|
|
市場調査レポート
商品コード
1424895
下大静脈フィルター(IVCF)市場:パイプラインレポート(開発段階、セグメント、地域・国、規制経路、主要企業)、2024年最新版Inferior Vena Cava Filters (IVCF) Pipeline Report including Stages of Development, Segments, Region and Countries, Regulatory Path and Key Companies, 2024 Update |
||||||
カスタマイズ可能
適宜更新あり
|
|||||||
| 下大静脈フィルター(IVCF)市場:パイプラインレポート(開発段階、セグメント、地域・国、規制経路、主要企業)、2024年最新版 |
|
出版日: 2024年01月31日
発行: GlobalData
ページ情報: 英文 41 Pages
納期: 即納可能
|
全表示
- 概要
- 図表
- 目次
下大静脈フィルター(IVCF)は、血栓が肺に移動するのを防ぐために使用される金属製の装置です。フィルターは下大静脈と呼ばれる太い静脈(下肢から心臓へ血液を運ぶ)の内側に挿入されます。IVCフィルターは、血栓が肺に到達する前に捕捉します。
当レポートでは、下大静脈フィルター(IVCF)市場について調査し、製品概要と開発中のパイプライン製品動向、様々な開発段階にある製品の比較分析や進行中の臨床試験に関する情報、参入企業の最近の動向などを提供しています。
目次
第1章 目次
第2章 イントロダクション
- 下大静脈フィルター(IVCF)の概要
第3章 開発中の製品
- 下大静脈フィルター(IVCF) - 開発段階別パイプライン製品
- 下大静脈フィルター(IVCF) - セグメント別パイプライン製品
- 下大静脈フィルター(IVCF) - 地域別パイプライン製品
- 下大静脈フィルター(IVCF) - 規制経路別パイプライン製品
- 下大静脈フィルター(IVCF) - 推定承認日別パイプライン製品
- 下大静脈フィルター(IVCF) - 進行中の臨床試験
第4章 下大静脈フィルター(IVCF)- 企業が開発中のパイプライン製品
- 下大静脈フィルター(IVCF)企業- 開発段階別のパイプライン製品
- 下大静脈フィルター(IVCF)- 開発段階別のパイプライン製品
第5章 下大静脈フィルター(IVCF)企業と製品概要
- Adient Medical Inc.
- APT Medical Inc
- Contego Medical LLC
- CyndRX LLC
- Interventional &Surgical Innovations, LLC
- Kaleidscope
- Koninklijke Philips NV
- Suzhou Tianhong Shengjie Medical Co Ltd
- University of Arizona
- University of Toledo
- Veniti Inc
- VueKlar Cardiovascular Ltd
- Zylox-Tonbridge Medical Technology Co Ltd
第6章 下大静脈フィルター(IVCF)- 最近の開発
第7章 付録
List of Tables
List of Tables
- Inferior Vena Cava Filters (IVCF) - Pipeline Products by Stage of Development
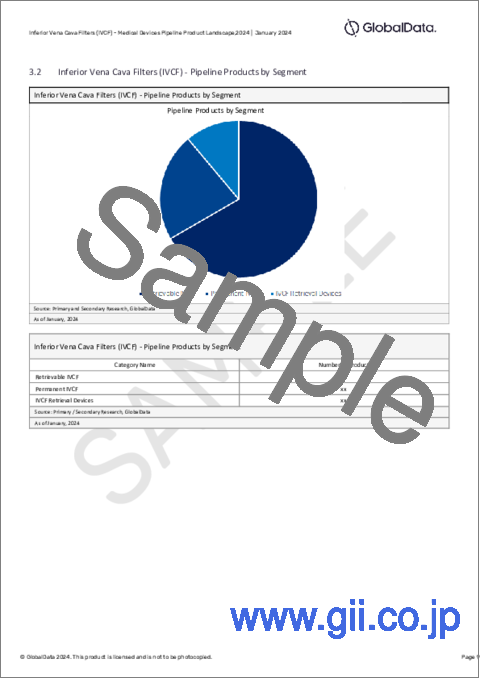
- Inferior Vena Cava Filters (IVCF) - Pipeline Products by Segment
- Inferior Vena Cava Filters (IVCF) - Pipeline Products by Territory
- Inferior Vena Cava Filters (IVCF) - Pipeline Products by Regulatory Path
- Inferior Vena Cava Filters (IVCF) - Pipeline Products by Estimated Approval Date
- Inferior Vena Cava Filters (IVCF) - Ongoing Clinical Trials
- Inferior Vena Cava Filters (IVCF) Companies - Pipeline Products by Stage of Development
- Inferior Vena Cava Filters (IVCF) - Pipeline Products by Stage of Development
- Adient Medical Inc. Pipeline Products & Ongoing Clinical Trials Overview
- Absorbable Vascular Filter - Product Status
- Absorbable Vascular Filter - Product Description
- Adient Medical Inc. - Ongoing Clinical Trials Overview
- Absorbable Vascular Filter - A Prospective, Multicenter, Pivotal Study with Randomized Controlled Prophylactic and Independent Therapeutic Cohorts Evaluate the Safety and Efficacy of an Absorbable Vena Cava Filter for Pulmonary Embolism Prevention
- APT Medical Inc Pipeline Products & Ongoing Clinical Trials Overview
- Retrievable Inferior Vena Cava Filter - Product Status
- Retrievable Inferior Vena Cava Filter - Product Description
- Contego Medical LLC Pipeline Products & Ongoing Clinical Trials Overview
- Temporary IVC Filter - Product Status
- Temporary IVC Filter - Product Description
- CyndRX LLC Pipeline Products & Ongoing Clinical Trials Overview
- Inferior Vena Cava (IVC) Filter - Product Status
- Inferior Vena Cava (IVC) Filter - Product Description
- Interventional & Surgical Innovations, LLC Pipeline Products & Ongoing Clinical Trials Overview
- Inferior Vena Cava Filter - Product Status
- Inferior Vena Cava Filter - Product Description
- Kaleidoscope Pipeline Products & Ongoing Clinical Trials Overview
- Reversible IVC Filter - Product Status
- Reversible IVC Filter - Product Description
- Koninklijke Philips NV Pipeline Products & Ongoing Clinical Trials Overview
- LUMIFI with Crux VCF System - Product Status
- LUMIFI with Crux VCF System - Product Description
- Suzhou Tianhong Shengjie Medical Co Ltd Pipeline Products & Ongoing Clinical Trials Overview
- Vena Cava Filter - Product Status
- Vena Cava Filter - Product Description
- University of Arizona Pipeline Products & Ongoing Clinical Trials Overview
- Inferior Vena Cava Filter - Product Status
- Inferior Vena Cava Filter - Product Description
- University of Toledo Pipeline Products & Ongoing Clinical Trials Overview
- IVC Filter Retrieval System - Product Status
- IVC Filter Retrieval System - Product Description
- Veniti Inc Pipeline Products & Ongoing Clinical Trials Overview
- VENITI VIDI Vena Cava Filter - Product Status
- VENITI VIDI Vena Cava Filter - Product Description
- VueKlar Cardiovascular Ltd (Inactive) Pipeline Products & Ongoing Clinical Trials Overview
- MR-Enhancing Retrievable Vena Cava Filter - Product Status
- MR-Enhancing Retrievable Vena Cava Filter - Product Description
- Zylox-Tonbridge Medical Technology Co Ltd Pipeline Products & Ongoing Clinical Trials Overview
- Implantable Inferior Vena Cava Filter - Product Status
- Implantable Inferior Vena Cava Filter - Product Description
- Zylox-Tonbridge Medical Technology Co Ltd - Ongoing Clinical Trials Overview
- Implantable Inferior Vena Cava Filter - Multi-center, Randomized and Non-inferiority Clinical Trial to Investigate the Efficacy and Safety of Retrievable Inferior Vena Cava Filter
- Glossary
List of Figures
List of Figures
- Inferior Vena Cava Filters (IVCF) - Pipeline Products by Stage of Development
- Inferior Vena Cava Filters (IVCF) - Pipeline Products by Segment
- Inferior Vena Cava Filters (IVCF) - Pipeline Products by Territory
- Inferior Vena Cava Filters (IVCF) - Pipeline Products by Regulatory Path
- Inferior Vena Cava Filters (IVCF) - Pipeline Products by Estimated Approval Date
- Inferior Vena Cava Filters (IVCF) - Ongoing Clinical Trials
GlobalData's Medical Devices sector report, "Inferior Vena Cava Filters (IVCF) Pipeline Report including Stages of Development, Segments, Region and Countries, Regulatory Path and Key Companies, 2024 Update" provides comprehensive information about the Inferior Vena Cava Filters (IVCF) pipeline products with comparative analysis of the products at various stages of development and information about the clinical trials which are in progress.
Inferior Vena Cava Filter (IVCF) is a metallic device used to prevent blood clots from traveling to lungs. The filter is inserted inside a large vein called the inferior vena cava (which carries blood from lower extremities to the heart). IVC filter traps blood clots before it reaches to the lungs.
Note: Certain sections in the report may be removed or altered based on the availability and relevance of data in relation to the equipment type.
Scope
- Extensive coverage of the Inferior Vena Cava Filters (IVCF) under development
- The report reviews details of major pipeline products which includes, product description, licensing and collaboration details and other developmental activities
- The report reviews the major players involved in the development of Inferior Vena Cava Filters (IVCF) and list all their pipeline projects
- The coverage of pipeline products based on various stages of development ranging from Early Development to Approved / Issued stage
- The report provides key clinical trial data of ongoing trials specific to pipeline products
- Recent developments in the segment / industry
Reasons to Buy
The report enables you to -
- Formulate significant competitor information, analysis, and insights to improve R&D strategies
- Identify emerging players with potentially strong product portfolio and create effective counter strategies to gain competitive advantage
- Identify and understand important and diverse types of Inferior Vena Cava Filters (IVCF) under development
- Develop market-entry and market expansion strategies
- Plan mergers and acquisitions effectively by identifying major players with the most promising pipeline
- In-depth analysis of the product's current stage of development, territory and estimated launch date
Table of Contents
1 Table of Contents
- 1.1 List of Tables
- 1.2 List of Figures
2 Introduction
- 2.1 Inferior Vena Cava Filters (IVCF) Overview
3 Products under Development
- 3.1 Inferior Vena Cava Filters (IVCF) - Pipeline Products by Stage of Development
- 3.2 Inferior Vena Cava Filters (IVCF) - Pipeline Products by Segment
- 3.3 Inferior Vena Cava Filters (IVCF) - Pipeline Products by Territory
- 3.4 Inferior Vena Cava Filters (IVCF) - Pipeline Products by Regulatory Path
- 3.5 Inferior Vena Cava Filters (IVCF) - Pipeline Products by Estimated Approval Date
- 3.6 Inferior Vena Cava Filters (IVCF) - Ongoing Clinical Trials
4 Inferior Vena Cava Filters (IVCF) - Pipeline Products under Development by Companies
- 4.1 Inferior Vena Cava Filters (IVCF) Companies - Pipeline Products by Stage of Development
- 4.2 Inferior Vena Cava Filters (IVCF) - Pipeline Products by Stage of Development
5 Inferior Vena Cava Filters (IVCF) Companies and Product Overview
- 5.1 Adient Medical Inc. Company Overview
- 5.1.1 Adient Medical Inc. Pipeline Products & Ongoing Clinical Trials Overview
- 5.2 APT Medical Inc Company Overview
- 5.2.1 APT Medical Inc Pipeline Products & Ongoing Clinical Trials Overview
- 5.3 Contego Medical LLC Company Overview
- 5.3.1 Contego Medical LLC Pipeline Products & Ongoing Clinical Trials Overview
- 5.4 CyndRX LLC Company Overview
- 5.4.1 CyndRX LLC Pipeline Products & Ongoing Clinical Trials Overview
- 5.5 Interventional & Surgical Innovations, LLC Company Overview
- 5.5.1 Interventional & Surgical Innovations, LLC Pipeline Products & Ongoing Clinical Trials Overview
- 5.6 Kaleidoscope Company Overview
- 5.6.1 Kaleidoscope Pipeline Products & Ongoing Clinical Trials Overview
- 5.7 Koninklijke Philips NV Company Overview
- 5.7.1 Koninklijke Philips NV Pipeline Products & Ongoing Clinical Trials Overview
- 5.8 Suzhou Tianhong Shengjie Medical Co Ltd Company Overview
- 5.8.1 Suzhou Tianhong Shengjie Medical Co Ltd Pipeline Products & Ongoing Clinical Trials Overview
- 5.9 University of Arizona Company Overview
- 5.9.1 University of Arizona Pipeline Products & Ongoing Clinical Trials Overview
- 5.10 University of Toledo Company Overview
- 5.10.1 University of Toledo Pipeline Products & Ongoing Clinical Trials Overview
- 5.11 Veniti Inc Company Overview
- 5.11.1 Veniti Inc Pipeline Products & Ongoing Clinical Trials Overview
- 5.12 VueKlar Cardiovascular Ltd (Inactive) Company Overview
- 5.12.1 VueKlar Cardiovascular Ltd (Inactive) Pipeline Products & Ongoing Clinical Trials Overview
- 5.13 Zylox-Tonbridge Medical Technology Co Ltd Company Overview
- 5.13.1 Zylox-Tonbridge Medical Technology Co Ltd Pipeline Products & Ongoing Clinical Trials Overview
6 Inferior Vena Cava Filters (IVCF)- Recent Developments
- 6.1 Jan 05, 2024: Boston Scientific Announces Conference Call Discussing Fourth Quarter 2023 Results
- 6.2 Oct 26, 2023: Boston Scientific Announces Results for Third Quarter 2023
- 6.3 Sep 29, 2023: Barry Slowey Named Vice President and Chief Sustainability Officer
- 6.4 Sep 15, 2023: Amber Beauchamp Named Vice President of Global Human Resources
- 6.5 Aug 10, 2023: Vena Cava Filter System Saexten Approved by NMPA
- 6.6 Jul 19, 2023: Boston Scientific lays off 52 workers as it closes another Silicon Valley facility
- 6.7 Jun 04, 2023: Cook Medical receives additional 510(k) clearance for Gunther Tulip Vena Cava Filter Retrieval Set
7 Appendix
- 7.1 Methodology
- 7.2 About GlobalData
- 7.3 Contact Us
- 7.4 Disclaimer