|
|
市場調査レポート
商品コード
1597905
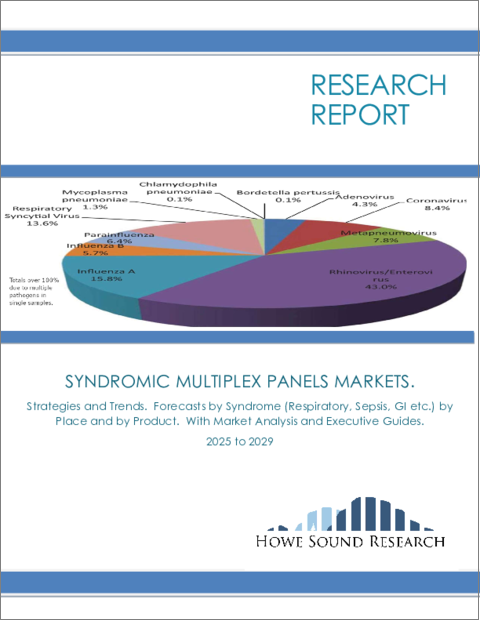
症候群マルチプレックスパネルの世界市場:戦略と動向 - 症候群別、場所別、製品別、国別予測:市場分析およびエグゼクティブガイド付き(2025年~2029年)Syndromic Multiplex Panels Markets. Strategies and Trends. Forecasts by Syndrome (Respiratory, Sepsis, GI etc.) by Place, by Product and by Country. With Market Analysis and Executive Guides. 2025 to 2029 |
||||||
|
|||||||
| 症候群マルチプレックスパネルの世界市場:戦略と動向 - 症候群別、場所別、製品別、国別予測:市場分析およびエグゼクティブガイド付き(2025年~2029年) |
|
出版日: 2024年11月15日
発行: Howe Sound Research
ページ情報: 英文 454 Pages
納期: 即日から翌営業日
|
全表示
- 概要
- 図表
- 目次
概要
当レポートは、世界の症候群マルチプレックスパネル市場について調査し、市場の概要とともに、症候群別、場所別、製品別、国別動向、および市場に参入する企業のプロファイルなどを提供しています。
目次
第1章 市場ガイド
第2章 イントロダクションと市場の定義
- 症候群マルチプレックスパネルとは何か
- 症候群検査- 診断における静かな革命
- 市場の定義
- 調査手法
- 視点:ヘルスケアとIVD業界
第3章 感染症
- 感染症- 全体像
- 病原体
- 症状と症候群
- 病気
- 感染
- 感染症診断
- 感染症の定義
- 症候群性感染症
第4章 業界の概要
- 市場参入機関
- 学術研究機関
- 診断試験開発者
- 計装サプライヤー
- 薬品・試薬メーカー
- 病理サプライヤー
- 独立系臨床検査室
- 公共の国立/地域研究所
- 病院検査室
- 臨床検査室
- 監査機関
- 認証機関
- 臨床検査市場のセグメント
- 業界構造
第5章 市場動向
- 成長促進要因
- 成長抑制要因
- 計測、自動化、診断の動向
第6章 症候群検査の最近の動向
第7章 主要企業のプロファイル
- Abacus Diagnostica
- Abbott Laboratories
- Accelerate Diagnostics
- Ador Diagnostics
- ADT Biotech
- Akonni Biosystems
- Alveo Technologies
- Antelope Dx
- Applied BioCode
- Aureum Diagnostics
- Aus Diagnostics
- Baebies
- Beckman Coulter Diagnostics
- Becton, Dickinson and Company
- Binx Health
- Biocartis
- BioFire Diagnostics(bioMerieux)
- bioMerieux Diagnostics
- Bio-Rad Laboratories, Inc
- Bosch Healthcare Solutions GmbH
- Cepheid(Danaher)
- Credo Diagnostics Biomedical
- Cue Health
- Curetis N.V./Curetis GmbH
- Detect
- Diagenode Diagnostics
- Diasorin S.p.A.
- Domus Diagnostics
- Enzo Biochem
- Eurofins Scientific
- Fluxergy
- Fusion Genomics.
- Genetic Signatures
- GenMark Dx(Roche)
- Grip Molecular Technologies
- Hibergene Diagnostics
- Hologic
- Immunexpress
- Inflammatix
- Invetech
- Janssen Diagnostics
- Karius
- Lexagene
- LightDeck Diagnostics
- Lucira Health
- Luminex Corp(DiaSorin)
- Maxim Biomedical
- Meridian Bioscience
- Mesa Biotech(Thermo Fisher)
- MicroGem
- Millipore Sigma
- Minute Molecular
- Mobidiag(Hologic)
- Molbio Diagnostics
- Nanomix
- Novel Microdevices
- OnsiteGene
- Operon
- Oxford Nanopore Technologies
- Panagene
- Perkin Elmer
- Primerdesign(Novacyt)
- Prominex
- Proof Diagnostics
- Qiagen
- QuantuMDx
- QuidelOrtho
- Roche Molecular Diagnostics
- Saw Diagnostics
- Scope Fluidics
- Seegene
- Siemens Healthineers
- Sona Nanotech
- SpeeDx
- T2 Biosystems
- Talis Biomedical
- Thermo Fisher Scientific Inc.
- Vela Diagnostics
- Veramarx
- Visby Medical
- XCR Diagnostics
第8章 世界の症候群マルチプレックス診断市場
- 世界市場概要、国別
- 世界市場概要、症候群別
- 世界市場概要、場所別
- 世界市場概要、製品別
第9章 世界の症候群マルチプレックス市場- 症候群別
- 呼吸器
- 胃腸
- 血液
- 髄膜炎/脳炎
- 性感染症
- その他
第10章 世界の症候群マルチプレックス市場- 場所別
- 病院研究室
- 外来検査室
- ポイントオブケア
- その他
第11章 世界の症候群マルチプレックス市場- 製品別
- 機器
- カートリッジ
- 試薬
- その他
第12章 付録
図表
Table of Tables
- Table 1: Market Players by Type
- Table 2: Clinical Laboratory Departments and Segments
- Table 3: Laboratory Management Focus - Different Approaches
- Table 4: Key Segmentation Variables Going Forward
- Table 5: Possible Market Segments of Syndromic Multiplex Market
- Table 6: Five Factors Driving Growth
- Table 7: How SMT Improves Outcomes
- Table 8: Four Factors Limiting Growth
- Table 9: Seven Key Diagnostic Laboratory Technology Trends
- Table 10 - Global Market by Region
- Table 11: Global Market by Syndrome
- Table 12: Global Market by Place
- Table 13: Global Market by Product
- Table 14: Respiratory by Country
- Table 15: Gastrointestinal by Country
- Table 16: Blood by Country
- Table 17: Meningitis/Encephalitis by Country
- Table 18: Sexually Transmitted Disease by Country
- Table 19: Other by Country
- Table 20: Hospital Lab by Country
- Table 21: Outpatient Lab by Country
- Table 22: Point of Care by Country
- Table 23: Other by Country
- Table 24: Instruments by Country
- Table 25: Cartridges by Country
- Table 26: Reagents by Country
- Table 27: Other Product by Country
- Table 28: Clinical Lab Fee Schedule
- Table 29: The Most Common Assays
- Table 30: Largest Revenue Assays
Table of Figures
- Figure 1: Global Healthcare Spending
- Figure 2: The Lab Test Pie
- Figure 3: The Road to Diagnostics
- Figure 4: Chart Death Rates and Infectious Disease Decline
- Figure 5: Centralized vs. Decentralized Laboratory Service
- Figure 6: A Highly Multiplexed Syndromic Testing Unit
- Figure 7: The Real Cost to Sequence the Human Genome
- Figure 8: The Codevelopment Process
- Figure 9: Comparing Syndromic and Targeted Testing
- Figure 17: The Multiplex Paradigm Shift
- Figure 11: Global Market Share Chart
- Figure 12: Global Market by Syndrome - Base vs. Final
- Figure 13: Global Market by Syndrome Base Year
- Figure 14: Global Market by Syndrome End Year
- Figure 15: Syndrome Share by Year
- Figure 16: Syndrome Segments Growth
- Figure 17: Global Market by Place - Base vs. Final
- Figure 18: Global Market by Place Base Year
- Figure 19: Global Market by Place End Year
- Figure 20: Place Share by Year
- Figure 21: Place Segments Growth
- Figure 22: Global Market by Product - Base vs. Final
- Figure 23: Global Market by Product Base Year
- Figure 24: Global Market by Product End Year
- Figure 25: Product Share by Year
- Figure 26: Product Segments Growth
- Figure 27: Respiratory Growth
- Figure 28: Gastrointestinal Diagnostics Growth
- Figure 29: Blood Growth
- Figure 30: Meningitis/Encephalitis Growth
- Figure 31: Sexually Transmitted Disease Growth
- Figure 32: Other Growth
- Figure 33: Hospital Lab Growth
- Figure 34: Outpatient Lab Growth
- Figure 35: Point of Care Growth
- Figure 36: Other Growth
- Figure 37: Instruments Growth
- Figure 38: Cartridges Growth
- Figure 39: Reagents Growth
- Figure 40: Other Product Growth
目次
Report Overview:
Infectious disease Dx is changing and will change more in the future. Can a rapidly growing market expand even faster? Find out all about it in this comprehensive report on Syndromic Multiplex Diagnostics.
Will diagnostics replace physicians? When will Infectious Disease testing move into the Physician's Office or even the Home?
Learn about this market including the issues and outlooks. The two key trends of Point of Care Testing and Molecular Diagnostics are merging with spectacular success. It could possibly displace most frontline test protocols AND save money at the same time. The report forecasts the market size out for five years and even further with a later updated report with next years numbers.
All report data is available in Excel format on request.
Table of Contents
1. Market Guides
- 1.1. Situation Analysis
- 1.2. Guide for Executives and Marketing Staff
- 1.3. Guide for Investment Analysts and Management Consultants
2. Introduction and Market Definition
- 2.1. What are Syndromic Multiplex Tests?
- 2.2. Syndromic Testing - the quiet revolution in diagnostics
- 2.2.1. Syndromic Testing - more than Panels
- 2.3. Market Definition
- 2.3.1. Multiplex Market Size
- 2.3.2. Currency
- 2.3.3. Years
- 2.4. Methodology
- 2.4.1. Methodology
- 2.4.2. Sources
- 2.4.3. Authors
- 2.5. Perspective: Healthcare and the IVD Industry
- 2.5.1. Global Healthcare Spending
- 2.5.2. Spending on Diagnostics
- 2.5.3. Important Role of Insurance for Diagnostics
3. Infectious Disease
- 3.1. Infections - The Big Picture
- 3.2. The Pathogens
- 3.2.1. Bacteria
- 3.2.2. Fungi and Protozoa
- 3.2.3. Viruses
- 3.2.4. Prions
- 3.3. Symptoms and Syndromes
- 3.3.1. Bacterial or viral
- 3.3.2. Typical Viral Symptoms
- 3.3.3. Typical Bacterial Symptoms
- 3.4. Disease
- 3.5. Transmission
- 3.6. Infectious Disease Diagnostics
- 3.6.1. Microbial culture
- 3.6.2. Microscopy
- 3.6.3. Biochemical tests
- 3.6.4. PCR-based diagnostics
- 3.6.5. Metagenomic sequencing
- 3.7. Defining Infections
- 3.8. Syndromic Infection
4. Industry Overview
- 4.1. Players in a Dynamic Market
- 4.1.1. Academic Research Lab
- 4.1.2. Diagnostic Test Developer
- 4.1.3. Instrumentation Supplier
- 4.1.4. Chemical/Reagent Supplier
- 4.1.5. Pathology Supplier
- 4.1.6. Independent Clinical Laboratory
- 4.1.7. Public National/regional Laboratory
- 4.1.8. Hospital Laboratory
- 4.1.9. Physicians Office Lab (POLS)
- 4.1.10. Audit Body
- 4.1.11. Certification Body
- 4.2. The Clinical Laboratory Market Segments
- 4.2.1. Traditional Market Segmentation
- 4.2.2. Laboratory Focus and Segmentation
- 4.2.3. Segmenting the Syndromic Testing Market
- 4.3. Industry Structure
- 4.3.1. Hospital Testing Share
- 4.3.2. Economies of Scale
- 4.3.2.1. Hospital vs. Central Lab
- 4.3.3. Physician Office Lab's
- 4.3.4. Physician's and POCT
5. Market Trends
- 5.1. Factors Driving Growth
- 5.1.1. Speed of Diagnosis
- 5.1.2. Effect of Syndromic Testing on Costs
- 5.1.3. Point of Care Advantage
- 5.1.4. Accuracy and Diagnostic Risk
- 5.1.5. Single Visits
- 5.1.6. Improvement in Outcomes
- 5.1.7. Impact of the Pandemic
- 5.2. Factors Limiting Growth
- 5.2.1. Lower Prices
- 5.2.2. Administration/reimbursement
- 5.2.3. Infectious Disease is Declining But..
- 5.2.4. Wellness Hurts
- 5.2.5. Economic Growth improves Living Standards
- 5.2.6. Impact of the Pandemic
- 5.3. Instrumentation, Automation and Diagnostic Trends
- 5.3.1. Traditional Automation and Centralization
- 5.3.2. The New Automation, Decentralization and Point of Care
- 5.3.3. Instruments Key to Market Share
- 5.3.4. Bioinformatics Plays a Role
- 5.3.5. PCR Takes Command
- 5.3.6. Next Generation Sequencing Fuels a Revolution
- 5.3.7. NGS Impact on Pricing
- 5.3.8. Whole Genome Sequencing, A Brave New World
- 5.3.9. Companion Diagnostics Blurs Diagnosis and Treatment
- 5.3.10. Comparing Syndrome and Targeted Testing
- 5.3.11. The Multiplex Paradigm Shift
- 5.3.12. The Sepsis Testing Market - Bellwether for Syndromics
- 5.3.13. The Single Visit and AntiMicrobial Resistance
- 5.3.14. Syndromics drives POCT adoption.
6. Syndromic Testing Recent Developments
- 6.1. Recent Developments - Importance and How to Use This Section
- 6.1.1. Importance of These Developments
- 6.1.2. How to Use This Section
- 6.2. Credo Preps MDx System, Multiplex Respiratory Test for US Launch
- 6.3. Diasorin Nabs Clearance for Expanded Respiratory Panel
- 6.4. Bosch, R-Biopharm to Invest Euro-150M in PCR Tests for Vivalytic MDx Platform
- 6.5. Cepheid Gets Waiver for Multiplex Vaginal Panel
- 6.6. BioMerieux Submits Application for Respiratory, Sore Throat Panel
- 6.7. Sensible Diagnostics Nabs Award for Respiratory Panel
- 6.8. Aptitude Medical Systems to Develop At-Home STI Test
- 6.9. Multiplex Meningitis/Encephalitis Panel Market Expanding
- 6.10. Burst Dx to Deliver Lab-Accurate ELISA-in-a-Cartridge
- 6.11. OpGen Seeking Approval for UTI Panel
- 6.12. Altratech to Enter MDx, POC Space by Supplanting PCR
- 6.13. BIOFIRE Announces New SPOTFIRE-R System
- 6.14. Cambridge Nucleomics to Develop 'Nanobait' Tech
- 6.15. Takara Bio Developing Multiplex Panels
- 6.16. Rover Diagnostics Developing Optical Multiplex PCR
- 6.17. Nanopath Secures Funding to Develop POC Dx
- 6.18. Genetic Signatures Wins Public Health Wales Contract
- 6.19. Qiagen Looks to Long-Term Growth
- 6.20. LexaGene Gets Investment
- 6.21. Seegene Multiplex COVID-19/Flu/RSV Test Receives Approval
- 6.22. BforCure to Adapt Rapid PCR Platform for Cancer
- 6.23. Accelerated Growth for Decentralized Testing
- 6.24. QuantuMDx Developing Syndromic Panels for European Launch
- 6.25. Quidel Gains CE Mark for Savanna Analyzer, Respiratory Panel
- 6.26. QuantumDx Gets CE Mark for Rapid PoC PCR System
- 6.27. Bio-Rad Laboratories, Seegene Partner for MDx Development
- 6.28. Baebies to Expand Finder Platform
- 6.29. With Luminex Acquisition, DiaSorin to Broaden MDx Portfolio
- 6.30. Binx Health Targeting Clinics, DTC & OTC With STI Tests
- 6.31. Luminex Lands BARDA Grant to Develop Test for SARS-CoV-2, Flu, RSV
- 6.32. Molzym, Fraunhofer Developing Rapid Sepsis Diagnostic
- 6.33. MiRxes Receives Approval for Multiplex SARS-CoV-2, Flu Test
- 6.34. New Approach Involves Silicon-Based Test for Infectious Disease Screening
- 6.35. Scanogen's Portable Battery Operated Instrument for 90 Minute Multiplex Test
- 6.36. Qiagen sees NeuMoDx as Growth Vehicle
- 6.37. Torus Biosystems Developing Syndromic Test with 30-Minute Turnaround
7. Profiles of Key Syndromic Testing Companies
- 7.1. Abacus Diagnostica
- 7.2. Abbott Laboratories
- 7.3. Accelerate Diagnostics
- 7.4. Ador Diagnostics
- 7.5. ADT Biotech
- 7.6. Akonni Biosystems
- 7.7. Alveo Technologies
- 7.8. Antelope Dx
- 7.9. Applied BioCode
- 7.10. Aureum Diagnostics
- 7.11. Aus Diagnostics
- 7.12. Baebies
- 7.13. Beckman Coulter Diagnostics
- 7.14. Becton, Dickinson and Company
- 7.15. Binx Health
- 7.16. Biocartis
- 7.17. BioFire Diagnostics (bioMerieux)
- 7.18. bioMerieux Diagnostics
- 7.19. Bio-Rad Laboratories, Inc
- 7.20. Bosch Healthcare Solutions GmbH
- 7.21. Cepheid (Danaher)
- 7.22. Credo Diagnostics Biomedical
- 7.23. Cue Health
- 7.24. Curetis N.V. / Curetis GmbH
- 7.25. Detect
- 7.26. Diagenode Diagnostics
- 7.27. Diasorin S.p.A.
- 7.28. Domus Diagnostics
- 7.29. Enzo Biochem
- 7.30. Eurofins Scientific
- 7.31. Fluxergy
- 7.32. Fusion Genomics.
- 7.33. Genetic Signatures
- 7.34. GenMark Dx (Roche)
- 7.35. Grip Molecular Technologies
- 7.36. Hibergene Diagnostics
- 7.37. Hologic
- 7.38. Immunexpress
- 7.39. Inflammatix
- 7.40. Invetech
- 7.41. Janssen Diagnostics
- 7.42. Karius
- 7.43. Lexagene
- 7.44. LightDeck Diagnostics
- 7.45. Lucira Health
- 7.46. Luminex Corp (DiaSorin)
- 7.47. Maxim Biomedical
- 7.48. Meridian Bioscience
- 7.49. Mesa Biotech (Thermo Fisher)
- 7.50. MicroGem
- 7.51. Millipore Sigma
- 7.52. Minute Molecular
- 7.53. Mobidiag (Hologic)
- 7.54. Molbio Diagnostics
- 7.55. Nanomix
- 7.56. Novel Microdevices
- 7.57. OnsiteGene
- 7.58. Operon
- 7.59. Oxford Nanopore Technologies
- 7.60. Panagene
- 7.61. Perkin Elmer
- 7.62. Primerdesign (Novacyt)
- 7.63. Prominex
- 7.64. Proof Diagnostics
- 7.65. Qiagen
- 7.66. QuantuMDx
- 7.67. QuidelOrtho
- 7.68. Roche Molecular Diagnostics
- 7.69. Saw Diagnostics
- 7.70. Scope Fluidics
- 7.71. Seegene
- 7.72. Siemens Healthineers
- 7.73. Sona Nanotech
- 7.74. SpeeDx
- 7.75. T2 Biosystems
- 7.76. Talis Biomedical
- 7.77. Thermo Fisher Scientific Inc.
- 7.78. Vela Diagnostics
- 7.79. Veramarx
- 7.80. Visby Medical
- 7.81. XCR Diagnostics
8. The Global Market for Syndromic Multiplex Diagnostics
- 8.1. Global Market Overview by Country
- 8.1.1. Table - Global Market by Country
- 8.1.2. Chart - Global Market by Country
- 8.2. Global Market by Syndrome - Overview
- 8.2.1. Table - Global Market by Syndrome
- 8.2.2. Chart - Global Market by Syndrome - Base/Final Year Comparison
- 8.2.3. Chart - Global Market by Syndrome - Base Year
- 8.2.4. Chart - Global Market by Syndrome - End Year
- 8.2.5. Chart - Global Market by Syndrome - Share by Year
- 8.2.6. Chart - Global Market by Syndrome - Segments Growth
- 8.3. Global Market by Place - Overview
- 8.3.1. Table - Global Market by Place
- 8.3.2. Chart - Global Market by Place - Base/Final Year Comparison
- 8.3.3. Chart - Global Market by Place - Base Year
- 8.3.4. Chart - Global Market by Place - End Year
- 8.3.5. Chart - Global Market by Place - Share by Year
- 8.3.6. Chart - Global Market by Place - Segments Growth
- 8.4. Global Market by Product - Overview
- 8.4.1. Table - Global Market by Product
- 8.4.2. Chart - Global Market by Product - Base/Final Year Comparison
- 8.4.3. Chart - Global Market by Product - Base Year
- 8.4.4. Chart - Global Market by Product - End Year
- 8.4.5. Chart - Global Market by Product - Share by Year
- 8.4.6. Chart - Global Market by Product - Segments Growth
9. Global Syndromic Multiplex Markets - By Syndrome
- 9.1. Respiratory
- 9.1.1. Table Respiratory - by Country
- 9.1.2. Chart - Respiratory Growth
- 9.2. Gastrointestinal
- 9.2.1. Table Gastrointestinal - by Country
- 9.2.2. Chart - Gastrointestinal Growth
- 9.3. Blood
- 9.3.1. Table Blood - by Country
- 9.3.2. Chart - Blood Growth
- 9.4. Meningitis/Encephalitis
- 9.4.1. Table Meningitis/Encephalitis - by Country
- 9.4.2. Chart - Meningitis/Encephalitis Growth
- 9.5. Sexually Transmitted Disease
- 9.5.1. Table Sexually Transmitted Disease - by Country
- 9.5.2. Chart - Sexually Transmitted Disease Growth
- 9.6. Other Syndrome
- 9.6.1. Table Other - by Country
- 9.6.2. Chart - Other Growth
10. Global MDx Infectious Disease Markets - by Place
- 10.1. Hospital Lab
- 10.1.1. Table Hospital Lab - by Country
- 10.1.2. Chart - Hospital Lab Growth
- 10.2. Outpatient Lab
- 10.2.1. Table Outpatient Lab - by Country
- 10.2.2. Chart - Outpatient Lab Growth
- 10.3. Point of Care
- 10.3.1. Table Point of Care - by Country
- 10.3.2. Chart - Point of Care Growth
- 10.4. Other Place
- 10.4.1. Table Other - by Country
- 10.4.2. Chart - Other Place Growth
11. Global MDx Infectious Disease Markets - by Product
- 11.1. Instruments
- 11.1.1. Table Instruments - by Country
- 11.1.2. Chart - Instruments Growth
- 11.2. Cartridges
- 11.2.1. Table Cartridges - by Country
- 11.2.2. Chart - Cartridges Growth
- 11.3. Reagents
- 11.3.1. Table Reagents - by Country
- 11.3.2. Chart - Reagents Growth
- 11.4. Other Product
- 11.4.1. Table Other Product - by Country
- 11.4.2. Chart - Other Product Growth
12. Appendices
- 12.1. United States Medicare System: Clinical Laboratory Fees Schedule
- 12.2. The Most Used IVD Assays
- 12.3. The Highest Grossing Assays