|
|
市場調査レポート
商品コード
1191714
RNAワクチン市場:進歩と成長の機会Advances and Growth Opportunities in RNA Vaccines |
||||||
| RNAワクチン市場:進歩と成長の機会 |
|
出版日: 2022年12月20日
発行: Frost & Sullivan
ページ情報: 英文 77 Pages
納期: 即日から翌営業日
|
- 全表示
- 概要
- 目次
RNA治療薬やワクチンは以前から存在していましたが、COVID-19のパンデミックによって大量に市場に投入されるようになりました。業界関係者は、COVID-19のために開発されたmRNAワクチンが、集団におけるSARS-CoV-2ウイルスの影響を軽減するために有効であると考えたのです。
当レポートでは、世界のRNAワクチン市場について調査し、市場の概要とともに、戦略的インペラティブ、成長の機会などを提供しています。
目次
戦略的インペラティブ
- 成長がますます困難になるのはなぜか
- 戦略的インペラティブ
- RNAワクチン業界に対する上位3つの戦略的インペラティブの影響
- 成長の機会が成長パイプラインエンジンを加速させる
成長機会分析
- 分析範囲
- セグメンテーション
- RNAワクチンの開発への移行が進む
- RNAワクチンは従来のワクチンの限界を克服
- RNAワクチン開発の世界の動向
- 促進要因
- 成長抑制
イノベーションと研究開発シナリオ
RNAワクチン開発の情勢:予防および治療用ワクチン
利害関係者のエコシステム
- RNAワクチンの成長の可能性を示す動的な世界エコシステム
- 革新的なワクチン製造ユニットの開発と生産能力の拡大
- RNAワクチン開発者間のコラボレーション
成長の機会
- 成長の機会1:RNAワクチンの製造と配送のための世界のインフラ開発
- 成長の機会2:RNAの変更とデザインの改善
- 成長の機会3:デリバリーシステムと製剤の改善
付録
次のステップ
Technology Innovations and Investments Bolster Next-generation Prophylactic and Therapeutic RNA Vaccines
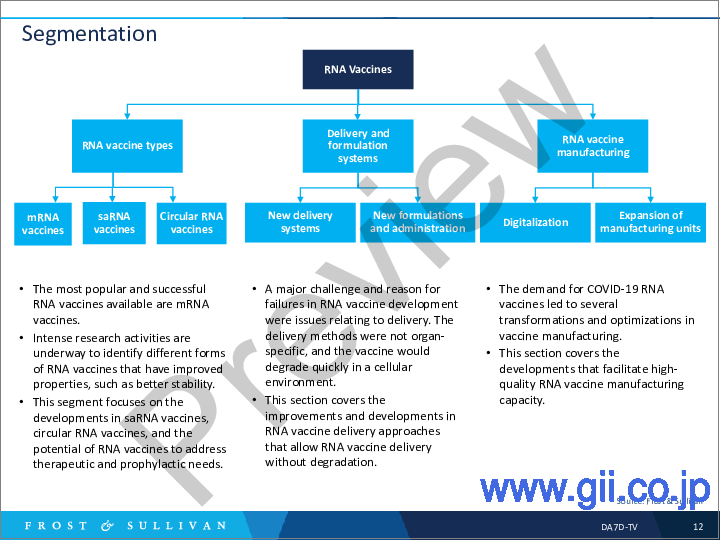
RNA therapeutics and vaccines have been around for a long time, but the COVID-19 pandemic enabled their mass-market commercialization. Industry players saw mRNA vaccines developed for COVID-19 as effective for reducing the impact of the SARS-CoV-2 virus in populations. Moderna emerged as the first player to develop and commercialize an mRNA vaccine for COVID-19, followed by others such as Pfizer and BioNTech. mRNA is the first form of RNA vaccine to complete clinical trials and commercialization. Frost & Sullivan's analysis shows industry participants are developing mRNA vaccines for influenza, HIV, and cancer.
RNA vaccine development is research intensive, with newer RNA modifications giving rise to more unique forms like self-amplifying mRNA (saRNA) and circular RNA vaccines. RNA design and optimization are essential to obtain RNA forms that suit vaccine development and provide the expected outcomes. saRNA vaccines are considered the next-generation RNA vaccines with improved properties and more advantages over mRNA vaccines. Meanwhile, circular RNA vaccines are emerging due to enhanced properties such as stability and higher protein expressions. It is a new RNA vaccine with high potential in the early stages of R&D and clinical trials.
Apart from research on RNA forms, RNA vaccine delivery systems and route of administration are also deciding factors in their success. To achieve maximum potential, researchers should deliver RNA vaccines through effective delivery systems that retain their potency.
Increased global demands for COVID-19 vaccines led to the manufacturing of mRNA vaccines in a short time to cater to large populations. Vaccine developers adopted digital technologies, expanded manufacturing units and capabilities, and increased collaborations with contract development and manufacturing companies to achieve such humongous targets. Expanding RNA vaccine manufacturing will increase the vaccine capacity and reduce large-scale manufacturing costs. This will ensure that the vaccine is affordable and allow vaccine developers to provide them to developing countries at a subsidy.
This report includes the following:
- Reasons for RNA vaccines emerging as a promising category of therapeutics
- Technological developments
- Digital biomanufacturing-driven changes in RNA vaccine development
- Newer modes of delivery
- Progress of clinical trials since the approval of the first RNA vaccine
- Global adoption rate
- Prospects for RNA vaccine developers
Table of Contents
Strategic Imperatives
- Why Is It Increasingly Difficult to Grow?The Strategic Imperative 8™: Factors Creating Pressure on Growth
- The Strategic Imperative 8™
- The Impact of the Top 3 Strategic Imperatives on the Ribonucleic Acid (RNA) Vaccine Industry
- Growth Opportunities Fuel the Growth Pipeline Engine™
- Research Methodology
Growth Opportunity Analysis
- Scope of Analysis
- Segmentation
- A Growing Shift Toward Developing RNA Vaccines
- RNA Vaccines Overcome Limitations of Conventional Vaccines
- Global Trends in RNA Vaccine Development
- Growth Drivers
- Growth Restraints
Innovations and R&D Scenario
- Types of RNA Vaccines and Technology Readiness Level (TRL)
- Design and Modifications Improve Synthetic RNA Vaccine Production
- Industry Players with RNA Vaccine Modification Platforms
- Advanced Platforms Improve RNA Vaccine Designs and Processes to Produce High-quality Vaccines
- Industry Players with AI-based RNA Design Platforms
- The Lack of Well-developed Manufacturing Processes Slow RNA Vaccines Production Rates
- Overcoming RNA Vaccine Manufacturing Bottlenecks to Meet Increased Demand
- New Initiatives in RNA Vaccine Development and Manufacturing Platforms
- Digitalization Will Transform RNA Vaccine Manufacturing
- Developments in RNA Vaccine Delivery Systems
- Developments in RNA Vaccine Delivery Systems (continued)
- Industry Players Developing RNA Vaccine Delivery Platforms
- New Formulations Increase the Shelf-life and Maximize the Delivery Potential of the RNA Vaccine
- Newer Developments in RNA Vaccine Administration Routes
- Needle-free Vaccine Delivery Administration Routes for mRNA Vaccines
RNA Vaccines Development Landscape: Prophylactic and Therapeutic Vaccines
- RNA Vaccines Clinical Pipelines
- Increased RNA Vaccine Programs Globally
- Prophylactic RNA Vaccines
- Therapeutic RNA Vaccines
- mRNA Vaccines
- Ongoing Developments by mRNA Vaccine Developers
- saRNA Vaccines
- Ongoing Developments by saRNA Vaccine Developers
- Circular RNA Vaccines
- Priority Disease Areas for RNA Vaccine Developers
- Disease Focus Areas and Players Developing RNA Vaccines
- RNA Vaccine Development and Manufacturing Industry Players
- Future Opportunities for RNA Vaccines
Stakeholder Ecosystem
- A Dynamic Global Ecosystem Showcasing the Growth Potential of RNA Vaccines
- A Dynamic Global Ecosystem Showcasing the Growth Potential of RNA Vaccines (continued)
- Development of Innovative Vaccine Manufacturing Units and Capacity Expansion
- Collaborations Among RNA Vaccine Developers
- Collaborations Among RNA Vaccine Developers (continued)
Growth Opportunity Universe
- Growth Opportunity 1: Global Infrastructure Development for RNA Vaccine Manufacturing and Delivery
- Growth Opportunity 1: Global Infrastructure Development for RNA Vaccine Manufacturing and Delivery (continued)
- Growth Opportunity 2: RNA Modifications and Design Improvements
- Growth Opportunity 2: RNA Modifications and Design Improvements (continued)
- Growth Opportunity 3: Improving Delivery Systems and Formulations
- Growth Opportunity 3: Improving Delivery Systems and Formulations (continued)
Appendix
- Technology Readiness Levels (TRL): Explanation
- Active RNA Vaccine Pipelines
- Active RNA Vaccine Pipelines (continued)
- Active RNA Vaccine Pipelines (continued)
- Active RNA Vaccine Pipelines (continued)
- Active RNA Vaccine Pipelines (continued)
- Active RNA Vaccine Pipelines (continued)
- Active RNA Vaccine Pipelines (continued)
- Active RNA Vaccine Pipelines (continued)
- Overcoming Delivery Challenges in RNA Vaccines
Next Steps
- Your Next Steps
- Why Frost, Why Now?
- Legal Disclaimer