|
|
市場調査レポート
商品コード
1109515
胃バンドとバルーンのパイプラインレポート:開発段階、セグメント、地域と国、規制パス、主要企業-2022年最新版Gastric Bands and Balloons Pipeline Report including Stages of Development, Segments, Region and Countries, Regulatory Path and Key Companies, 2022 Update |
||||||
|
● お客様のご希望に応じて、既存データの加工や未掲載情報(例:国別セグメント)の追加などの対応が可能です。 詳細はお問い合わせください。 |
|||||||
| 胃バンドとバルーンのパイプラインレポート:開発段階、セグメント、地域と国、規制パス、主要企業-2022年最新版 |
|
出版日: 2022年06月20日
発行: GlobalData
ページ情報: 英文 90 Pages
納期: 即納可能
|
- 全表示
- 概要
- 図表
- 目次
当レポートでは、世界の胃バンドとバルーン市場について調査分析し、研究開発戦略を向上させるための重要な競合情報・分析・洞察、効果的な対抗戦略を立てて競争優位を獲得する強力な製品ポートフォリオを持つ新興企業、開発中の重要かつ多様なタイプの胃バンドとバルーン、市場参入および市場拡大戦略の策定、効果的なM&Aを計画するための最も有望なパイプラインを持つ主要企業、製品の現在の開発段階、地域、発売予定日についての詳細な分析等に関する情報を提供しています。
目次
第1章 目次
- テーブル一覧
- 図一覧
第2章 イントロダクション
- 胃バンドとバルーンの概要
第3章:開発中の製品
- 胃バンドとバルーン:開発段階別パイプライン製品
- 胃バンドとバルーン:セグメント別パイプライン製品
- 胃バンドとバルーン:地域別パイプライン製品
- 胃バンドとバルーン:規制パス別パイプライン製品
- 胃バンドとバルーン:承認予定日別パイプライン製品
- 胃バンドとバルーン:進行中の臨床試験
第4章 胃バンドとバルーン:企業が開発中のパイプライン製品
- 胃バンドとバルーン企業:開発段階別パイプライン製品
- 胃バンドとバルーン:開発段階別パイプライン製品
第5章 胃バンドとバルーンの企業と製品の概要
- 3DT Holdings LLC 企業概要
- Allurion Technologies, Inc. 企業概要
- Apollo Endosurgery Inc 企業概要
- Applied Medical Resources Corporation 企業概要
- Asensus Surgical Inc 企業概要
- Columbia University 企業概要
- CR Bard Inc 企業概要
- EndoRetics Inc. 企業概要
- EndoVx Inc 企業概要
- Gelesis Inc 企業概要
- GI Dynamics Inc 企業概要
- Hangzhou Tangji Medical Technology Co Ltd 企業概要
- Hebrew University of Jerusalem 企業概要
- Innovia LLC 企業概要
- Johns Hopkins University 企業概要
- Mayo Clinic 企業概要
- Medi-Globe GmbH 企業概要
- MetaModix, Inc 企業概要
- Nanyang Technological University 企業概要
- Nitinotes Surgical Ltd 企業概要
- PandaMed 企業概要
- Precision Medical Devices Inc 企業概要
- ReShape Lifesciences Inc 企業概要
- Satiety Inc (Inactive) 企業概要
- Sensate LLC 企業概要
- Silhouette Medical Inc 企業概要
- SLIMEDICS LTD 企業概要
- The Sheikh Zayed Institute for Pediatric Surgical Innovation 企業概要
- Tulip Medical Ltd. 企業概要
- University of Florida 企業概要
- University of Miami 企業概要
- ValenTx Inc 企業概要
- Vibrynt, Inc. (Inactive) 企業概要
第6章 胃バンドとバルーン:最近の開発
第7章 付録
- 調査手法
- GlobalDataについて
- お問い合わせ
- 免責事項
List of Tables
List of Tables
- Gastric Bands & Balloons - Pipeline Products by Stage of Development
- Gastric Bands & Balloons - Pipeline Products by Segment
- Gastric Bands & Balloons - Pipeline Products by Territory
- Gastric Bands & Balloons - Pipeline Products by Regulatory Path
- Gastric Bands & Balloons - Pipeline Products by Estimated Approval Date
- Gastric Bands & Balloons - Ongoing Clinical Trials
- Gastric Bands & Balloons Companies - Pipeline Products by Stage of Development
- Gastric Bands & Balloons - Pipeline Products by Stage of Development
- 3DT Holdings LLC Pipeline Products & Ongoing Clinical Trials Overview
- Gastric Sleeve Magnetic Implant - Product Status
- Gastric Sleeve Magnetic Implant - Product Description
- Allurion Technologies, Inc. Pipeline Products & Ongoing Clinical Trials Overview
- Elipse Balloon - Product Status
- Elipse Balloon - Product Description
- Allurion Technologies, Inc. - Ongoing Clinical Trials Overview
- Elipse Balloon - The BALLOON- (Balloon Treatment for Obesity in Norway) Pilot Study
- Apollo Endosurgery Inc Pipeline Products & Ongoing Clinical Trials Overview
- ORBERA Intragastric Balloon System - NASH - Product Status
- ORBERA Intragastric Balloon System - NASH - Product Description
- Applied Medical Resources Corporation Pipeline Products & Ongoing Clinical Trials Overview
- Next Generation Bariatric Surgical Device - Product Status
- Next Generation Bariatric Surgical Device - Product Description
- Asensus Surgical Inc Pipeline Products & Ongoing Clinical Trials Overview
- Intraluminal Gastroplasty Device - Obesity - Product Status
- Intraluminal Gastroplasty Device - Obesity - Product Description
- Columbia University Pipeline Products & Ongoing Clinical Trials Overview
- Gastric Bypass Shunt - Product Status
- Gastric Bypass Shunt - Product Description
- CR Bard Inc Pipeline Products & Ongoing Clinical Trials Overview
- Restore Suturing System - Product Status
- Restore Suturing System - Product Description
- EndoRetics Inc. Pipeline Products & Ongoing Clinical Trials Overview
- Endoretics Device - Product Status
- Endoretics Device - Product Description
- EndoVx Inc Pipeline Products & Ongoing Clinical Trials Overview
- EndoVx Device - Product Status
- EndoVx Device - Product Description
- Gelesis Inc Pipeline Products & Ongoing Clinical Trials Overview
- Gelesis100 - Adolescent - Product Status
- Gelesis100 - Adolescent - Product Description
- PLENITY - Product Status
- PLENITY - Product Description
- GI Dynamics Inc Pipeline Products & Ongoing Clinical Trials Overview
- EndoBarrier Flow Restrictor - Product Status
- EndoBarrier Flow Restrictor - Product Description
- EndoBarrier Liner With EndoBarrier Restrictor - Product Status
- EndoBarrier Liner With EndoBarrier Restrictor - Product Description
- GI Dynamics Inc - Ongoing Clinical Trials Overview
- EndoBarrier Liner With EndoBarrier Restrictor - Endoscopic Treatment of Obesity with a Duodenal-jejunal Bypass Sleeve: Does Impairment of Fat Absorption Explain Weight Loss
- Hangzhou Tangji Medical Technology Co Ltd Pipeline Products & Ongoing Clinical Trials Overview
- Gastric Bypass Stent System - Product Status
- Gastric Bypass Stent System - Product Description
- Hangzhou Tangji Medical Technology Co Ltd - Ongoing Clinical Trials Overview
- Gastric Bypass Stent System - A Prospective, Open-label, Multicenter, Single-arm Clinical Study to Evaluate the Safety and Performance of the Gastric Bypass Stent System as a Weight Loss Treatment for Obesity
- Hebrew University of Jerusalem Pipeline Products & Ongoing Clinical Trials Overview
- MetaboShield - Product Status
- MetaboShield - Product Description
- Innovia LLC Pipeline Products & Ongoing Clinical Trials Overview
- Gastric Balloon - Product Status
- Gastric Balloon - Product Description
- Laparoscopic Band - Product Status
- Laparoscopic Band - Product Description
- Johns Hopkins University Pipeline Products & Ongoing Clinical Trials Overview
- Image-guided Bariatric Arterial Embolization Device - Product Status
- Image-guided Bariatric Arterial Embolization Device - Product Description
- Mayo Clinic Pipeline Products & Ongoing Clinical Trials Overview
- Endoscopic Therapy Device - Obesity - Product Status
- Endoscopic Therapy Device - Obesity - Product Description
- Medi-Globe GmbH Pipeline Products & Ongoing Clinical Trials Overview
- Medi-Globe Gastric Balloon - Product Status
- Medi-Globe Gastric Balloon - Product Description
- MetaModix, Inc Pipeline Products & Ongoing Clinical Trials Overview
- Endosleeve - Product Status
- Endosleeve - Product Description
- Second Generation Liner Device - Product Status
- Second Generation Liner Device - Product Description
- Nanyang Technological University Pipeline Products & Ongoing Clinical Trials Overview
- Gastric Clip - Product Status
- Gastric Clip - Product Description
- Nitinotes Surgical Ltd Pipeline Products & Ongoing Clinical Trials Overview
- EndoZip System - Product Status
- EndoZip System - Product Description
- Nitinotes Surgical Ltd - Ongoing Clinical Trials Overview
- EndoZip System - Evaluation of EndoZip System in Obese Patients who Failed to Reduce Weight with Non-surgical Weight-loss Methods
- PandaMed Pipeline Products & Ongoing Clinical Trials Overview
- Intragastric Balloon System - Product Status
- Intragastric Balloon System - Product Description
- Precision Medical Devices Inc Pipeline Products & Ongoing Clinical Trials Overview
- Flow Control Device - Obesity - Product Status
- Flow Control Device - Obesity - Product Description
- ReShape Lifesciences Inc Pipeline Products & Ongoing Clinical Trials Overview
- ReShape Vest - Product Status
- ReShape Vest - Product Description
- ReShape Lifesciences Inc - Ongoing Clinical Trials Overview
- ReShape Vest - An Investigative, Prospective, Non-randomized, Multi-center Study to Assess the Safety and Effectiveness of a Novel Gastric Restrictive Device, Call the ReShape Vest, in People Who Are Obese
- Satiety Inc (Inactive) Pipeline Products & Ongoing Clinical Trials Overview
- TOGA System - Product Status
- TOGA System - Product Description
- Sensate LLC Pipeline Products & Ongoing Clinical Trials Overview
- Sensate Anchored Gastric Device - Product Status
- Sensate Anchored Gastric Device - Product Description
- Silhouette Medical Inc Pipeline Products & Ongoing Clinical Trials Overview
- nObese Device - Product Status
- nObese Device - Product Description
- SLIMEDICS LTD Pipeline Products & Ongoing Clinical Trials Overview
- Intra Gastric Implant - Product Status
- Intra Gastric Implant - Product Description
- The Sheikh Zayed Institute for Pediatric Surgical Innovation Pipeline Products & Ongoing Clinical Trials Overview
- Obesity Balloon - Product Status
- Obesity Balloon - Product Description
- Tulip Medical Ltd. Pipeline Products & Ongoing Clinical Trials Overview
- Tulip Capsule - Product Status
- Tulip Capsule - Product Description
- University of Florida Pipeline Products & Ongoing Clinical Trials Overview
- Mutated Gene Repair System - Obesity - Product Status
- Mutated Gene Repair System - Obesity - Product Description
- University of Miami Pipeline Products & Ongoing Clinical Trials Overview
- EEA Anvil Placement Device - Product Status
- EEA Anvil Placement Device - Product Description
- ValenTx Inc Pipeline Products & Ongoing Clinical Trials Overview
- ValenTx Endoluminal Bypass System - Product Status
- ValenTx Endoluminal Bypass System - Product Description
- ValenTx Inc - Ongoing Clinical Trials Overview
- ValenTx Endoluminal Bypass System - An Open Label Trial of the Safety and Effectiveness of the ValenTx Endo Bypass System and Use of the Endoscopic Anchor Placement Device in Obese Subjects
- ValenTx Endoluminal Bypass System - Safety and Efficacy of the ValenTx EndoPass System for the Treatment of Obese Subjects: The ePass Clinical Trial
- Vibrynt, Inc. (Inactive) Pipeline Products & Ongoing Clinical Trials Overview
- PREVAIL Implant System - Product Status
- PREVAIL Implant System - Product Description
- Glossary
List of Figures
List of Figures
- Gastric Bands & Balloons - Pipeline Products by Stage of Development
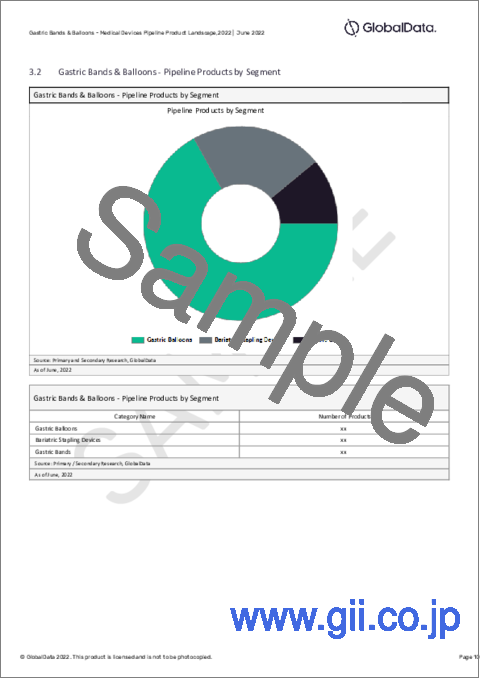
- Gastric Bands & Balloons - Pipeline Products by Segment
- Gastric Bands & Balloons - Pipeline Products by Territory
- Gastric Bands & Balloons - Pipeline Products by Regulatory Path
- Gastric Bands & Balloons - Pipeline Products by Estimated Approval Date
- Gastric Bands & Balloons - Ongoing Clinical Trials
GlobalData's Medical Devices sector report, "Gastric Bands and Balloons Pipeline Report including Stages of Development, Segments, Region and Countries, Regulatory Path and Key Companies, 2022 Update" provides comprehensive information about the Gastric Bands & Balloons pipeline products with comparative analysis of the products at various stages of development and information about the clinical trials which are in progress.
Medical devices that are used to perform bariatric surgery are referred to as bariatric surgery devices.
Note: Certain sections in the report may be removed or altered based on the availability and relevance of data in relation to the equipment type.
Scope
- Extensive coverage of the Gastric Bands & Balloons under development
- The report reviews details of major pipeline products which includes, product description, licensing and collaboration details and other developmental activities
- The report reviews the major players involved in the development of Gastric Bands & Balloons and list all their pipeline projects
- The coverage of pipeline products based on various stages of development ranging from Early Development to Approved / Issued stage
- The report provides key clinical trial data of ongoing trials specific to pipeline products
- Recent developments in the segment / industry
Reasons to Buy
The report enables you to -
- Formulate significant competitor information, analysis, and insights to improve R&D strategies
- Identify emerging players with potentially strong product portfolio and create effective counter strategies to gain competitive advantage
- Identify and understand important and diverse types of Gastric Bands & Balloons under development
- Develop market-entry and market expansion strategies
- Plan mergers and acquisitions effectively by identifying major players with the most promising pipeline
- In-depth analysis of the product's current stage of development, territory and estimated launch date
Table of Contents
1 Table of Contents
- 1.1 List of Tables
- 1.2 List of Figures
2 Introduction
- 2.1 Gastric Bands & Balloons Overview
3 Products under Development
- 3.1 Gastric Bands & Balloons - Pipeline Products by Stage of Development
- 3.2 Gastric Bands & Balloons - Pipeline Products by Segment
- 3.3 Gastric Bands & Balloons - Pipeline Products by Territory
- 3.4 Gastric Bands & Balloons - Pipeline Products by Regulatory Path
- 3.5 Gastric Bands & Balloons - Pipeline Products by Estimated Approval Date
- 3.6 Gastric Bands & Balloons - Ongoing Clinical Trials
4 Gastric Bands & Balloons - Pipeline Products under Development by Companies
- 4.1 Gastric Bands & Balloons Companies - Pipeline Products by Stage of Development
- 4.2 Gastric Bands & Balloons - Pipeline Products by Stage of Development
5 Gastric Bands & Balloons Companies and Product Overview
- 5.1 3DT Holdings LLC Company Overview
- 5.2 Allurion Technologies, Inc. Company Overview
- 5.3 Apollo Endosurgery Inc Company Overview
- 5.4 Applied Medical Resources Corporation Company Overview
- 5.5 Asensus Surgical Inc Company Overview
- 5.6 Columbia University Company Overview
- 5.7 CR Bard Inc Company Overview
- 5.8 EndoRetics Inc. Company Overview
- 5.9 EndoVx Inc Company Overview
- 5.10 Gelesis Inc Company Overview
- 5.11 GI Dynamics Inc Company Overview
- 5.12 Hangzhou Tangji Medical Technology Co Ltd Company Overview
- 5.13 Hebrew University of Jerusalem Company Overview
- 5.14 Innovia LLC Company Overview
- 5.15 Johns Hopkins University Company Overview
- 5.16 Mayo Clinic Company Overview
- 5.17 Medi-Globe GmbH Company Overview
- 5.18 MetaModix, Inc Company Overview
- 5.19 Nanyang Technological University Company Overview
- 5.20 Nitinotes Surgical Ltd Company Overview
- 5.21 PandaMed Company Overview
- 5.22 Precision Medical Devices Inc Company Overview
- 5.23 ReShape Lifesciences Inc Company Overview
- 5.24 Satiety Inc (Inactive) Company Overview
- 5.25 Sensate LLC Company Overview
- 5.26 Silhouette Medical Inc Company Overview
- 5.27 SLIMEDICS LTD Company Overview
- 5.28 The Sheikh Zayed Institute for Pediatric Surgical Innovation Company Overview
- 5.29 Tulip Medical Ltd. Company Overview
- 5.30 University of Florida Company Overview
- 5.31 University of Miami Company Overview
- 5.32 ValenTx Inc Company Overview
- 5.33 Vibrynt, Inc. (Inactive) Company Overview
6 Gastric Bands & Balloons- Recent Developments
- 6.1 May 27, 2022: Medtronic reports full year and fourth quarter fiscal year 2022 financial results
- 6.2 May 26, 2022: Medtronic reports full year and fourth quarter fiscal year 2022 financial results announces 8% dividend increase
- 6.3 May 23, 2022: ReShape Lifesciences Reports First Quarter 2022 Financial Results and Provides Corporate Update
- 6.4 May 18, 2022: Gelesis to Participate in Upcoming Investor Conferences
- 6.5 May 16, 2022: ReShape Lifesciences to Announce Financial Results for the First Quarter Ended March 31, 2022 and Provide Corporate Update
- 6.6 May 09, 2022: Gelesis to Report First Quarter 2022 Financial Results on May 12, 2022
- 6.7 May 04, 2022: Munson Healthcare Names Primary Care Service Line Medical Director
- 6.8 Apr 28, 2022: Boston Scientific reports net sales of $3.03bn in Q1 2022
- 6.10 Mar 31, 2022: Vizient Medical Device tech watch provides trends in transcatheter valve replacement and a model for assessing new technology in musculoskeletal care
- 6.11 Mar 31, 2022: ReShape Lifesciences™ to Announce Financial Results for the First Quarter Ended March 31, 2022 and Provide Corporate Update
- 6.12 Mar 28, 2022: ReShape Lifesciences Reports Year Ended 2021 Financial and Operating Results and Provides Corporate Update
- 6.13 Feb 10, 2022: TriSalus Life Sciences expands clinical executive team and scientific advisors in support of company's focus to overcome liver and pancreatic cancer treatment barriers and enable more patients to benefit from immunotherapy
- 6.14 Jan 04, 2022: Medtronic Chairman and CEO Geoff Martha to speak at J.P. Morgan healthcare conference
- 6.15 Nov 11, 2021: ReShape Lifesciences Reports Third Quarter and Nine Month 2021 Financial and Operational Results
- 6.16 Nov 08, 2021: ReShape Lifesciences to Report Third Quarter 2021 Financial and Operational Results on Thursday, November 11, 2021
- 6.17 Nov 08, 2021: Boston Scientific Announces Upcoming Conference Schedule
- 6.18 Oct 27, 2021: Boston Scientific Announces Results For Third Quarter 2021
- 6.19 Oct 15, 2021: PENTAX announces expansion of Vizient contract to include pediatric endoscopic products and solutions
- 6.20 Sep 13, 2021: ReShape Lifesciences to Host Exhibit at Bariatric Summit
- 6.21 Sep 09, 2021: Boston Scientific Announces 2021 Investor Day Meeting
7 Appendix
- 7.1 Methodology
- 7.2 About GlobalData
- 7.3 Contact Us
- 7.4 Disclaimer