|
|
市場調査レポート
商品コード
1309713
オピオイド使用障害(OUD)の世界市場規模、シェア、産業動向分析レポート:薬剤クラス別(ブプレノルフィン、メタドン、ナルトレキソン)、投与経路別、流通チャネル別、地域別展望および予測、2023年~2030年Global Opioid Use Disorder (OUD) Market Size, Share & Industry Trends Analysis Report By Drug Class (Buprenorphine, Methadone and Naltrexone), By Route of Administration, By Distribution Channel, By Regional Outlook and Forecast, 2023 - 2030 |
||||||
|
|||||||
| オピオイド使用障害(OUD)の世界市場規模、シェア、産業動向分析レポート:薬剤クラス別(ブプレノルフィン、メタドン、ナルトレキソン)、投与経路別、流通チャネル別、地域別展望および予測、2023年~2030年 |
|
出版日: 2023年06月30日
発行: KBV Research
ページ情報: 英文 204 Pages
納期: 即納可能
|
- 全表示
- 概要
- 図表
- 目次
オピオイド使用障害(OUD)市場規模は、予測期間中にCAGR 9.9%で上昇し、2030年までに64億米ドルに達すると予測されます。
しかし、これらの薬剤は筋肉痛、吐き気、嘔吐、下痢、便秘、呼吸器障害、骨・関節痛、膀胱痛、腹部けいれんなどを頻繁に引き起こします。さらに、薬剤反応が強い場合、患者はうつ病やその他の心理的問題を経験することもあります。身体依存や中毒に対する臨床的な懸念が適切な処方を妨げ、疼痛管理が不十分になる可能性があります。こうした弊害が市場の拡大を制限する可能性があります。COVID-19のパンデミックは、需要によって製薬企業に有利にも不利にも影響を与えました。病院や診療所のようなヘルスケア施設に行くリスクを冒す人が減ったため、一部の医薬品は需要が減少しました。各政府はウイルスの蔓延を食い止めるため、全国的に厳しい封鎖措置を実施しました。COVID-19に関連する混乱の結果、売上は急激に減少し続けた。その結果、パンデミックの間、世界経済は衰退傾向にあっています。
薬剤クラスの展望
薬剤によって、市場はブプレノルフィン、メタドン、ナルトレキソンに分類されます。2022年には、ブプレノルフィン・セグメントが最大の収益シェアを占めて市場を独占しました。同市場では、ブプレノルフィンパッチ治療の需要が増加しています。これらのパッチは、オピオイド使用障害を治療するための効率的な治療法とみなされています。経皮パッチには、ドラッグデリバリーのメカニズムの改善や痛みの軽減など、いくつかの一般的かつ自己投与的な利点があります。これらの経皮パッチは、OUDのために24時間365日のオピオイド投薬が必要な人の代替薬にもなります。サブロケイド、サブキソン、ズブソルブは、ブプレノルフィンの重要な治療薬です。
投与経路の展望
投与経路によって、市場は経口剤と非経口剤に細分化されます。2022年の市場では、非経口投与セグメントが最も高い売上シェアを占めています。非経口投与法は、カロリーの補給や薬剤の迅速な送達に使用できます。非経口投与では、薬剤が血流に速やかに到達するため、作用発現が速いです。このことは、オピオイドの離脱症状を管理し、OUD患者を即座に救済する上で特に重要です。非経口投与は、ヘルスケア専門家によって薬剤が投与されるため、薬剤の転用や誤用のリスクを最小限に抑えることができます。非経口投与のこうした利点が、この分野の市場成長を支えるものと予測されます。
流通チャネルの展望
流通チャネル別では、病院薬局、小売薬局・店舗、オンライン薬局に分類されます。オンライン薬局セグメントは、2022年の市場において突出した収益シェアを予測しました。これは、病院やその他のヘルスケア施設における電子処方箋の利用が増加していることに加え、インターネット利用者の増加、オンラインサービスへのアクセシビリティの向上などが要因となっています。オンライン薬局の成長は、遠隔診察や診断サポートの拡大によっても促進されています。現在では、オンライン薬局で薬を購入する際に使用できる電子処方箋は、遠隔診察によって作成することができます。
地域別展望
地域別に見ると、市場は北米、欧州、アジア太平洋、LAMEAで分析されます。2022年には、北米地域が市場で最も高い収益シェアを獲得して市場をリードしました。北米の最も大きな収益シェアに影響を与えている主な要因の1つは、米国とカナダにおけるオピオイドの流行です。蔓延しつつある慢性疾患は、疼痛管理のためのオピオイド使用量増加の主な要因です。このため、OUDの有病率が上昇し、同地域の成長が見込まれると予測されています。
目次
第1章 市場範囲と調査手法
- 市場の定義
- 目的
- 市場範囲
- セグメンテーション
- 調査手法
第2章 市場概要
- イントロダクション
- 概要
- 市場構成とシナリオ
- 概要
- 市場に影響を与える主な要因
- 市場促進要因
- 市場抑制要因
第3章 オピオイド使用障害(OUD)市場で展開される戦略
第4章 世界のオピオイド使用障害(OUD)市場:薬剤クラス別
- 世界のブプレノルフィン市場:地域別
- 世界のメタドン市場:地域別
- 世界のナルトレキソン市場:地域別
第5章 世界のオピオイド使用障害(OUD)市場:投与経路別
- 世界の非経口薬市場:地域別
- 世界の口腔市場:地域別
第6章 世界のオピオイド使用障害(OUD)市場:流通チャネル別
- 世界の病院薬局市場:地域別
- 世界の小売薬局および店舗市場:地域別
- 世界のオンライン薬局市場:地域別
第7章 世界のオピオイド使用障害(OUD)市場:地域別
- 北米
- 北米の市場:国別
- 米国
- カナダ
- メキシコ
- その他北米地域
- 北米の市場:国別
- 欧州
- 欧州の市場:国別
- ドイツ
- 英国
- フランス
- ロシア
- スペイン
- イタリア
- その他欧州地域
- 欧州の市場:国別
- アジア太平洋
- アジア太平洋の市場:国別
- 中国
- 日本
- インド
- 韓国
- シンガポール
- マレーシア
- その他アジア太平洋地域
- アジア太平洋の市場:国別
- ラテンアメリカ・中東・アフリカ
- ラテンアメリカ・中東・アフリカの市場:国別
- ブラジル
- アルゼンチン
- アラブ首長国連邦
- サウジアラビア
- 南アフリカ
- ナイジェリア
- その他ラテンアメリカ・中東・アフリカ地域
- ラテンアメリカ・中東・アフリカの市場:国別
第8章 企業プロファイル
- Indivior PLC(Reckitt Benckiser Group plc)
- Collegium Pharmaceutical, Inc(BioDelivery Sciences International, Inc.)
- Orexo AB(Orexo US, Inc)
- Alkermes PLC
- Titan Pharmaceuticals, Inc
- Camurus AB
- AstraZeneca PLC
- Hikma Pharmaceuticals PLC
- Mallinckrodt PLC
- Viatris, Inc
LIST OF TABLES
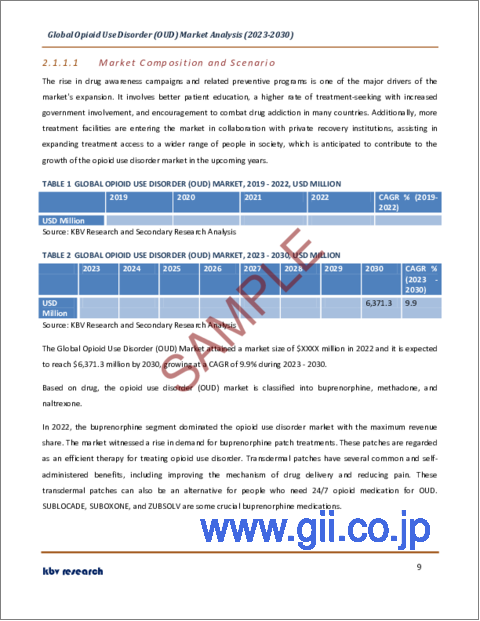
- TABLE 1 Global Opioid Use Disorder (OUD) Market, 2019 - 2022, USD Million
- TABLE 2 Global Opioid Use Disorder (OUD) Market, 2023 - 2030, USD Million
- TABLE 3 Global Opioid Use Disorder (OUD) Market by Drug Class, 2019 - 2022, USD Million
- TABLE 4 Global Opioid Use Disorder (OUD) Market by Drug Class, 2023 - 2030, USD Million
- TABLE 5 Global Buprenorphine Market by Region, 2019 - 2022, USD Million
- TABLE 6 Global Buprenorphine Market by Region, 2023 - 2030, USD Million
- TABLE 7 Global Methadone Market by Region, 2019 - 2022, USD Million
- TABLE 8 Global Methadone Market by Region, 2023 - 2030, USD Million
- TABLE 9 Global Naltrexone Market by Region, 2019 - 2022, USD Million
- TABLE 10 Global Naltrexone Market by Region, 2023 - 2030, USD Million
- TABLE 11 Global Opioid Use Disorder (OUD) Market by Route of Administration, 2019 - 2022, USD Million
- TABLE 12 Global Opioid Use Disorder (OUD) Market by Route of Administration, 2023 - 2030, USD Million
- TABLE 13 Global Parenteral Market by Region, 2019 - 2022, USD Million
- TABLE 14 Global Parenteral Market by Region, 2023 - 2030, USD Million
- TABLE 15 Global Oral Market by Region, 2019 - 2022, USD Million
- TABLE 16 Global Oral Market by Region, 2023 - 2030, USD Million
- TABLE 17 Global Opioid Use Disorder (OUD) Market by Distribution Channel, 2019 - 2022, USD Million
- TABLE 18 Global Opioid Use Disorder (OUD) Market by Distribution Channel, 2023 - 2030, USD Million
- TABLE 19 Global Hospital Pharmacies Market by Region, 2019 - 2022, USD Million
- TABLE 20 Global Hospital Pharmacies Market by Region, 2023 - 2030, USD Million
- TABLE 21 Global Retail Pharmacies & Stores Market by Region, 2019 - 2022, USD Million
- TABLE 22 Global Retail Pharmacies & Stores Market by Region, 2023 - 2030, USD Million
- TABLE 23 Global Online Pharmacies Market by Region, 2019 - 2022, USD Million
- TABLE 24 Global Online Pharmacies Market by Region, 2023 - 2030, USD Million
- TABLE 25 Global Opioid Use Disorder (OUD) Market by Region, 2019 - 2022, USD Million
- TABLE 26 Global Opioid Use Disorder (OUD) Market by Region, 2023 - 2030, USD Million
- TABLE 27 North America Opioid Use Disorder (OUD) Market, 2019 - 2022, USD Million
- TABLE 28 North America Opioid Use Disorder (OUD) Market, 2023 - 2030, USD Million
- TABLE 29 North America Opioid Use Disorder (OUD) Market by Drug Class, 2019 - 2022, USD Million
- TABLE 30 North America Opioid Use Disorder (OUD) Market by Drug Class, 2023 - 2030, USD Million
- TABLE 31 North America Buprenorphine Market by Country, 2019 - 2022, USD Million
- TABLE 32 North America Buprenorphine Market by Country, 2023 - 2030, USD Million
- TABLE 33 North America Methadone Market by Country, 2019 - 2022, USD Million
- TABLE 34 North America Methadone Market by Country, 2023 - 2030, USD Million
- TABLE 35 North America Naltrexone Market by Country, 2019 - 2022, USD Million
- TABLE 36 North America Naltrexone Market by Country, 2023 - 2030, USD Million
- TABLE 37 North America Opioid Use Disorder (OUD) Market by Route of Administration, 2019 - 2022, USD Million
- TABLE 38 North America Opioid Use Disorder (OUD) Market by Route of Administration, 2023 - 2030, USD Million
- TABLE 39 North America Parenteral Market by Country, 2019 - 2022, USD Million
- TABLE 40 North America Parenteral Market by Country, 2023 - 2030, USD Million
- TABLE 41 North America Oral Market by Country, 2019 - 2022, USD Million
- TABLE 42 North America Oral Market by Country, 2023 - 2030, USD Million
- TABLE 43 North America Opioid Use Disorder (OUD) Market by Distribution Channel, 2019 - 2022, USD Million
- TABLE 44 North America Opioid Use Disorder (OUD) Market by Distribution Channel, 2023 - 2030, USD Million
- TABLE 45 North America Hospital Pharmacies Market by Country, 2019 - 2022, USD Million
- TABLE 46 North America Hospital Pharmacies Market by Country, 2023 - 2030, USD Million
- TABLE 47 North America Retail Pharmacies & Stores Market by Country, 2019 - 2022, USD Million
- TABLE 48 North America Retail Pharmacies & Stores Market by Country, 2023 - 2030, USD Million
- TABLE 49 North America Online Pharmacies Market by Country, 2019 - 2022, USD Million
- TABLE 50 North America Online Pharmacies Market by Country, 2023 - 2030, USD Million
- TABLE 51 North America Opioid Use Disorder (OUD) Market by Country, 2019 - 2022, USD Million
- TABLE 52 North America Opioid Use Disorder (OUD) Market by Country, 2023 - 2030, USD Million
- TABLE 53 US Opioid Use Disorder (OUD) Market, 2019 - 2022, USD Million
- TABLE 54 US Opioid Use Disorder (OUD) Market, 2023 - 2030, USD Million
- TABLE 55 US Opioid Use Disorder (OUD) Market by Drug Class, 2019 - 2022, USD Million
- TABLE 56 US Opioid Use Disorder (OUD) Market by Drug Class, 2023 - 2030, USD Million
- TABLE 57 US Opioid Use Disorder (OUD) Market by Route of Administration, 2019 - 2022, USD Million
- TABLE 58 US Opioid Use Disorder (OUD) Market by Route of Administration, 2023 - 2030, USD Million
- TABLE 59 US Opioid Use Disorder (OUD) Market by Distribution Channel, 2019 - 2022, USD Million
- TABLE 60 US Opioid Use Disorder (OUD) Market by Distribution Channel, 2023 - 2030, USD Million
- TABLE 61 Canada Opioid Use Disorder (OUD) Market, 2019 - 2022, USD Million
- TABLE 62 Canada Opioid Use Disorder (OUD) Market, 2023 - 2030, USD Million
- TABLE 63 Canada Opioid Use Disorder (OUD) Market by Drug Class, 2019 - 2022, USD Million
- TABLE 64 Canada Opioid Use Disorder (OUD) Market by Drug Class, 2023 - 2030, USD Million
- TABLE 65 Canada Opioid Use Disorder (OUD) Market by Route of Administration, 2019 - 2022, USD Million
- TABLE 66 Canada Opioid Use Disorder (OUD) Market by Route of Administration, 2023 - 2030, USD Million
- TABLE 67 Canada Opioid Use Disorder (OUD) Market by Distribution Channel, 2019 - 2022, USD Million
- TABLE 68 Canada Opioid Use Disorder (OUD) Market by Distribution Channel, 2023 - 2030, USD Million
- TABLE 69 Mexico Opioid Use Disorder (OUD) Market, 2019 - 2022, USD Million
- TABLE 70 Mexico Opioid Use Disorder (OUD) Market, 2023 - 2030, USD Million
- TABLE 71 Mexico Opioid Use Disorder (OUD) Market by Drug Class, 2019 - 2022, USD Million
- TABLE 72 Mexico Opioid Use Disorder (OUD) Market by Drug Class, 2023 - 2030, USD Million
- TABLE 73 Mexico Opioid Use Disorder (OUD) Market by Route of Administration, 2019 - 2022, USD Million
- TABLE 74 Mexico Opioid Use Disorder (OUD) Market by Route of Administration, 2023 - 2030, USD Million
- TABLE 75 Mexico Opioid Use Disorder (OUD) Market by Distribution Channel, 2019 - 2022, USD Million
- TABLE 76 Mexico Opioid Use Disorder (OUD) Market by Distribution Channel, 2023 - 2030, USD Million
- TABLE 77 Rest of North America Opioid Use Disorder (OUD) Market, 2019 - 2022, USD Million
- TABLE 78 Rest of North America Opioid Use Disorder (OUD) Market, 2023 - 2030, USD Million
- TABLE 79 Rest of North America Opioid Use Disorder (OUD) Market by Drug Class, 2019 - 2022, USD Million
- TABLE 80 Rest of North America Opioid Use Disorder (OUD) Market by Drug Class, 2023 - 2030, USD Million
- TABLE 81 Rest of North America Opioid Use Disorder (OUD) Market by Route of Administration, 2019 - 2022, USD Million
- TABLE 82 Rest of North America Opioid Use Disorder (OUD) Market by Route of Administration, 2023 - 2030, USD Million
- TABLE 83 Rest of North America Opioid Use Disorder (OUD) Market by Distribution Channel, 2019 - 2022, USD Million
- TABLE 84 Rest of North America Opioid Use Disorder (OUD) Market by Distribution Channel, 2023 - 2030, USD Million
- TABLE 85 Europe Opioid Use Disorder (OUD) Market, 2019 - 2022, USD Million
- TABLE 86 Europe Opioid Use Disorder (OUD) Market, 2023 - 2030, USD Million
- TABLE 87 Europe Opioid Use Disorder (OUD) Market by Drug Class, 2019 - 2022, USD Million
- TABLE 88 Europe Opioid Use Disorder (OUD) Market by Drug Class, 2023 - 2030, USD Million
- TABLE 89 Europe Buprenorphine Market by Country, 2019 - 2022, USD Million
- TABLE 90 Europe Buprenorphine Market by Country, 2023 - 2030, USD Million
- TABLE 91 Europe Methadone Market by Country, 2019 - 2022, USD Million
- TABLE 92 Europe Methadone Market by Country, 2023 - 2030, USD Million
- TABLE 93 Europe Naltrexone Market by Country, 2019 - 2022, USD Million
- TABLE 94 Europe Naltrexone Market by Country, 2023 - 2030, USD Million
- TABLE 95 Europe Opioid Use Disorder (OUD) Market by Route of Administration, 2019 - 2022, USD Million
- TABLE 96 Europe Opioid Use Disorder (OUD) Market by Route of Administration, 2023 - 2030, USD Million
- TABLE 97 Europe Parenteral Market by Country, 2019 - 2022, USD Million
- TABLE 98 Europe Parenteral Market by Country, 2023 - 2030, USD Million
- TABLE 99 Europe Oral Market by Country, 2019 - 2022, USD Million
- TABLE 100 Europe Oral Market by Country, 2023 - 2030, USD Million
- TABLE 101 Europe Opioid Use Disorder (OUD) Market by Distribution Channel, 2019 - 2022, USD Million
- TABLE 102 Europe Opioid Use Disorder (OUD) Market by Distribution Channel, 2023 - 2030, USD Million
- TABLE 103 Europe Hospital Pharmacies Market by Country, 2019 - 2022, USD Million
- TABLE 104 Europe Hospital Pharmacies Market by Country, 2023 - 2030, USD Million
- TABLE 105 Europe Retail Pharmacies & Stores Market by Country, 2019 - 2022, USD Million
- TABLE 106 Europe Retail Pharmacies & Stores Market by Country, 2023 - 2030, USD Million
- TABLE 107 Europe Online Pharmacies Market by Country, 2019 - 2022, USD Million
- TABLE 108 Europe Online Pharmacies Market by Country, 2023 - 2030, USD Million
- TABLE 109 Europe Opioid Use Disorder (OUD) Market by Country, 2019 - 2022, USD Million
- TABLE 110 Europe Opioid Use Disorder (OUD) Market by Country, 2023 - 2030, USD Million
- TABLE 111 Germany Opioid Use Disorder (OUD) Market, 2019 - 2022, USD Million
- TABLE 112 Germany Opioid Use Disorder (OUD) Market, 2023 - 2030, USD Million
- TABLE 113 Germany Opioid Use Disorder (OUD) Market by Drug Class, 2019 - 2022, USD Million
- TABLE 114 Germany Opioid Use Disorder (OUD) Market by Drug Class, 2023 - 2030, USD Million
- TABLE 115 Germany Opioid Use Disorder (OUD) Market by Route of Administration, 2019 - 2022, USD Million
- TABLE 116 Germany Opioid Use Disorder (OUD) Market by Route of Administration, 2023 - 2030, USD Million
- TABLE 117 Germany Opioid Use Disorder (OUD) Market by Distribution Channel, 2019 - 2022, USD Million
- TABLE 118 Germany Opioid Use Disorder (OUD) Market by Distribution Channel, 2023 - 2030, USD Million
- TABLE 119 UK Opioid Use Disorder (OUD) Market, 2019 - 2022, USD Million
- TABLE 120 UK Opioid Use Disorder (OUD) Market, 2023 - 2030, USD Million
- TABLE 121 UK Opioid Use Disorder (OUD) Market by Drug Class, 2019 - 2022, USD Million
- TABLE 122 UK Opioid Use Disorder (OUD) Market by Drug Class, 2023 - 2030, USD Million
- TABLE 123 UK Opioid Use Disorder (OUD) Market by Route of Administration, 2019 - 2022, USD Million
- TABLE 124 UK Opioid Use Disorder (OUD) Market by Route of Administration, 2023 - 2030, USD Million
- TABLE 125 UK Opioid Use Disorder (OUD) Market by Distribution Channel, 2019 - 2022, USD Million
- TABLE 126 UK Opioid Use Disorder (OUD) Market by Distribution Channel, 2023 - 2030, USD Million
- TABLE 127 France Opioid Use Disorder (OUD) Market, 2019 - 2022, USD Million
- TABLE 128 France Opioid Use Disorder (OUD) Market, 2023 - 2030, USD Million
- TABLE 129 France Opioid Use Disorder (OUD) Market by Drug Class, 2019 - 2022, USD Million
- TABLE 130 France Opioid Use Disorder (OUD) Market by Drug Class, 2023 - 2030, USD Million
- TABLE 131 France Opioid Use Disorder (OUD) Market by Route of Administration, 2019 - 2022, USD Million
- TABLE 132 France Opioid Use Disorder (OUD) Market by Route of Administration, 2023 - 2030, USD Million
- TABLE 133 France Opioid Use Disorder (OUD) Market by Distribution Channel, 2019 - 2022, USD Million
- TABLE 134 France Opioid Use Disorder (OUD) Market by Distribution Channel, 2023 - 2030, USD Million
- TABLE 135 Russia Opioid Use Disorder (OUD) Market, 2019 - 2022, USD Million
- TABLE 136 Russia Opioid Use Disorder (OUD) Market, 2023 - 2030, USD Million
- TABLE 137 Russia Opioid Use Disorder (OUD) Market by Drug Class, 2019 - 2022, USD Million
- TABLE 138 Russia Opioid Use Disorder (OUD) Market by Drug Class, 2023 - 2030, USD Million
- TABLE 139 Russia Opioid Use Disorder (OUD) Market by Route of Administration, 2019 - 2022, USD Million
- TABLE 140 Russia Opioid Use Disorder (OUD) Market by Route of Administration, 2023 - 2030, USD Million
- TABLE 141 Russia Opioid Use Disorder (OUD) Market by Distribution Channel, 2019 - 2022, USD Million
- TABLE 142 Russia Opioid Use Disorder (OUD) Market by Distribution Channel, 2023 - 2030, USD Million
- TABLE 143 Spain Opioid Use Disorder (OUD) Market, 2019 - 2022, USD Million
- TABLE 144 Spain Opioid Use Disorder (OUD) Market, 2023 - 2030, USD Million
- TABLE 145 Spain Opioid Use Disorder (OUD) Market by Drug Class, 2019 - 2022, USD Million
- TABLE 146 Spain Opioid Use Disorder (OUD) Market by Drug Class, 2023 - 2030, USD Million
- TABLE 147 Spain Opioid Use Disorder (OUD) Market by Route of Administration, 2019 - 2022, USD Million
- TABLE 148 Spain Opioid Use Disorder (OUD) Market by Route of Administration, 2023 - 2030, USD Million
- TABLE 149 Spain Opioid Use Disorder (OUD) Market by Distribution Channel, 2019 - 2022, USD Million
- TABLE 150 Spain Opioid Use Disorder (OUD) Market by Distribution Channel, 2023 - 2030, USD Million
- TABLE 151 Italy Opioid Use Disorder (OUD) Market, 2019 - 2022, USD Million
- TABLE 152 Italy Opioid Use Disorder (OUD) Market, 2023 - 2030, USD Million
- TABLE 153 Italy Opioid Use Disorder (OUD) Market by Drug Class, 2019 - 2022, USD Million
- TABLE 154 Italy Opioid Use Disorder (OUD) Market by Drug Class, 2023 - 2030, USD Million
- TABLE 155 Italy Opioid Use Disorder (OUD) Market by Route of Administration, 2019 - 2022, USD Million
- TABLE 156 Italy Opioid Use Disorder (OUD) Market by Route of Administration, 2023 - 2030, USD Million
- TABLE 157 Italy Opioid Use Disorder (OUD) Market by Distribution Channel, 2019 - 2022, USD Million
- TABLE 158 Italy Opioid Use Disorder (OUD) Market by Distribution Channel, 2023 - 2030, USD Million
- TABLE 159 Rest of Europe Opioid Use Disorder (OUD) Market, 2019 - 2022, USD Million
- TABLE 160 Rest of Europe Opioid Use Disorder (OUD) Market, 2023 - 2030, USD Million
- TABLE 161 Rest of Europe Opioid Use Disorder (OUD) Market by Drug Class, 2019 - 2022, USD Million
- TABLE 162 Rest of Europe Opioid Use Disorder (OUD) Market by Drug Class, 2023 - 2030, USD Million
- TABLE 163 Rest of Europe Opioid Use Disorder (OUD) Market by Route of Administration, 2019 - 2022, USD Million
- TABLE 164 Rest of Europe Opioid Use Disorder (OUD) Market by Route of Administration, 2023 - 2030, USD Million
- TABLE 165 Rest of Europe Opioid Use Disorder (OUD) Market by Distribution Channel, 2019 - 2022, USD Million
- TABLE 166 Rest of Europe Opioid Use Disorder (OUD) Market by Distribution Channel, 2023 - 2030, USD Million
- TABLE 167 Asia Pacific Opioid Use Disorder (OUD) Market, 2019 - 2022, USD Million
- TABLE 168 Asia Pacific Opioid Use Disorder (OUD) Market, 2023 - 2030, USD Million
- TABLE 169 Asia Pacific Opioid Use Disorder (OUD) Market by Drug Class, 2019 - 2022, USD Million
- TABLE 170 Asia Pacific Opioid Use Disorder (OUD) Market by Drug Class, 2023 - 2030, USD Million
- TABLE 171 Asia Pacific Buprenorphine Market by Country, 2019 - 2022, USD Million
- TABLE 172 Asia Pacific Buprenorphine Market by Country, 2023 - 2030, USD Million
- TABLE 173 Asia Pacific Methadone Market by Country, 2019 - 2022, USD Million
- TABLE 174 Asia Pacific Methadone Market by Country, 2023 - 2030, USD Million
- TABLE 175 Asia Pacific Naltrexone Market by Country, 2019 - 2022, USD Million
- TABLE 176 Asia Pacific Naltrexone Market by Country, 2023 - 2030, USD Million
- TABLE 177 Asia Pacific Opioid Use Disorder (OUD) Market by Route of Administration, 2019 - 2022, USD Million
- TABLE 178 Asia Pacific Opioid Use Disorder (OUD) Market by Route of Administration, 2023 - 2030, USD Million
- TABLE 179 Asia Pacific Parenteral Market by Country, 2019 - 2022, USD Million
- TABLE 180 Asia Pacific Parenteral Market by Country, 2023 - 2030, USD Million
- TABLE 181 Asia Pacific Oral Market by Country, 2019 - 2022, USD Million
- TABLE 182 Asia Pacific Oral Market by Country, 2023 - 2030, USD Million
- TABLE 183 Asia Pacific Opioid Use Disorder (OUD) Market by Distribution Channel, 2019 - 2022, USD Million
- TABLE 184 Asia Pacific Opioid Use Disorder (OUD) Market by Distribution Channel, 2023 - 2030, USD Million
- TABLE 185 Asia Pacific Hospital Pharmacies Market by Country, 2019 - 2022, USD Million
- TABLE 186 Asia Pacific Hospital Pharmacies Market by Country, 2023 - 2030, USD Million
- TABLE 187 Asia Pacific Retail Pharmacies & Stores Market by Country, 2019 - 2022, USD Million
- TABLE 188 Asia Pacific Retail Pharmacies & Stores Market by Country, 2023 - 2030, USD Million
- TABLE 189 Asia Pacific Online Pharmacies Market by Country, 2019 - 2022, USD Million
- TABLE 190 Asia Pacific Online Pharmacies Market by Country, 2023 - 2030, USD Million
- TABLE 191 Asia Pacific Opioid Use Disorder (OUD) Market by Country, 2019 - 2022, USD Million
- TABLE 192 Asia Pacific Opioid Use Disorder (OUD) Market by Country, 2023 - 2030, USD Million
- TABLE 193 China Opioid Use Disorder (OUD) Market, 2019 - 2022, USD Million
- TABLE 194 China Opioid Use Disorder (OUD) Market, 2023 - 2030, USD Million
- TABLE 195 China Opioid Use Disorder (OUD) Market by Drug Class, 2019 - 2022, USD Million
- TABLE 196 China Opioid Use Disorder (OUD) Market by Drug Class, 2023 - 2030, USD Million
- TABLE 197 China Opioid Use Disorder (OUD) Market by Route of Administration, 2019 - 2022, USD Million
- TABLE 198 China Opioid Use Disorder (OUD) Market by Route of Administration, 2023 - 2030, USD Million
- TABLE 199 China Opioid Use Disorder (OUD) Market by Distribution Channel, 2019 - 2022, USD Million
- TABLE 200 China Opioid Use Disorder (OUD) Market by Distribution Channel, 2023 - 2030, USD Million
- TABLE 201 Japan Opioid Use Disorder (OUD) Market, 2019 - 2022, USD Million
- TABLE 202 Japan Opioid Use Disorder (OUD) Market, 2023 - 2030, USD Million
- TABLE 203 Japan Opioid Use Disorder (OUD) Market by Drug Class, 2019 - 2022, USD Million
- TABLE 204 Japan Opioid Use Disorder (OUD) Market by Drug Class, 2023 - 2030, USD Million
- TABLE 205 Japan Opioid Use Disorder (OUD) Market by Route of Administration, 2019 - 2022, USD Million
- TABLE 206 Japan Opioid Use Disorder (OUD) Market by Route of Administration, 2023 - 2030, USD Million
- TABLE 207 Japan Opioid Use Disorder (OUD) Market by Distribution Channel, 2019 - 2022, USD Million
- TABLE 208 Japan Opioid Use Disorder (OUD) Market by Distribution Channel, 2023 - 2030, USD Million
- TABLE 209 India Opioid Use Disorder (OUD) Market, 2019 - 2022, USD Million
- TABLE 210 India Opioid Use Disorder (OUD) Market, 2023 - 2030, USD Million
- TABLE 211 India Opioid Use Disorder (OUD) Market by Drug Class, 2019 - 2022, USD Million
- TABLE 212 India Opioid Use Disorder (OUD) Market by Drug Class, 2023 - 2030, USD Million
- TABLE 213 India Opioid Use Disorder (OUD) Market by Route of Administration, 2019 - 2022, USD Million
- TABLE 214 India Opioid Use Disorder (OUD) Market by Route of Administration, 2023 - 2030, USD Million
- TABLE 215 India Opioid Use Disorder (OUD) Market by Distribution Channel, 2019 - 2022, USD Million
- TABLE 216 India Opioid Use Disorder (OUD) Market by Distribution Channel, 2023 - 2030, USD Million
- TABLE 217 South Korea Opioid Use Disorder (OUD) Market, 2019 - 2022, USD Million
- TABLE 218 South Korea Opioid Use Disorder (OUD) Market, 2023 - 2030, USD Million
- TABLE 219 South Korea Opioid Use Disorder (OUD) Market by Drug Class, 2019 - 2022, USD Million
- TABLE 220 South Korea Opioid Use Disorder (OUD) Market by Drug Class, 2023 - 2030, USD Million
- TABLE 221 South Korea Opioid Use Disorder (OUD) Market by Route of Administration, 2019 - 2022, USD Million
- TABLE 222 South Korea Opioid Use Disorder (OUD) Market by Route of Administration, 2023 - 2030, USD Million
- TABLE 223 South Korea Opioid Use Disorder (OUD) Market by Distribution Channel, 2019 - 2022, USD Million
- TABLE 224 South Korea Opioid Use Disorder (OUD) Market by Distribution Channel, 2023 - 2030, USD Million
- TABLE 225 Singapore Opioid Use Disorder (OUD) Market, 2019 - 2022, USD Million
- TABLE 226 Singapore Opioid Use Disorder (OUD) Market, 2023 - 2030, USD Million
- TABLE 227 Singapore Opioid Use Disorder (OUD) Market by Drug Class, 2019 - 2022, USD Million
- TABLE 228 Singapore Opioid Use Disorder (OUD) Market by Drug Class, 2023 - 2030, USD Million
- TABLE 229 Singapore Opioid Use Disorder (OUD) Market by Route of Administration, 2019 - 2022, USD Million
- TABLE 230 Singapore Opioid Use Disorder (OUD) Market by Route of Administration, 2023 - 2030, USD Million
- TABLE 231 Singapore Opioid Use Disorder (OUD) Market by Distribution Channel, 2019 - 2022, USD Million
- TABLE 232 Singapore Opioid Use Disorder (OUD) Market by Distribution Channel, 2023 - 2030, USD Million
- TABLE 233 Malaysia Opioid Use Disorder (OUD) Market, 2019 - 2022, USD Million
- TABLE 234 Malaysia Opioid Use Disorder (OUD) Market, 2023 - 2030, USD Million
- TABLE 235 Malaysia Opioid Use Disorder (OUD) Market by Drug Class, 2019 - 2022, USD Million
- TABLE 236 Malaysia Opioid Use Disorder (OUD) Market by Drug Class, 2023 - 2030, USD Million
- TABLE 237 Malaysia Opioid Use Disorder (OUD) Market by Route of Administration, 2019 - 2022, USD Million
- TABLE 238 Malaysia Opioid Use Disorder (OUD) Market by Route of Administration, 2023 - 2030, USD Million
- TABLE 239 Malaysia Opioid Use Disorder (OUD) Market by Distribution Channel, 2019 - 2022, USD Million
- TABLE 240 Malaysia Opioid Use Disorder (OUD) Market by Distribution Channel, 2023 - 2030, USD Million
- TABLE 241 Rest of Asia Pacific Opioid Use Disorder (OUD) Market, 2019 - 2022, USD Million
- TABLE 242 Rest of Asia Pacific Opioid Use Disorder (OUD) Market, 2023 - 2030, USD Million
- TABLE 243 Rest of Asia Pacific Opioid Use Disorder (OUD) Market by Drug Class, 2019 - 2022, USD Million
- TABLE 244 Rest of Asia Pacific Opioid Use Disorder (OUD) Market by Drug Class, 2023 - 2030, USD Million
- TABLE 245 Rest of Asia Pacific Opioid Use Disorder (OUD) Market by Route of Administration, 2019 - 2022, USD Million
- TABLE 246 Rest of Asia Pacific Opioid Use Disorder (OUD) Market by Route of Administration, 2023 - 2030, USD Million
- TABLE 247 Rest of Asia Pacific Opioid Use Disorder (OUD) Market by Distribution Channel, 2019 - 2022, USD Million
- TABLE 248 Rest of Asia Pacific Opioid Use Disorder (OUD) Market by Distribution Channel, 2023 - 2030, USD Million
- TABLE 249 LAMEA Opioid Use Disorder (OUD) Market, 2019 - 2022, USD Million
- TABLE 250 LAMEA Opioid Use Disorder (OUD) Market, 2023 - 2030, USD Million
- TABLE 251 LAMEA Opioid Use Disorder (OUD) Market by Drug Class, 2019 - 2022, USD Million
- TABLE 252 LAMEA Opioid Use Disorder (OUD) Market by Drug Class, 2023 - 2030, USD Million
- TABLE 253 LAMEA Buprenorphine Market by Country, 2019 - 2022, USD Million
- TABLE 254 LAMEA Buprenorphine Market by Country, 2023 - 2030, USD Million
- TABLE 255 LAMEA Methadone Market by Country, 2019 - 2022, USD Million
- TABLE 256 LAMEA Methadone Market by Country, 2023 - 2030, USD Million
- TABLE 257 LAMEA Naltrexone Market by Country, 2019 - 2022, USD Million
- TABLE 258 LAMEA Naltrexone Market by Country, 2023 - 2030, USD Million
- TABLE 259 LAMEA Opioid Use Disorder (OUD) Market by Route of Administration, 2019 - 2022, USD Million
- TABLE 260 LAMEA Opioid Use Disorder (OUD) Market by Route of Administration, 2023 - 2030, USD Million
- TABLE 261 LAMEA Parenteral Market by Country, 2019 - 2022, USD Million
- TABLE 262 LAMEA Parenteral Market by Country, 2023 - 2030, USD Million
- TABLE 263 LAMEA Oral Market by Country, 2019 - 2022, USD Million
- TABLE 264 LAMEA Oral Market by Country, 2023 - 2030, USD Million
- TABLE 265 LAMEA Opioid Use Disorder (OUD) Market by Distribution Channel, 2019 - 2022, USD Million
- TABLE 266 LAMEA Opioid Use Disorder (OUD) Market by Distribution Channel, 2023 - 2030, USD Million
- TABLE 267 LAMEA Hospital Pharmacies Market by Country, 2019 - 2022, USD Million
- TABLE 268 LAMEA Hospital Pharmacies Market by Country, 2023 - 2030, USD Million
- TABLE 269 LAMEA Retail Pharmacies & Stores Market by Country, 2019 - 2022, USD Million
- TABLE 270 LAMEA Retail Pharmacies & Stores Market by Country, 2023 - 2030, USD Million
- TABLE 271 LAMEA Online Pharmacies Market by Country, 2019 - 2022, USD Million
- TABLE 272 LAMEA Online Pharmacies Market by Country, 2023 - 2030, USD Million
- TABLE 273 LAMEA Opioid Use Disorder (OUD) Market by Country, 2019 - 2022, USD Million
- TABLE 274 LAMEA Opioid Use Disorder (OUD) Market by Country, 2023 - 2030, USD Million
- TABLE 275 Brazil Opioid Use Disorder (OUD) Market, 2019 - 2022, USD Million
- TABLE 276 Brazil Opioid Use Disorder (OUD) Market, 2023 - 2030, USD Million
- TABLE 277 Brazil Opioid Use Disorder (OUD) Market by Drug Class, 2019 - 2022, USD Million
- TABLE 278 Brazil Opioid Use Disorder (OUD) Market by Drug Class, 2023 - 2030, USD Million
- TABLE 279 Brazil Opioid Use Disorder (OUD) Market by Route of Administration, 2019 - 2022, USD Million
- TABLE 280 Brazil Opioid Use Disorder (OUD) Market by Route of Administration, 2023 - 2030, USD Million
- TABLE 281 Brazil Opioid Use Disorder (OUD) Market by Distribution Channel, 2019 - 2022, USD Million
- TABLE 282 Brazil Opioid Use Disorder (OUD) Market by Distribution Channel, 2023 - 2030, USD Million
- TABLE 283 Argentina Opioid Use Disorder (OUD) Market, 2019 - 2022, USD Million
- TABLE 284 Argentina Opioid Use Disorder (OUD) Market, 2023 - 2030, USD Million
- TABLE 285 Argentina Opioid Use Disorder (OUD) Market by Drug Class, 2019 - 2022, USD Million
- TABLE 286 Argentina Opioid Use Disorder (OUD) Market by Drug Class, 2023 - 2030, USD Million
- TABLE 287 Argentina Opioid Use Disorder (OUD) Market by Route of Administration, 2019 - 2022, USD Million
- TABLE 288 Argentina Opioid Use Disorder (OUD) Market by Route of Administration, 2023 - 2030, USD Million
- TABLE 289 Argentina Opioid Use Disorder (OUD) Market by Distribution Channel, 2019 - 2022, USD Million
- TABLE 290 Argentina Opioid Use Disorder (OUD) Market by Distribution Channel, 2023 - 2030, USD Million
- TABLE 291 UAE Opioid Use Disorder (OUD) Market, 2019 - 2022, USD Million
- TABLE 292 UAE Opioid Use Disorder (OUD) Market, 2023 - 2030, USD Million
- TABLE 293 UAE Opioid Use Disorder (OUD) Market by Drug Class, 2019 - 2022, USD Million
- TABLE 294 UAE Opioid Use Disorder (OUD) Market by Drug Class, 2023 - 2030, USD Million
- TABLE 295 UAE Opioid Use Disorder (OUD) Market by Route of Administration, 2019 - 2022, USD Million
- TABLE 296 UAE Opioid Use Disorder (OUD) Market by Route of Administration, 2023 - 2030, USD Million
- TABLE 297 UAE Opioid Use Disorder (OUD) Market by Distribution Channel, 2019 - 2022, USD Million
- TABLE 298 UAE Opioid Use Disorder (OUD) Market by Distribution Channel, 2023 - 2030, USD Million
- TABLE 299 Saudi Arabia Opioid Use Disorder (OUD) Market, 2019 - 2022, USD Million
- TABLE 300 Saudi Arabia Opioid Use Disorder (OUD) Market, 2023 - 2030, USD Million
- TABLE 301 Saudi Arabia Opioid Use Disorder (OUD) Market by Drug Class, 2019 - 2022, USD Million
- TABLE 302 Saudi Arabia Opioid Use Disorder (OUD) Market by Drug Class, 2023 - 2030, USD Million
- TABLE 303 Saudi Arabia Opioid Use Disorder (OUD) Market by Route of Administration, 2019 - 2022, USD Million
- TABLE 304 Saudi Arabia Opioid Use Disorder (OUD) Market by Route of Administration, 2023 - 2030, USD Million
- TABLE 305 Saudi Arabia Opioid Use Disorder (OUD) Market by Distribution Channel, 2019 - 2022, USD Million
- TABLE 306 Saudi Arabia Opioid Use Disorder (OUD) Market by Distribution Channel, 2023 - 2030, USD Million
- TABLE 307 South Africa Opioid Use Disorder (OUD) Market, 2019 - 2022, USD Million
- TABLE 308 South Africa Opioid Use Disorder (OUD) Market, 2023 - 2030, USD Million
- TABLE 309 South Africa Opioid Use Disorder (OUD) Market by Drug Class, 2019 - 2022, USD Million
- TABLE 310 South Africa Opioid Use Disorder (OUD) Market by Drug Class, 2023 - 2030, USD Million
- TABLE 311 South Africa Opioid Use Disorder (OUD) Market by Route of Administration, 2019 - 2022, USD Million
- TABLE 312 South Africa Opioid Use Disorder (OUD) Market by Route of Administration, 2023 - 2030, USD Million
- TABLE 313 South Africa Opioid Use Disorder (OUD) Market by Distribution Channel, 2019 - 2022, USD Million
- TABLE 314 South Africa Opioid Use Disorder (OUD) Market by Distribution Channel, 2023 - 2030, USD Million
- TABLE 315 Nigeria Opioid Use Disorder (OUD) Market, 2019 - 2022, USD Million
- TABLE 316 Nigeria Opioid Use Disorder (OUD) Market, 2023 - 2030, USD Million
- TABLE 317 Nigeria Opioid Use Disorder (OUD) Market by Drug Class, 2019 - 2022, USD Million
- TABLE 318 Nigeria Opioid Use Disorder (OUD) Market by Drug Class, 2023 - 2030, USD Million
- TABLE 319 Nigeria Opioid Use Disorder (OUD) Market by Route of Administration, 2019 - 2022, USD Million
- TABLE 320 Nigeria Opioid Use Disorder (OUD) Market by Route of Administration, 2023 - 2030, USD Million
- TABLE 321 Nigeria Opioid Use Disorder (OUD) Market by Distribution Channel, 2019 - 2022, USD Million
- TABLE 322 Nigeria Opioid Use Disorder (OUD) Market by Distribution Channel, 2023 - 2030, USD Million
- TABLE 323 Rest of LAMEA Opioid Use Disorder (OUD) Market, 2019 - 2022, USD Million
- TABLE 324 Rest of LAMEA Opioid Use Disorder (OUD) Market, 2023 - 2030, USD Million
- TABLE 325 Rest of LAMEA Opioid Use Disorder (OUD) Market by Drug Class, 2019 - 2022, USD Million
- TABLE 326 Rest of LAMEA Opioid Use Disorder (OUD) Market by Drug Class, 2023 - 2030, USD Million
- TABLE 327 Rest of LAMEA Opioid Use Disorder (OUD) Market by Route of Administration, 2019 - 2022, USD Million
- TABLE 328 Rest of LAMEA Opioid Use Disorder (OUD) Market by Route of Administration, 2023 - 2030, USD Million
- TABLE 329 Rest of LAMEA Opioid Use Disorder (OUD) Market by Distribution Channel, 2019 - 2022, USD Million
- TABLE 330 Rest of LAMEA Opioid Use Disorder (OUD) Market by Distribution Channel, 2023 - 2030, USD Million
- TABLE 331 Key Information - Indivior PLC (Reckitt Benckiser Group plc)
- TABLE 332 Key Information - collegium pharmaceutical
- TABLE 333 Key Information - Orexo AB
- TABLE 334 Key Information - Alkermes PLC
- TABLE 335 Key Information - Titan Pharmaceuticals, Inc.
- TABLE 336 Key Information - Camurus AB
- TABLE 337 KEY INFORMATION - AstraZeneca PLC
- TABLE 338 Key Information - Hikma Pharmaceuticals PLC
- TABLE 339 Key information - Mallinckrodt PLC
- TABLE 340 key information - Viatris, Inc.
List of Figures
- FIG 1 Methodology for the research
- FIG 2 Global Opioid Use Disorder (OUD) Market share by Drug Class, 2022
- FIG 3 Global Opioid Use Disorder (OUD) Market share by Drug Class, 2030
- FIG 4 Global Opioid Use Disorder (OUD) Market by Drug Class, 2019 - 2030, USD Million
- FIG 5 Global Opioid Use Disorder (OUD) Market share by Route of Administration, 2022
- FIG 6 Global Opioid Use Disorder (OUD) Market share by Route of Administration, 2030
- FIG 7 Global Opioid Use Disorder (OUD) Market by Route of Administration, 2019 - 2030, USD Million
- FIG 8 Global Opioid Use Disorder (OUD) Market share by Distribution Channel, 2022
- FIG 9 Global Opioid Use Disorder (OUD) Market share by Distribution Channel, 2030
- FIG 10 Global Opioid Use Disorder (OUD) Market by Distribution Channel, 2019 - 2030, USD Million
- FIG 11 Global Opioid Use Disorder (OUD) Market share by Region, 2022
- FIG 12 Global Opioid Use Disorder (OUD) Market share by Region, 2030
- FIG 13 Global Opioid Use Disorder (OUD) Market by Region, 2019 - 2030, USD Million
The Global Opioid Use Disorder (OUD) Market size is expected to reach $6.4 billion by 2030, rising at a market growth of 9.9% CAGR during the forecast period.
Asia Pacific region healthcare sector is anticipated to rise due to the general public's increased knowledge of opioid disorders and the region's rising healthcare spending. Consequently, APAC registered $706.9 million revenue in the market in 2022. Growing elderly populations, increased public knowledge of healthcare services, and an increase in chronic diseases are all factors responsible for the growth. On the other hand, the Methadone Maintenance Treatment Program, a multifaceted therapy method that aims to reduce the health and social problem caused by drug diseases, was successfully implemented by the China' government to address the severe drug problem in China. Some of the factors impacting the market are expanding the use of buprenorphine patches in therapy, growing focus of non-governmental & governmental institutions for raising awareness, and OUD Drugs' negative effects.
The increasing use of buprenorphine patches as a successful treatment for opioid use disorder is one of the forthcoming trends in the market. One of the most cutting-edge methods for treating opioid use disorder is the use of buprenorphine patches. Transdermal patches are a more comfortable drug delivery method that successfully lowers pain and can be self-administered by the patient than traditional treatment techniques such as injections. A significant aspect that is fueling the market's growth is the increasing involvement of governmental and non-governmental groups in raising awareness about opioid overdose and its dangers. A number of groups have expanded their efforts to address the issue as a result of the sharp rise in the number of opioid addicts.
However, these medications frequently cause muscle aches, nausea, vomiting, diarrhea, constipation, respiratory problems, bone/joint pain, bladder pain, and abdominal cramping. In addition, when a drug reaction is strong, the patient may also experience depression and other psychological issues. Clinical concerns about physical dependence and addiction may hinder appropriate prescribing, resulting in insufficient pain management. These negative effects may limit the expansion of the market. The COVID-19 pandemic affected pharmaceutical firms in both favorable and negative manner, depending on demand. Certain pharmaceutical products saw decreased demand as fewer individuals risked going to healthcare facilities like hospitals and clinics. The respective governments implemented strict national lockdowns to stop the spread of virus from spreading. As a result of COVID-19-related disruptions, sales continued to decline sharply. As a result, during the pandemic, there was a declining tendency in the worldwide economy.
Drug Class Outlook
Based on drug, the market is classified into buprenorphine, methadone, and naltrexone. In 2022, the buprenorphine segment dominated the market with the maximum revenue share. The market witnessed a rise in demand for buprenorphine patch treatments. These patches are regarded as an efficient therapy for treating opioid use disorder. Transdermal patches have several common and self-administered benefits, including improving the mechanism of drug delivery and reducing pain. These transdermal patches can also be an alternative for people who need 24/7 opioid medication for OUD. SUBLOCADE, SUBOXONE, and ZUBSOLV are some crucial buprenorphine medications.
Route of Administration Outlook
On the basis of route of administration, the market is fragmented into oral, and parenteral. The parenteral segment held the highest revenue share in the market in 2022. The parenteral method can be used to supplement calories and deliver medications quickly. Parenteral administration allows for the drug to reach the bloodstream quickly, resulting in a rapid onset of action. This is particularly important in managing opioid withdrawal symptoms and providing immediate relief to individuals with OUD. The parenteral route can help minimize the risk of drug diversion and misuse since the medication is administered by healthcare professionals. These benefits of parenteral administration are predicted to support the market growth in this segment.
Distribution Channel Outlook
By distribution channel, the market is categorized into hospital pharmacies, retail pharmacies & stores, and online pharmacies. The online pharmacies segment projected a prominent revenue share in the market in 2022. This is attributed to the increasing usage of e-prescriptions at hospitals along with other healthcare facilities as well as the growth in internet users, increased accessibility to online services, and other factors. The growth of online pharmacy is also fueled by the expansion of teleconsultation and diagnostic support. Nowadays, e-prescriptions that can be used to buy medications from online pharmacies can be created through remote consultation.
Regional Outlook
Region wise, the market is analyzed across North America, Europe, Asia Pacific, and LAMEA. In 2022, the North America region led the market by generating highest revenue share in the market. One of the main factors influencing North America's most significant revenue share is the opioid epidemic in the United States and Canada. Chronic diseases, which are becoming increasingly prevalent, are the primary drivers of the rising usage of opioids for the management of pain. This is predicted to rise the prevalence of OUD, thereby offering growth prospects in the region.
The market research report covers the analysis of key stake holders of the market. Key companies profiled in the report include Indivior PLC (Reckitt Benckiser Group plc), Collegium Pharmaceutical, Inc. (BioDelivery Sciences International, Inc.), Orexo AB (Orexo US, Inc.), Alkermes PLC, Titan Pharmaceuticals, Inc., Camurus AB, AstraZeneca PLC, Hikma Pharmaceuticals PLC, Mallinckrodt PLC, and Viatris, Inc.
Strategies Deployed in Opioid Use Disorder (OUD) Market
May-2023: Indivior PLC received approval from the U.S. Food and Drug Administration for OPVEE (nalmefene) nasal spray for the treatment of opioid overdose induced by natural or synthetic opioids. OPVEE contains nalmefene, an opioid receptor that delivers fast onset and long-duration reversal of opioid-induced respiratory depression.
May-2023: Camurus received approval from the US Food and Drug Administration (FDA) for its Brixadi (buprenorphine) extended-release injection for subcutaneous (SC) use, to treat moderate to severe opioid use disorder. The Brixadi would be used as a part of a complete treatment plan that includes counseling and psychosocial support.
Mar-2023: Indivior PLC took over Opiant Pharmaceuticals, Inc., a pharmaceutical company that develops treatments for drug overdose and addictions. The acquisition strengthens the addiction treatment and science portfolio of the company by adding Opiant's late-stage assets, notably OPNT003, an investigational opioid overdose treatment.
Jan-2023: Hikma Pharmaceuticals PLC revealed Naloxone Hydrochloride Injection, USP, 2mg/2mL, in the prefilled syringe (PFS) form. The drug is used to treat known or suspected opioid overdose. The launch expands the portfolio of addiction therapy treatments of the company.
Sep-2022: Orexo AB formed a partnership with Trinity Health, a comprehensive healthcare system. With this partnership, Trinity Health would offer Orexo's evidence-based digital therapies vorvida® and deprexis® to help patients manage depression and excessive drinking and expand patient access to digital therapeutics.
Jul-2022: Titan Pharmaceuticals, Inc. got approval for its Investigational New Drug ("IND") application from U.S. Food and Drug Administration ("FDA"), for the Phase 1 study of its subdermal formulation of nalmefene, an opioid antagonist which is to be used in the prevention of relapse following opioid detoxification in adults with Opioid Use Disorder ("OUD").
Jul-2021: AstraZeneca took over Alexion Pharmaceuticals, a global biopharmaceutical company. Through this acquisition, the company would be able to enter into medicines for rare diseases and the beginning of a new chapter. Further, the acquisition improved AstraZeneca's scientific presence in immunology and, through Alexion's new complement-biology platform and robust pipeline, would continue to pioneer the discovery & development of medicines for patients with rare diseases.
Jun-2021: U.S. Food and Drug Administration (FDA) has approved LYBALVI (olanzapine and samidorphan), an oral atypical antipsychotic of Alkermes plc, for the treatment of schizophrenia and bipolar I disorder.
Aug-2019: Orexo AB signed an agreement with GAIA AG, a health company that specializes in the development of eHealth and mHealth interventions. The agreement enables the company to have global commercial rights to the product. Both companies would leverage proven effective treatments and technology to bring new digital therapeutics products.
Jul-2019: Camurus AB signed a license agreement with Ra Pharmaceuticals, Inc., a clinical-stage biopharmaceutical company, for the use of Camurus's proprietary FluidCrystal® (FC) technology in the development, manufacturing, and commercializing formulation of zilucoplan. The agreement enables the company to develop new promising product candidates based on our unique FluidCrystal® technology.
Scope of the Study
Market Segments covered in the Report:
By Drug Class
- Buprenorphine
- Methadone
- Naltrexone
By Route of Administration
- Parenteral
- Oral
By Distribution Channel
- Hospital Pharmacies
- Retail Pharmacies & Stores
- Online Pharmacies
By Geography
- North America
- US
- Canada
- Mexico
- Rest of North America
- Europe
- Germany
- UK
- France
- Russia
- Spain
- Italy
- Rest of Europe
- Asia Pacific
- China
- Japan
- India
- South Korea
- Singapore
- Malaysia
- Rest of Asia Pacific
- LAMEA
- Brazil
- Argentina
- UAE
- Saudi Arabia
- South Africa
- Nigeria
- Rest of LAMEA
Companies Profiled
- Indivior PLC (Reckitt Benckiser Group plc)
- Collegium Pharmaceutical, Inc. (BioDelivery Sciences International, Inc.)
- Orexo AB (Orexo US, Inc.)
- Alkermes PLC
- Titan Pharmaceuticals, Inc.
- Camurus AB
- AstraZeneca PLC
- Hikma Pharmaceuticals PLC
- Mallinckrodt PLC
- Viatris, Inc.
Unique Offerings from KBV Research
- Exhaustive coverage
- Highest number of market tables and figures
- Subscription based model available
- Guaranteed best price
- Assured post sales research support with 10% customization free
Table of Contents
Chapter 1. Market Scope & Methodology
- 1.1 Market Definition
- 1.2 Objectives
- 1.3 Market Scope
- 1.4 Segmentation
- 1.4.1 Global Opioid Use Disorder (OUD) Market, by Drug Class
- 1.4.2 Global Opioid Use Disorder (OUD) Market, by Route of Administration
- 1.4.3 Global Opioid Use Disorder (OUD) Market, by Distribution Channel
- 1.4.4 Global Opioid Use Disorder (OUD) Market, by Geography
- 1.5 Methodology for the research
Chapter 2. Market Overview
- 2.1 Introduction
- 2.1.1 Overview
- 2.1.1.1 Market Composition and Scenario
- 2.1.1 Overview
- 2.2 Key Factors Impacting the Market
- 2.2.1 Market Drivers
- 2.2.2 Market Restraints
Chapter 3. Strategies Deployed in Opioid Use Disorder (OUD) Market
Chapter 4. Global Opioid Use Disorder (OUD) Market by Drug Class
- 4.1 Global Buprenorphine Market by Region
- 4.2 Global Methadone Market by Region
- 4.3 Global Naltrexone Market by Region
Chapter 5. Global Opioid Use Disorder (OUD) Market by Route of Administration
- 5.1 Global Parenteral Market by Region
- 5.2 Global Oral Market by Region
Chapter 6. Global Opioid Use Disorder (OUD) Market by Distribution Channel
- 6.1 Global Hospital Pharmacies Market by Region
- 6.2 Global Retail Pharmacies & Stores Market by Region
- 6.3 Global Online Pharmacies Market by Region
Chapter 7. Global Opioid Use Disorder (OUD) Market by Region
- 7.1 North America Opioid Use Disorder (OUD) Market
- 7.1.1 North America Opioid Use Disorder (OUD) Market by Drug Class
- 7.1.1.1 North America Buprenorphine Market by Country
- 7.1.1.2 North America Methadone Market by Country
- 7.1.1.3 North America Naltrexone Market by Country
- 7.1.2 North America Opioid Use Disorder (OUD) Market by Route of Administration
- 7.1.2.1 North America Parenteral Market by Country
- 7.1.2.2 North America Oral Market by Country
- 7.1.3 North America Opioid Use Disorder (OUD) Market by Distribution Channel
- 7.1.3.1 North America Hospital Pharmacies Market by Country
- 7.1.3.2 North America Retail Pharmacies & Stores Market by Country
- 7.1.3.3 North America Online Pharmacies Market by Country
- 7.1.4 North America Opioid Use Disorder (OUD) Market by Country
- 7.1.4.1 US Opioid Use Disorder (OUD) Market
- 7.1.4.1.1 US Opioid Use Disorder (OUD) Market by Drug Class
- 7.1.4.1.2 US Opioid Use Disorder (OUD) Market by Route of Administration
- 7.1.4.1.3 US Opioid Use Disorder (OUD) Market by Distribution Channel
- 7.1.4.2 Canada Opioid Use Disorder (OUD) Market
- 7.1.4.2.1 Canada Opioid Use Disorder (OUD) Market by Drug Class
- 7.1.4.2.2 Canada Opioid Use Disorder (OUD) Market by Route of Administration
- 7.1.4.2.3 Canada Opioid Use Disorder (OUD) Market by Distribution Channel
- 7.1.4.3 Mexico Opioid Use Disorder (OUD) Market
- 7.1.4.3.1 Mexico Opioid Use Disorder (OUD) Market by Drug Class
- 7.1.4.3.2 Mexico Opioid Use Disorder (OUD) Market by Route of Administration
- 7.1.4.3.3 Mexico Opioid Use Disorder (OUD) Market by Distribution Channel
- 7.1.4.4 Rest of North America Opioid Use Disorder (OUD) Market
- 7.1.4.4.1 Rest of North America Opioid Use Disorder (OUD) Market by Drug Class
- 7.1.4.4.2 Rest of North America Opioid Use Disorder (OUD) Market by Route of Administration
- 7.1.4.4.3 Rest of North America Opioid Use Disorder (OUD) Market by Distribution Channel
- 7.1.4.1 US Opioid Use Disorder (OUD) Market
- 7.1.1 North America Opioid Use Disorder (OUD) Market by Drug Class
- 7.2 Europe Opioid Use Disorder (OUD) Market
- 7.2.1 Europe Opioid Use Disorder (OUD) Market by Drug Class
- 7.2.1.1 Europe Buprenorphine Market by Country
- 7.2.1.2 Europe Methadone Market by Country
- 7.2.1.3 Europe Naltrexone Market by Country
- 7.2.2 Europe Opioid Use Disorder (OUD) Market by Route of Administration
- 7.2.2.1 Europe Parenteral Market by Country
- 7.2.2.2 Europe Oral Market by Country
- 7.2.3 Europe Opioid Use Disorder (OUD) Market by Distribution Channel
- 7.2.3.1 Europe Hospital Pharmacies Market by Country
- 7.2.3.2 Europe Retail Pharmacies & Stores Market by Country
- 7.2.3.3 Europe Online Pharmacies Market by Country
- 7.2.4 Europe Opioid Use Disorder (OUD) Market by Country
- 7.2.4.1 Germany Opioid Use Disorder (OUD) Market
- 7.2.4.1.1 Germany Opioid Use Disorder (OUD) Market by Drug Class
- 7.2.4.1.2 Germany Opioid Use Disorder (OUD) Market by Route of Administration
- 7.2.4.1.3 Germany Opioid Use Disorder (OUD) Market by Distribution Channel
- 7.2.4.2 UK Opioid Use Disorder (OUD) Market
- 7.2.4.2.1 UK Opioid Use Disorder (OUD) Market by Drug Class
- 7.2.4.2.2 UK Opioid Use Disorder (OUD) Market by Route of Administration
- 7.2.4.2.3 UK Opioid Use Disorder (OUD) Market by Distribution Channel
- 7.2.4.3 France Opioid Use Disorder (OUD) Market
- 7.2.4.3.1 France Opioid Use Disorder (OUD) Market by Drug Class
- 7.2.4.3.2 France Opioid Use Disorder (OUD) Market by Route of Administration
- 7.2.4.3.3 France Opioid Use Disorder (OUD) Market by Distribution Channel
- 7.2.4.4 Russia Opioid Use Disorder (OUD) Market
- 7.2.4.4.1 Russia Opioid Use Disorder (OUD) Market by Drug Class
- 7.2.4.4.2 Russia Opioid Use Disorder (OUD) Market by Route of Administration
- 7.2.4.4.3 Russia Opioid Use Disorder (OUD) Market by Distribution Channel
- 7.2.4.5 Spain Opioid Use Disorder (OUD) Market
- 7.2.4.5.1 Spain Opioid Use Disorder (OUD) Market by Drug Class
- 7.2.4.5.2 Spain Opioid Use Disorder (OUD) Market by Route of Administration
- 7.2.4.5.3 Spain Opioid Use Disorder (OUD) Market by Distribution Channel
- 7.2.4.6 Italy Opioid Use Disorder (OUD) Market
- 7.2.4.6.1 Italy Opioid Use Disorder (OUD) Market by Drug Class
- 7.2.4.6.2 Italy Opioid Use Disorder (OUD) Market by Route of Administration
- 7.2.4.6.3 Italy Opioid Use Disorder (OUD) Market by Distribution Channel
- 7.2.4.7 Rest of Europe Opioid Use Disorder (OUD) Market
- 7.2.4.7.1 Rest of Europe Opioid Use Disorder (OUD) Market by Drug Class
- 7.2.4.7.2 Rest of Europe Opioid Use Disorder (OUD) Market by Route of Administration
- 7.2.4.7.3 Rest of Europe Opioid Use Disorder (OUD) Market by Distribution Channel
- 7.2.4.1 Germany Opioid Use Disorder (OUD) Market
- 7.2.1 Europe Opioid Use Disorder (OUD) Market by Drug Class
- 7.3 Asia Pacific Opioid Use Disorder (OUD) Market
- 7.3.1 Asia Pacific Opioid Use Disorder (OUD) Market by Drug Class
- 7.3.1.1 Asia Pacific Buprenorphine Market by Country
- 7.3.1.2 Asia Pacific Methadone Market by Country
- 7.3.1.3 Asia Pacific Naltrexone Market by Country
- 7.3.2 Asia Pacific Opioid Use Disorder (OUD) Market by Route of Administration
- 7.3.2.1 Asia Pacific Parenteral Market by Country
- 7.3.2.2 Asia Pacific Oral Market by Country
- 7.3.3 Asia Pacific Opioid Use Disorder (OUD) Market by Distribution Channel
- 7.3.3.1 Asia Pacific Hospital Pharmacies Market by Country
- 7.3.3.2 Asia Pacific Retail Pharmacies & Stores Market by Country
- 7.3.3.3 Asia Pacific Online Pharmacies Market by Country
- 7.3.4 Asia Pacific Opioid Use Disorder (OUD) Market by Country
- 7.3.4.1 China Opioid Use Disorder (OUD) Market
- 7.3.4.1.1 China Opioid Use Disorder (OUD) Market by Drug Class
- 7.3.4.1.2 China Opioid Use Disorder (OUD) Market by Route of Administration
- 7.3.4.1.3 China Opioid Use Disorder (OUD) Market by Distribution Channel
- 7.3.4.2 Japan Opioid Use Disorder (OUD) Market
- 7.3.4.2.1 Japan Opioid Use Disorder (OUD) Market by Drug Class
- 7.3.4.2.2 Japan Opioid Use Disorder (OUD) Market by Route of Administration
- 7.3.4.2.3 Japan Opioid Use Disorder (OUD) Market by Distribution Channel
- 7.3.4.3 India Opioid Use Disorder (OUD) Market
- 7.3.4.3.1 India Opioid Use Disorder (OUD) Market by Drug Class
- 7.3.4.3.2 India Opioid Use Disorder (OUD) Market by Route of Administration
- 7.3.4.3.3 India Opioid Use Disorder (OUD) Market by Distribution Channel
- 7.3.4.4 South Korea Opioid Use Disorder (OUD) Market
- 7.3.4.4.1 South Korea Opioid Use Disorder (OUD) Market by Drug Class
- 7.3.4.4.2 South Korea Opioid Use Disorder (OUD) Market by Route of Administration
- 7.3.4.4.3 South Korea Opioid Use Disorder (OUD) Market by Distribution Channel
- 7.3.4.5 Singapore Opioid Use Disorder (OUD) Market
- 7.3.4.5.1 Singapore Opioid Use Disorder (OUD) Market by Drug Class
- 7.3.4.5.2 Singapore Opioid Use Disorder (OUD) Market by Route of Administration
- 7.3.4.5.3 Singapore Opioid Use Disorder (OUD) Market by Distribution Channel
- 7.3.4.6 Malaysia Opioid Use Disorder (OUD) Market
- 7.3.4.6.1 Malaysia Opioid Use Disorder (OUD) Market by Drug Class
- 7.3.4.6.2 Malaysia Opioid Use Disorder (OUD) Market by Route of Administration
- 7.3.4.6.3 Malaysia Opioid Use Disorder (OUD) Market by Distribution Channel
- 7.3.4.7 Rest of Asia Pacific Opioid Use Disorder (OUD) Market
- 7.3.4.7.1 Rest of Asia Pacific Opioid Use Disorder (OUD) Market by Drug Class
- 7.3.4.7.2 Rest of Asia Pacific Opioid Use Disorder (OUD) Market by Route of Administration
- 7.3.4.7.3 Rest of Asia Pacific Opioid Use Disorder (OUD) Market by Distribution Channel
- 7.3.4.1 China Opioid Use Disorder (OUD) Market
- 7.3.1 Asia Pacific Opioid Use Disorder (OUD) Market by Drug Class
- 7.4 LAMEA Opioid Use Disorder (OUD) Market
- 7.4.1 LAMEA Opioid Use Disorder (OUD) Market by Drug Class
- 7.4.1.1 LAMEA Buprenorphine Market by Country
- 7.4.1.2 LAMEA Methadone Market by Country
- 7.4.1.3 LAMEA Naltrexone Market by Country
- 7.4.2 LAMEA Opioid Use Disorder (OUD) Market by Route of Administration
- 7.4.2.1 LAMEA Parenteral Market by Country
- 7.4.2.2 LAMEA Oral Market by Country
- 7.4.3 LAMEA Opioid Use Disorder (OUD) Market by Distribution Channel
- 7.4.3.1 LAMEA Hospital Pharmacies Market by Country
- 7.4.3.2 LAMEA Retail Pharmacies & Stores Market by Country
- 7.4.3.3 LAMEA Online Pharmacies Market by Country
- 7.4.4 LAMEA Opioid Use Disorder (OUD) Market by Country
- 7.4.4.1 Brazil Opioid Use Disorder (OUD) Market
- 7.4.4.1.1 Brazil Opioid Use Disorder (OUD) Market by Drug Class
- 7.4.4.1.2 Brazil Opioid Use Disorder (OUD) Market by Route of Administration
- 7.4.4.1.3 Brazil Opioid Use Disorder (OUD) Market by Distribution Channel
- 7.4.4.2 Argentina Opioid Use Disorder (OUD) Market
- 7.4.4.2.1 Argentina Opioid Use Disorder (OUD) Market by Drug Class
- 7.4.4.2.2 Argentina Opioid Use Disorder (OUD) Market by Route of Administration
- 7.4.4.2.3 Argentina Opioid Use Disorder (OUD) Market by Distribution Channel
- 7.4.4.3 UAE Opioid Use Disorder (OUD) Market
- 7.4.4.3.1 UAE Opioid Use Disorder (OUD) Market by Drug Class
- 7.4.4.3.2 UAE Opioid Use Disorder (OUD) Market by Route of Administration
- 7.4.4.3.3 UAE Opioid Use Disorder (OUD) Market by Distribution Channel
- 7.4.4.4 Saudi Arabia Opioid Use Disorder (OUD) Market
- 7.4.4.4.1 Saudi Arabia Opioid Use Disorder (OUD) Market by Drug Class
- 7.4.4.4.2 Saudi Arabia Opioid Use Disorder (OUD) Market by Route of Administration
- 7.4.4.4.3 Saudi Arabia Opioid Use Disorder (OUD) Market by Distribution Channel
- 7.4.4.5 South Africa Opioid Use Disorder (OUD) Market
- 7.4.4.5.1 South Africa Opioid Use Disorder (OUD) Market by Drug Class
- 7.4.4.5.2 South Africa Opioid Use Disorder (OUD) Market by Route of Administration
- 7.4.4.5.3 South Africa Opioid Use Disorder (OUD) Market by Distribution Channel
- 7.4.4.6 Nigeria Opioid Use Disorder (OUD) Market
- 7.4.4.6.1 Nigeria Opioid Use Disorder (OUD) Market by Drug Class
- 7.4.4.6.2 Nigeria Opioid Use Disorder (OUD) Market by Route of Administration
- 7.4.4.6.3 Nigeria Opioid Use Disorder (OUD) Market by Distribution Channel
- 7.4.4.7 Rest of LAMEA Opioid Use Disorder (OUD) Market
- 7.4.4.7.1 Rest of LAMEA Opioid Use Disorder (OUD) Market by Drug Class
- 7.4.4.7.2 Rest of LAMEA Opioid Use Disorder (OUD) Market by Route of Administration
- 7.4.4.7.3 Rest of LAMEA Opioid Use Disorder (OUD) Market by Distribution Channel
- 7.4.4.1 Brazil Opioid Use Disorder (OUD) Market
- 7.4.1 LAMEA Opioid Use Disorder (OUD) Market by Drug Class
Chapter 8. Company Profiles
- 8.1 Indivior PLC (Reckitt Benckiser Group plc)
- 8.1.1 Company Overview
- 8.1.2 Financial Analysis
- 8.1.3 Product and Regional Analysis
- 8.1.4 Research & Development Expenses
- 8.1.5 Recent strategies and developments:
- 8.1.5.1 Partnerships, Collaborations, and Agreements:
- 8.1.5.2 Acquisition and Mergers:
- 8.2 Collegium Pharmaceutical, Inc. (BioDelivery Sciences International, Inc.)
- 8.2.1 Company Overview
- 8.2.2 Financial Analysis
- 8.2.3 Research & Development Expenses
- 8.3 Orexo AB (Orexo US, Inc.)
- 8.3.1 Company Overview
- 8.3.2 Financial Analysis
- 8.3.3 Segmental and Regional Analysis
- 8.3.4 Research & Development Expenses
- 8.3.5 Recent strategies and developments:
- 8.3.5.1 Partnerships, Collaborations, and Agreements:
- 8.4 Alkermes PLC
- 8.4.1 Company Overview
- 8.4.2 Financial Analysis
- 8.4.3 Regional Analysis
- 8.4.4 Research & Development Expenses
- 8.4.5 Recent strategies and developments:
- 8.4.5.1 Trials and Approvals:
- 8.5 Titan Pharmaceuticals, Inc.
- 8.5.1 Company Overview
- 8.5.2 Financial Analysis
- 8.5.3 Segmental and Regional Analysis
- 8.5.4 Research & Development Expenses
- 8.5.5 Recent strategies and developments:
- 8.5.5.1 Trials and Approvals:
- 8.6 Camurus AB
- 8.6.1 Company Overview
- 8.6.2 Financial Analysis
- 8.6.3 Segmental and Regional Analysis
- 8.6.4 Research & Development Expenses
- 8.6.5 Recent strategies and developments:
- 8.6.5.1 Partnerships, Collaborations, and Agreements:
- 8.6.5.2 Trials and Approvals:
- 8.7 AstraZeneca PLC
- 8.7.1 Company Overview
- 8.7.2 Financial Analysis
- 8.7.3 Regional Analysis
- 8.7.4 Research & Development Expenses
- 8.7.5 Recent strategies and developments:
- 8.7.5.1 Acquisition and Mergers:
- 8.8 Hikma Pharmaceuticals PLC
- 8.8.1 Company Overview
- 8.8.2 Financial Analysis
- 8.8.3 Segmental and Regional Analysis
- 8.8.4 Research & Development Expenses
- 8.8.5 Recent strategies and developments:
- 8.8.5.1 Product Launches and Product Expansions:
- 8.9 Mallinckrodt PLC
- 8.9.1 Company Overview
- 8.9.2 Financial Analysis
- 8.9.3 Segmental and Regional Analysis
- 8.9.4 Research & Development Expense
- 8.10. Viatris, Inc.
- 8.10.1 Company Overview
- 8.10.2 Financial Analysis
- 8.10.3 Segmental Analysis
- 8.10.4 Research & Development Expense