|
|
市場調査レポート
商品コード
1543812
軟骨肉腫の世界市場 - 競合情勢Chondrosarcoma: Competitive Landscape |
||||||
カスタマイズ可能
適宜更新あり
|
|||||||
| 軟骨肉腫の世界市場 - 競合情勢 |
|
出版日: 2024年07月04日
発行: GlobalData
ページ情報: 英文 63 Pages
納期: 即納可能
|
全表示
- 概要
- 目次
概要
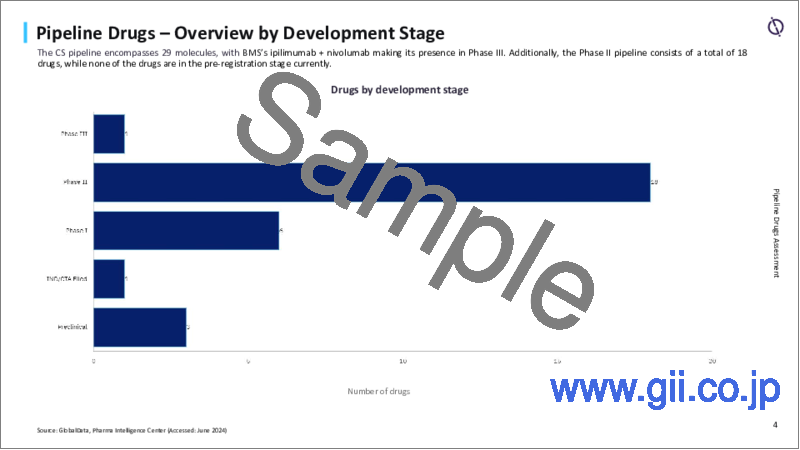
現在、軟骨肉腫市場において承認された疾患修飾療法はありません。軟骨肉腫の研究開発活動は緩やかで、パイプラインには29分子が存在しますが、そのうち第III相は1剤のみ、第II相は18剤です。
米国は軟骨肉腫の臨床試験を実施する主要国として台頭してきています。北米、欧州、アジア太平洋地域では、パートナーシップ別取引が主流です。
当レポートでは、世界の軟骨肉腫市場について調査し、疾患の概要とともに、治験動向、パイプライン概要、将来の見通しなどを提供しています。
目次
第1章 目次
第1章 序文
第2章 主な調査結果
第3章 疾患の情勢
- 疾患の概要
- 疫学の概要
- 治療の概要
第4章 上市済み薬剤の評価
- 主な上市済み薬剤
- 作用機序別概要
- 分子タイプ別概要
- 製品プロファイルと売上予測
第5章 価格設定と償還評価
- 年間治療費
- 価格設定と償還までの時間
第6章 パイプライン薬剤の評価
- 中期から後期段階のパイプライン薬剤
- 開発段階別概要
- 作用機序別概要
- 分子タイプ別概要
- 薬剤固有の相転移成功率(PTSR)と承認可能性(LoA)
- 治療領域と適応症別のPTSRとLoA
第7章 臨床試験の評価
- 過去の概要
- 相別概要
- ステータス別概要
- 進行中および計画中の試験の相別概要
- 仮想コンポーネントを使用した治験
- 地理的概要
- 地域別の単一国および多国籍試験
- 上位20のスポンサーと相別内訳
- 上位20のスポンサーのステータス別内訳
- エンドポイントステータス別概要
- 人種および民族別の概要
- 登録データ
- 治験サイトの上位20か国
- 世界のトップ20サイト
- 実現可能性分析- 地理的概要
- 実現可能性分析- ベンチマークモデル
第8章 取引情勢
第9章 商業的評価
- 主要な市場参入企業
第10章 将来の市場カタリスト
第11章 付録
目次
Product Code: GDHC165CL
This reports provides a data-driven overview of the current and future competitive landscape in Chondrosarcoma therapeutics.
- In 2024, more than 13,000 diagnosed incident cases of CS are anticipated in the 16 countries covered in GlobalData's epidemiology forecast for CS.
- Currently, no approved disease-modifying therapy is available globally in the market space for CS.
- R&D activities for CS are moderate with 29 molecules in the pipeline, out of which Phase III holds only one drug, whereas Phase II holds 18 drugs.
- The US is emerging as the key country for conducting CS trials.
- Partnership dominated the deal landscape in North America, Europe and Asia-Pacific.
Scope
GlobalData's Chondrosarcoma: Competitive Landscape combines data from the Pharma Intelligence Center with in-house analyst expertise to provide a competitive assessment of the disease marketplace.
Components of the report include -
- Disease Landscape
- Disease Overview
- Epidemiology Overview
- Treatment Overview
- Marketed Products Assessment
- Breakdown by Mechanism of Action, Route of Administration
- Product Profiles with Sales Forecast
- Pricing and Reimbursement Assessment
- Annual Therapy Cost
- Time to Pricing and Time to Reimbursement
- Pipeline Assessment
- Breakdown by Development Stage, Mechanism of Action, Molecule Type, Route of Administration
- Product Profiles with Sales Forecast
- Late-to-mid-stage Pipeline Drugs
- Phase Transition Success Rate and Likelihood of Approval
- Clinical Trials Assessment
- Breakdown of Trials by Phase, Status, Virtual Components, Sponsors, Geography, and Endpoint Status
- Enrolment Analytics, Site Analytics, Feasibility Analysis
- Deals Landscape
- Mergers, Acquisitions, and Strategic Alliances by Region
- Overview of Recent Deals
- Commercial Assessment
- Key Market Players
- Future Market Catalysts
Reasons to Buy
- Develop and design your in-licensing and out-licensing strategies through a review of pipeline products and technologies, and by identifying the companies with the most robust pipeline.
- Develop business strategies by understanding the trends shaping and driving the Chondrosarcoma market.
- Drive revenues by understanding the key trends, innovative products and technologies, and companies likely to impact the global Chondrosarcoma market in the future.
- Formulate effective sales and marketing strategies by understanding the competitive landscape and analyzing the performance of various competitors.
- Identify emerging players with potentially strong product portfolios and create effective counter-strategies to gain a competitive advantage.
- Organize your sales and marketing efforts by identifying the market categories that present the maximum opportunities for consolidations, investments, and strategic partnerships.
Table of Contents
1 Table of Contents (PowerPoint Deck)
1 Preface
- 1.1 Contents
- 1.2 Report Scope
- 1.3 List of Tables and Figures
- 1.4 Abbreviations
2 Key Findings
3 Disease Landscape
- 3.1 Disease Overview
- 3.2 Epidemiology Overview
- 3.3 Treatment Overview
4 Marketed Drugs Assessment
- 4.1 Leading Marketed Drugs
- 4.2 Overview by Mechanism of Action
- 4.3 Overview by Molecule Type
- 4.4 Product Profiles and Sales Forecast
5 Pricing and Reimbursement Assessment
- 5.1 Annual Cost of Therapy
- 5.2 Time to Pricing and Reimbursement
6 Pipeline Drugs Assessment
- 6.1 Mid-to-late-stage Pipeline Drugs
- 6.2 Overview by Development Stage
- 6.3 Overview by Mechanism of Action
- 6.4 Overview by Molecule Type
- 6.5 Drug Specific Phase Transition Success Rate (PTSR) and Likelihood of Approval (LoA)
- 6.6 Therapy Area and Indication-specific PTSR and LoA
7 Clinical Trials Assessment
- 7.1 Historical Overview
- 7.2 Overview by Phase
- 7.3 Overview by Status
- 7.4 Overview by Phase for Ongoing and Planned Trials
- 7.5 Trials with Virtual Components
- 7.6 Overview of Trials by Geography
- 7.7 Single-Country and Multinational Trials by Region
- 7.8 Top 20 Sponsors with Breakdown by Phase
- 7.9 Top 20 Sponsors with Breakdown by Status
- 7.10 Overview by Endpoint Status
- 7.11 Overview by Race and Ethnicity
- 7.12 Enrollment Data
- 7.13 Top 20 countries for Trial Sites
- 7.14 Top 20 Sites Globally
- 7.15 Feasibility Analysis - Geographic Overview
- 7.16 Feasibility Analysis - Benchmark Models
8 Deals Landscape
- 8.1 Mergers, Acquisitions, and Strategic Alliances by Region
- 8.2 Recent Mergers, Acquisitions, and Strategic Alliances
9 Commercial Assessment
- 9.1 Key Market Players
10 Future Market Catalysts
11 Appendix
- 11.1 Methodology
- 11.2 Methodology - Sales Forecast
- 11.3 Methodology - Pricing and Reimbursement
- 11.4 Methodology - PTSR and LoA Analysis
- 11.5 About the Authors
- 11.6 Contact Us
- 11.7 Disclaimer