|
|
市場調査レポート
商品コード
1116976
シェーグレン症候群の世界市場:市販薬とパイプライン薬品の評価、臨床試験、競合情勢Sjogrens Syndrome Marketed and Pipeline Drugs Assessment, Clinical Trials and Competitive Landscape |
||||||
|
● お客様のご希望に応じて、既存データの加工や未掲載情報(例:国別セグメント)の追加などの対応が可能です。 詳細はお問い合わせください。 |
|||||||
価格
| シェーグレン症候群の世界市場:市販薬とパイプライン薬品の評価、臨床試験、競合情勢 |
|
出版日: 2022年07月05日
発行: GlobalData
ページ情報: 英文 59 Pages
納期: 即納可能
|
ご注意事項 :
本レポートは最新情報反映のため適宜更新し、内容構成変更を行う場合があります。ご検討の際はお問い合わせください。
- 全表示
- 概要
- 目次
概要
当レポートでは、世界のシェーグレン症候群市場について調査分析し、市販薬とパイプライン薬品、臨床試験の評価、競合情勢などを提供しています。
目次
目次
第1章 序文
第2章 主な調査結果
第3章 疾患の情勢
- 疾患の概要
- 疫学の概要
- 治療の概要
第4章 市販薬の評価
第5章 パイプライン薬品の評価
- 後期パイプライン薬品
- 概要:開発段階別
- 概要:作用機序別
- 概要:投与経路別
- 概要:分子タイプ別
- 薬品固有の相転移成功率(PTSR)と承認の可能性(LoA)
- 治療領域と適応症固有のPTSR・LoA
第6章 臨床試験の評価
- 過去の概要
- 概要:フェーズ別
- 概要:ステータス別
- 概要:進行中・計画中の試験のフェーズ別
- 仮想コンポーネントを使用した試験
- 地理的概要
- 単一国・多国間試験:地域別
- 上位20のスポンサー:フェーズ別
- 上位20のスポンサー:ステータス別
- 概要:エンドポイントステータス別
- 概要:人種・民族別
- 登録データ
- 試験施設の上位20か国
- 世界の上位20施設
- 実現可能性分析 - 地理的概要
- 実現可能性分析 - ベンチマークモデル
第7章 取引情勢
- 合併、買収、戦略的提携:地域別
- 最近の合併、買収、戦略的提携
第8章 商業的評価
- 主要な市場企業
第9章 将来の市場カタリスト
第10章 付録
目次
Product Code: GDHC073CL
This reports provides a data-driven overview of the current and future competitive landscape in Sjogren's Syndrome therapeutics.
Key Highlights
- In 2022, there will be more than 3.9 million diagnosed prevalence cases of Sjogren's Syndrome across 16 pharmaceutical markets.
- No FDA-approved marketed drugs have been available for Sjogren's Syndrome in the last two decades.
- The Sjogren's Syndrome pipeline consists of 49 pharmaceuticals spanning all stages of development, with approximately 36% of drugs in mid- to late-stage development.
- Commercial sponsors dominate clinical trial development in Sjogren's Syndrome, with the US emerging as the key countries for conducting trials in Sjogren's Syndrome.
- Deals involving partnerships were the most common type of deals globally.
- Therapeutic options for Sjogren's Syndrome patients continue to be limited as only two new product approvals are expected within the next 5 years.
Scope
GlobalData's Sjogrens Syndrome Marketed and Pipeline Drugs Assessment, Clinical Trials and Competitive Landscape combines data from the Pharma Intelligence Center with in-house analyst expertise to provide a competitive assessment of the disease marketplace.
Components of the report include -
- Disease Landscape
- Disease Overview
- Epidemiology Overview
- Treatment Overview
- Marketed Products Assessment
- Breakdown by Mechanism of Action, Molecule Type and Route of Administration
- Product Profiles with Sales Forecast
- Pipeline Assessment
- Breakdown by Development Stage, Mechanism of Action, Molecule Type, Route of Administration
- Late-to-mid-stage Pipeline Drugs
- Phase Transition Success Rate and Likelihood of Approval
- Clinical Trials Assessment
- Breakdown of Trials by Phase, Status, Virtual Components, Sponsors, Geography, and Endpoint Status
- Enrolment Analytics, Site Analytics, Feasibility Analysis
- Deals Landscape
- Mergers, Acquisitions, and Strategic Alliances by Region
- Overview of Recent Deals
- Commercial Assessment
- Key Market Players
- Future Market Catalysts
Reasons to Buy
- Develop and design your in-licensing and out-licensing strategies through a review of pipeline products and technologies, and by identifying the companies with the most robust pipeline.
- Develop business strategies by understanding the trends shaping and driving the Sjogren's Syndrome market.
- Drive revenues by understanding the key trends, innovative products and technologies, market segments, and companies likely to impact the global Sjogren's Syndrome market in the future.
- Formulate effective sales and marketing strategies by understanding the competitive landscape and by analyzing the performance of various competitors.
- Identify emerging players with potentially strong product portfolios and create effective counter-strategies to gain a competitive advantage.
- Organize your sales and marketing efforts by identifying the market categories and segments that present maximum opportunities for consolidations, investments, and strategic partnerships.
Table of Contents
Table of Contents
1 Preface
- 1.1 Contents
- 1.2 Report Scope
- 1.3 List of Tables and Figures
- 1.4 Abbreviations
2 Key Findings
3 Disease Landscape
- 3.1 Disease Overview
- 3.2 Epidemiology Overview
- 3.3 Treatment Overview
4 Marketed Drugs Assessment
5 Pipeline Drugs Assessment
- 5.1 Late-stage Pipeline Drugs
- 5.2 Overview by Development Stage
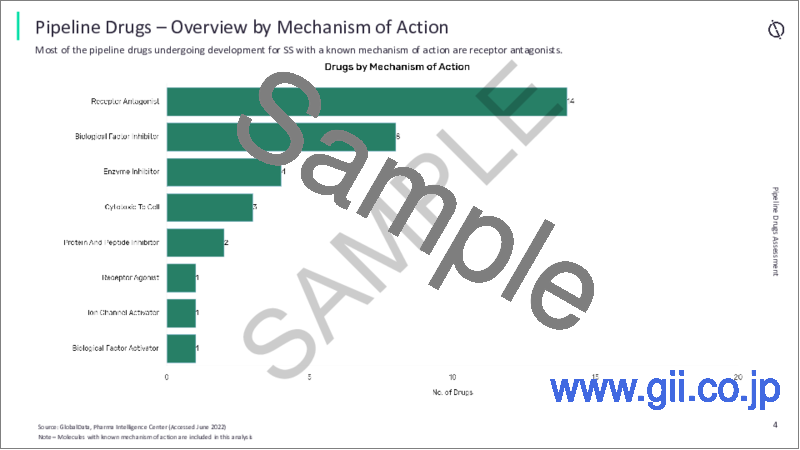
- 5.3 Overview by Mechanism of Action
- 5.4 Overview by Route of Administration
- 5.5 Overview by Molecule Type
- 5.6 Drug Specific Phase Transition Success Rate (PTSR) and Likelihood of Approval (LoA)
- 5.7 Therapy Area and Indication-specific PTSR and LoA
6 Clinical Trials Assessment
- 6.1 Historical Overview
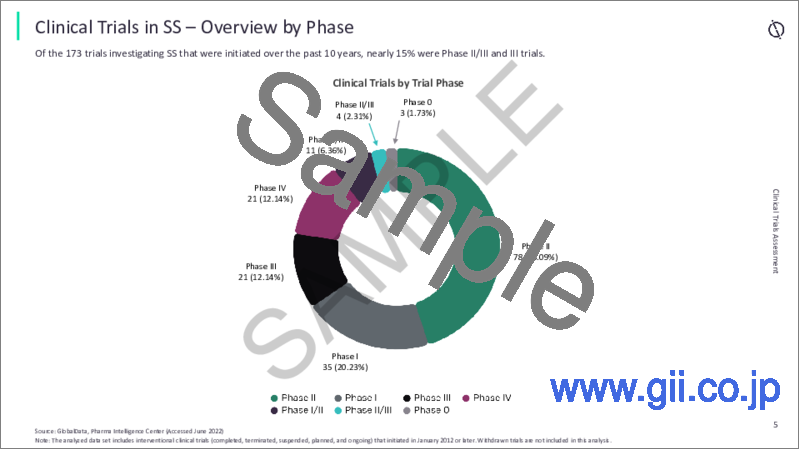
- 6.2 Overview by Phase
- 6.3 Overview by Status
- 6.4 Overview by Phase for Ongoing and Planned Trials
- 6.5 Trials with Virtual Components
- 6.6 Geographic Overview
- 6.7 Single-Country and Multinational Trials by Region
- 6.8 Top 20 Sponsors with Breakdown by Phase
- 6.9 Top 20 Sponsors with Breakdown by Status
- 6.10 Overview by Endpoint Status
- 6.11 Overview by Race and Ethnicity
- 6.12 Enrollment Data
- 6.13 Top 20 countries for Trial Sites
- 6.14 Top 20 Sites Globally
- 6.15 Feasibility Analysis - Geographic Overview
- 6.16 Feasibility Analysis - Benchmark Models
7 Deals Landscape
- 7.1 Mergers, Acquisitions, and Strategic Alliances by Region
- 7.2 Recent Mergers, Acquisitions, and Strategic Alliances
8 Commercial Assessment
- 8.1 Key Market Players
9 Future Market Catalysts
10 Appendix
- 10.1 Methodology
- 10.2 Methodology - PTSR and LoA Analysis
- 10.3 About the Authors
- 10.4 Contact Us
- 10.5 Disclaimer
お電話でのお問い合わせ
044-952-0102
( 土日・祝日を除く )