistance to treatment. ATR inhibition can help overcome resistance mechanisms that cancer cells development against treatment, therefore improving patient outcomes. Further, ATR inhibitors can also enable a more personalized approach to cancer treatment by targeting specific genetic vulnerabilities in patients. In the realm of neurodegenerative diseases and viral infections, ATR inhibition holds the promise of slowing or preventing disease progression and improving the quality of life of affected individuals.
Despite the optimism surrounding ATR inhibitors as outlined by their advantages over existing therapies, challenges exist such as the need for refined patient section criteria and a deeper understanding of the potential side effects. However, their future potential is vast. As ATR inhibitors continue to show promise, their integration into combination therapies is expected to become more common, potentially reshaping cancer treatment regimens. Moreover, advances in molecular profiling and genetic testing may enable a more targeted ATR inhibition.
The ongoing clinical and market trends for ATR inhibitors present a compelling narrative in the pharmaceutical domain. The novel approach to targeting DNA damage response pathways holds the promise of revolutionizing cancer treatment, as well as entering the treatment protocols for neurodegenerative and infectious diseases. With ongoing research studies revealing more about ATR kinase and its implications in different indications, it is understood the full potential of ATR inhibition is yet to be realized. As this happens, a new wave of development in the global pharmaceutical market is anticipated, which makes the ATR inhibition an exciting field.
Table of Contents
1. Introduction to ATR Inhibition
- 1.1. Overview of ATR Protein & ATR Inhibition
- 1.2. History & Evolution of ATR Inhibitors
2. ATR Inhibitors: Research & Clinical Trials Overview by Indication
- 2.1. Solid Cancers
- 2.2. Hematological Cancers
3. Global ATR Inhibitors Market Outlook
- 3.1. Current Market Trends & Developments
- 3.2. Future Growth Avenues
4. ATR Inhibitors Development Trends by Region
- 4.1. US
- 4.2. EU
- 4.3. UK
- 4.4. Canada
- 4.5. China
- 4.6. Japan
- 4.7. Australia
5. Global ATR Protein Inhibitors Clinical Trials Overview
- 5.1. By Country
- 5.2. Indication
- 5.3. Phase
- 5.4. Therapy Class
6. Global ATR Protein Inhibitors Clinical Pipeline By Company, Indication & Phase
- 6.1. Research
- 6.2. Preclinical
- 6.3. Phase-I
- 6.4. Phase-I/II
- 6.5. Phase-II
- 6.6. Phase-III
7. Global ATR Inhibitors Market Drivers & Challenges
- 7.1. Drivers & Opportunities
- 7.2. Challenges & Restraints
8. Competitive Landscape
- 8.1. Antengene Corporation
- 8.2. Aprea
- 8.3. AstraZeneca
- 8.4. Bayer
- 8.5. Beijing Tide Pharmaceutical
- 8.6. Biocity Biopharmaceutics
- 8.7. Chipscreen Biosciences
- 8.8. IMPACT Therapeutics
- 8.9. Laevoroc Neuro-Oncology
- 8.10. Repare Therapeutics
- 8.11. Shanghai De Novo Pharmatech
- 8.12. Shanghai Junshi Biosciences
- 8.13. ShangPharma
- 8.14. Vertex Pharmaceuticals