|
|
市場調査レポート
商品コード
1127250
mRNAがん治療の特許情勢分析(2022年)mRNA Cancer Therapies Patent Landscape Analysis 2022 |
||||||
| mRNAがん治療の特許情勢分析(2022年) |
|
出版日: 2022年09月20日
発行: KnowMade
ページ情報: 英文 100 Slide PDF, Excel File - 570 patent families
納期: 即日から翌営業日
|
- 全表示
- 概要
- 目次
現在、600件以上のmRNAの臨床試験が進行中であり、この件数は、関心の高まりと企業にとっての経済的な利害を反映して、増加し続けています。しかし、現在までに市場に出た製品はまだありませんが、主な特許に対してすでにいくつかの特許異議申し立てが開始されています。ここでは、約20件の異議申立手続が確認されており、そのほとんどが過去5年間に出願され、現在も係争中です。
進化する状況において、他の企業の知的財産の位置付けと戦略を理解することは極めて重要です。こうした知識は、ビジネスリスクやビジネスチャンスを察知し、新たな技術を予測し、市場での地位を強化するための戦略的決定を可能にします。
当レポートでは、mRNA癌治療薬の特許情勢について調査し、公開特許の時系列変化、特許出願国、特許の法的地位などのIP動向、主要企業のプロファイル等の情報を提供しています。
当レポート掲載の企業
BioNTech、Cellectis、CoImmune、CureVac、eTheRNA immunotherapies、Factor Bioscience、Gritstone bio、ImmunityBio、Moderna、Myeloid Therapeutics、Novartis、RIKEN、Sanofi、Shanghai Telebio Biomedical、Tricision Biotechnology、Nutcracker Therapeutics、Orna Therapeutics、など
目次
イントロダクション
- mRNA、がん治療の新たな治療領域
- 調査範囲
- 主な特許出願人
調査手法
- 特許の検索、選択、分析
- 検索戦略
- 特許分析の用語
- 強度とブロッキングの可能性
エグゼクティブサマリー
特許情勢の概要
- 特許公開の時間推移
- 出願国別の時間推移
- 最も多くの特許出願者のランキング
- 主要企業の現在の法的地位
- コーパスの特許法的地位
- 現在の主なIP所有者のマッピング
- 知財リーディングカンパニーの特許活動
- 主な特許出願人の経時変化
新規参入企業
- スタートアップ企業
- 設立された会社
コラボレーション
主な出願人の知財位置
- 特許出願者の知財リーダーシップ
- 特許出願人をブロックする知財先行技術
- 特許ポートフォリオの強み指数
- 主な特許
主なEP反対意見
特許のセグメンテーション
- 定義
- 技術別:パテントファミリー件数
- 技術別主な出願人
- クロスマトリックスセグメント
- 特定のRNAタイプ:circRNA
- 特定のRNAタイプ:saRNA
- RNAの修飾と安定化
- キャリア:ポリマー
- キャリア:脂質
- 投与経路:腫瘍
- 投与経路:非経口
- 療法:抗体
- 療法:免疫調節剤
- 療法:DC負荷別AG
- 療法:直接注射別AG
- 療法:改変抗原受容体
主要企業のIPプロファイル
- BioNTech
- TRON
- CureVac
- Moderna
- Novartis
- University of Pennsylvania
結論
既知のプレゼンテーション
コンタクト
Report's Key Features:
- PDF > 100 slides
- Excel database containing all patents analyzed in the report (>570 patent families), including patent segmentations and hyperlinks to an updated online database.
- IP trends, including time evolution of published patents, countries of patent filings and patents' legal status
- Ranking of main patent assignees
- Key players' IP position and relative strength of their patent portfolios
- Summary of the IP related to RNAs and carriers: specific RNA type (circRNA, saRNA), RNA modification & stabilization, carriers (vesicle, virus, peptide, polymer, lipid).
- Summary of the IP related to treatment: antibody, immuno-modulator, antigen (direct or via DC), modified antigen receptor.
- Analysis of collaboration and EP patent oppositions.
Context of the report
For nearly three decades, extensive efforts have been made to increase RNA intracellular stability, translational efficiency and uptake of mRNA. Several players have taken an interest in this issue, such as mRNA technology leaders CureVac, Moderna and BioNTech. These optimizations were achieved by modifying RNA non-coding elements (5' cap, 5'- and 3'- UTRs, 3' poly-A tail) and RNA coding region, and through the development of formulation technologies with specific carriers. In cancer immunotherapy, the most advanced application of mRNA is therapeutic vaccination with mRNA encoding antigens, i.e., direct cancer vaccines or dendritic cell vaccines. Moreover, for more than a decade, industrialists and academics have been developing personalized cancer vaccines, based on the specific molecular features of patients' tumors, to elicit an immune response against neoantigens produced by cancer cells. Other applications of mRNA in cancer immunotherapy include engineering T cells with modified antigen receptors such as CAR-T, immunomodulator production such as cytokines, co-stimulatory ligands and receptors, and antibody delivery. Currently, there are more than 600 clinical trials in progress, and this number continues to increase, reflecting the growing interest in this topic as well as the economic stakes for companies. However, to date, there is still no product on the market, but several patent oppositions have already been initiated against key patents. Around 20 opposition proceedings have been identified herein, most of which were filed in the past five years and still pending. By winning the race for the Covid-19 vaccine, mRNA vaccines have proven their worth, and demonstrated that RNA-based therapies can deliver on their promises. Additionally, it highlighted the advantages of mRNA over conventional therapies: active at a relatively low dose range, can be developed rapidly, and GMP-compliant manufacturing processes easily upscaled for rapid availability of large numbers of doses. All progress made in the development of Covid-19 vaccines will certainly help advance the development of mRNA-based cancer therapies. In this evolving context, it is crucial to understand the intellectual property position and strategy of these different players. Such knowledge can help detect business risks and opportunities, anticipate emerging technologies, and enable strategic decisions to strengthen market position.

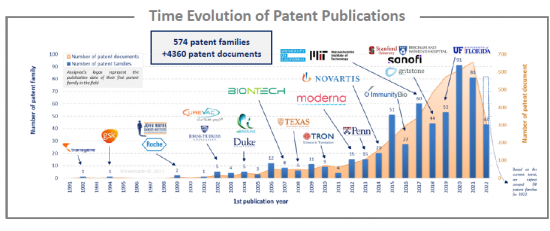
Analysis of the time evolution of patent publications shows that mRNA cancer therapies started to be investigated in 1992, but the technology emerged in the early 2000s with European companies such as CureVac and BioNTech, and American Universities such as Johns Hopkins University, Duke University and Texas University. In 2015 and 2017, two peaks are observed, resulting in an increase in the number of patent applicants. Moreover, in 2017, Moderna published 11 patent families and CureVac 7 patent families. As of 2019, this trend continues. Indeed, the increase in patent publications mainly corresponds to an increase in the number of assignees rather than an increase in the number of patent families for a player, even if BioNTech published 15 then 11 patent families in 2020 and 2021 respectively.
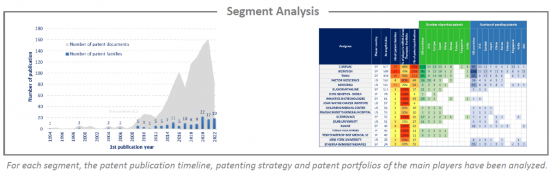
Analysis by segment
mRNA cancer therapies have been investigated and the selected patent families labeled according to the technologies to which they relate. This IP landscape features the following five types of segmentation: specific RNA type (circRNA, saRNA), RNA modification & stabilization, carriers (vesicle, virus, peptide, polymer, lipid), delivery route (tumoral, parenteral, other) and therapy (mRNA encoded antibody, immunomodulator, antigen via DC loading, antigen via direct injection, modified antigen receptor).

EP oppositions
There is a significant number of EP oppositions, which reflects the strategic issues of mRNA cancer therapies for companies. Most of the proceedings are recent, filed in the past five years, and are still pending. For each opposed patent, the application date, assignee, opponent, opposition year and results are detailed.
Identifying the companies that have recently emerged in the IP landscape
Among the players owning patent families related to mRNA cancer therapies, 50 newcomers were identified. These companies are either start-up firms (20) or established companies (30) developing their first technology in the mRNA field. These technologies are mainly related to carriers or to mRNA encoded specific proteins. Numerous IP newcomers are based in the US and in Asia, while some are based in Europe. It is possible that one of these innovative companies could become one of the next healthcare unicorns that the big corporations will be tempted to acquire.
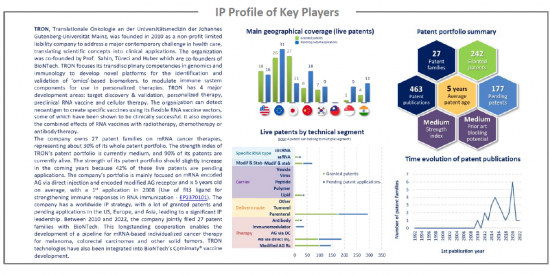
IP profile of key players
This IP study includes a selection and description of the key players. The patent portfolio analysis of key players includes a description of the assignee, key numbers in terms of patents, time evolution of patent publication, main geographical coverage and live patents by technical segment. This IP profile overview is followed by the description of all the assignee's key patents and by a table with its clinical trials.

Moreover, the report also includes an Excel database with the 574 patent families analyzed in this study. This useful patent database allows for multi-criteria searches and includes patent publication numbers, hyperlinks to the original documents, priority dates, titles, abstracts, patent assignees, each patent's current legal status and segmentation.
Companies mentioned in this report (non-exhaustive list)
BioNTech, Cellectis, CoImmune, CureVac, eTheRNA immunotherapies, Factor Bioscience, Gritstone bio, ImmunityBio, Moderna, Myeloid Therapeutics, Novartis, RIKEN, Sanofi, Shanghai Telebio Biomedical, Tricision Biotechnology, Nutcracker Therapeutics, Orna Therapeutics, etc.
TABLE OF CONTENTS
INTRODUCTION
- mRNA, a new therapeutic area in cancer treatment
- Scope of the report
- Main patent assignees
METHODOLOGY
- Patent search, selection and analysis
- Search strategy
- Terminologies for patent analysis
- Strength and blocking potential
EXECUTIVE SUMMARY
PATENT LANDSCAPE OVERVIEW
- Time evolution of patent publications
- Time evolution by country of filing
- Ranking of most prolific patent applicants
- Current legal status of the main players
- Patent legal status of the corpus
- Mapping of main current IP holders
- Patenting activity of IP leading companies
- Time evolution of main patent assignees
NEWCOMERS
- Start-up companies
- Established companies
COLLABORATIONS
IP POSITION OF MAIN APPLICANTS
- IP leadership of patent applicants
- IP prior art blocking potential of patent applicants
- Strength index of patent portfolios
- Key patents
MAIN EP OPPOSITIONS
PATENT SEGMENTATION
- Definition
- Number of patent families by technology
- Main assignees by technology
- Cross matrix segments
- Specific RNA type: circRNA
- Specific RNA type: saRNA
- RNA modification & stabilization
- Carrier: polymer
- Carrier: lipid
- Route of administration: tumoral
- Route of administration: parenteral
- Therapy: antibody
- Therapy: immunomodulator
- Therapy: AG via DC loading
- Therapy: AG via direct injection
- Therapy: modified antigen receptor
IP PROFILE OF KEY PLAYERS
- BioNTech
- TRON
- CureVac
- Moderna
- Novartis
- University of Pennsylvania