|
|
市場調査レポート
商品コード
1415495
てんかん市場 - 市場の洞察、疫学、市場予測:2032年Epilepsy - Market Insight, Epidemiology and Market Forecast -2032 |
||||||
カスタマイズ可能
|
|||||||
| てんかん市場 - 市場の洞察、疫学、市場予測:2032年 |
|
出版日: 2023年12月30日
発行: DelveInsight
ページ情報: 英文 395 Pages
納期: 1~3営業日
|
- 全表示
- 概要
- 図表
- 目次
米国では人口の増加と疾患に対する意識の高まりにより、てんかんの有病率が増加しています。てんかんはあらゆる年齢層に発症しますが、若年者や高齢者ではリスクが高いです。てんかんの臨床症状や発作型は多様であるため、てんかんの診断は困難です。てんかんの多様なサブタイプに対するバイオマーカーの同定は、現在進行中の課題です。
てんかんの管理には、発作のコントロール、QOLの改善、関連する問題への対処を目的とした包括的なアプローチが含まれます。てんかん患者の治療薬として、いくつかの抗てんかん薬(AED)が承認されていますが、いずれも治療薬ではありません。承認されているAEDのほとんどは、ラモトリギン、バルプロ酸ナトリウム、カルバマゼピン、レベチラセタム、トピラマートなどのジェネリック医薬品がすでに市販されています。
最近承認された治療薬には、XCOPRI、FYCOMPA、NAYZILAM、EPIDIOLEX、BRIVIACT、SYMPAZANなどがあります。
世界保健機関(WHO)は、てんかんを、あらゆる年齢層の個人を罹患させ、世界の負担の一因となっている慢性的な非伝染性脳疾患であると説明しています。てんかんは、再発性の非誘発性発作を特徴とする神経疾患です。てんかん発作は、突然起こる制御不能な脳の電気的障害であり、けいれん、意識変容、異常な感覚、行動など、さまざまな形で現れます。
てんかんの診断は、臨床検査、身体診察、画像解析(MRI、CTスキャン)、脳波、血液検査などの各種診断検査を組み合わせて行います。脳の電気的活動や構造的な異常は、磁気共鳴画像(MRI)、脳波、コンピューター断層撮影などの脳スキャンを用いて測定することができます。血液検査は、てんかんを引き起こしている基礎疾患を調べるために行われます。
主要7ヶ国におけるてんかんの市場規模は、2022年に85億2,890万米ドルとなりました。同市場は2023年~2032年の予測期間に拡大すると予測されています。
当レポートでは、主要7ヶ国におけるてんかん市場について調査し、市場の概要とともに、疫学、患者動向、新たな治療法、2032年までの市場規模予測、および医療のアンメットニーズなどを提供しています。
目次
第1章 重要な洞察
第2章 レポートのイントロダクション
第3章 てんかん市場概要
第4章 てんかんの疫学の調査手法と市場
第5章 てんかんのエグゼクティブサマリー
第6章 主要な出来事
第7章 疾患の背景と概要
- イントロダクション
- 発作の種類
- 臨床症状
- 原因
- てんかんの種類
- 危険因子
- 病態生理学
- 診断
- 治療
第8章 患者動向
第9章 疫学と患者数
- 主な調査結果
- 前提条件と根拠:主要7ヶ国
- 主要7ヶ国で診断されたてんかんの有病者数の合計
- 米国
- EU4ヶ国と英国
- 日本
第10章 上市済み薬剤
第11章 新興薬剤
第12章 てんかん:市場分析
- 主な調査結果
- 主要な市場予測の前提条件
- 市場の見通し
- コンジョイント分析
- 主要7ヶ国におけるてんかんの総市場規模
- 主要7ヶ国におけるてんかんの総市場規模、治療法別
- 米国におけるてんかんの市場規模
- EU4ヶ国および英国におけるてんかんの市場規模
- 日本のてんかん市場規模
第13章 主要なオピニオンリーダーの見解
第14章 SWOT分析
第15章 アンメットニーズ
第16章 市場アクセスと償還
- 米国
- EU4ヶ国と英国
- 日本
第17章 付録
第18章 DelveInsightのサービス内容
第19章 免責事項
第20章 DelveInsightについて
List of Tables
- Table 1: Summary of Market and Epidemiology (2019-2032)
- Table 2: Diagnosis and assessment of epilepsy
- Table 3: The French National Authority for Health (HAS) (2020) Guidelines
- Table 4: German Society for Neurology (DGN) Guidelines
- Table 5: Key Recommendations for Practice
- Table 6: Classification of Common Seizure Disorders and Recommended First-Line Therapy
- Table 7: Treating childhood-onset epilepsies
- Table 8: Recommendations For Treating Status Epilepticus, Repeated Or Cluster Seizures, And Prolonged Seizures
- Table 9: Nonpharmacological treatments
- Table 10: Psychological, Neurobehavioral, Cognitive, and Developmental Comorbidities in Epilepsy
- Table 11: Total Diagnosed Prevalent Cases of Epilepsy in the 7MM (2019-2032)
- Table 12: Total Diagnosed Prevalent Cases of Epilepsy in the US (2019-2032)
- Table 13: Gender-specific Diagnosed Prevalent Cases of Epilepsy in the US (2019-2032)
- Table 14: Diagnosed Prevalent Cases of Epilepsy Based on Seizure Type in the US (2019-2032)
- Table 15: Diagnosed Cases of Other Types of Epilepsies and Associated Diseases in the US (2019-2032)
- Table 16: Total Diagnosed Prevalent Cases of Epilepsy in EU4 and the UK(2019-2032)
- Table 17: Gender-specific Diagnosed Prevalent Cases of Epilepsy in EU4 and the UK (2019-2032)
- Table 18: Diagnosed Prevalent Cases of Epilepsy Based on Seizure Type in EU4 and the UK (2019-2032)
- Table 19: Diagnosed Cases of Other Types of Epilepsies and Associated Diseases in EU4 and the UK (2019-2032)
- Table 20: Total Diagnosed Prevalent Cases of Epilepsy in Japan (2019-2032)
- Table 21: Gender-specific Diagnosed Prevalent Cases of Epilepsy in Japan (2019-2032)
- Table 22: Diagnosed Prevalent Cases of Epilepsy Based on Seizure Type in Japan (2019-2032)
- Table 23: Diagnosed Cases of Other Types of Epilepsies and Associated Diseases in Japan (2019-2032)
- Table 24: Key Cross of Marketed Drugs
- Table 25: EPIDIOLEX/EPIDYOLEX (cannabidiol), Clinical Trial Description, 2023
- Table 26: XCOPRI/ONTOZRY (cenobamate), Clinical Trial Description, 2023
- Table 27: FINTEPLA (fenfluramine), Clinical Trial Description, 2023
- Table 28: NAYZILAM (midazolam), Clinical Trial Description, 2023
- Table 29: VALTOCO (diazepam nasal spray), Clinical Trial Description, 2023
- Table 30: ZTALMY (ganaxolone), Clinical Trial Description, 2023
- Table 31: BRIVIACT (brivaracetam), Clinical Trial Description, 2023
- Table 32: FYCOMPA (perampanel), Clinical Trial Description, 2023
- Table 33: OXTELLAR XR (oxcarbazepine), Clinical Trial Description, 2023
- Table 34: VIMPAT (lacosamide), Clinical Trial Description, 2023
- Table 35: DIACOMIT (stiripentol), Clinical Trial Description, 2023
- Table 36: AFINITOR DISPERZ/VOTUBIA (everolimus tablets for oral suspension) Clinical Trial Description, 2023
- Table 37: MOTPOLY XR (lacosamide), Clinical Trial Description, 2023
- Table 38: KIGABEQ (vigabatrin), Clinical Trial Description, 2023
- Table 39: APTIOM/ZEBINIX (eslicarbazepine acetate), Clinical Trial Description, 2023
- Table 40: Comparison of Emerging Drugs for Treatment
- Table 41: XEN1101, Clinical Trial Description, 2023
- Table 42: LIBERVANT (diazepam buccal film), Clinical Trial Description, 2023
- Table 43: Soticlestat (TAK-935), Clinical Trial Description, 2023
- Table 44: COMFYDE (carisbamate), Clinical Trial Description, 2023
- Table 45: Lorcaserin (E2023), Clinical Trial Description, 2023
- Table 46: BHV-7000 (KB-3061), Clinical Trial Description, 2023
- Table 47: STACCATO alprazolam (benzodiazepine), Clinical Trial Description, 2023
- Table 48: NBI-827104 (ACT-709478), Clinical Trial Description, 2023
- Table 49: NBI-921352, Clinical Trial Description, 2023
- Table 50: Ivermectin (EQU-001), Clinical Trial Description, 2023
- Table 51: Key Market Forecast Assumptions for LIBERVANT (diazepam buccal film)
- Table 52: Key Market Forecast Assumptions for XEN1101
- Table 53: Key Market Forecast Assumptions for COMFYDE (carisbamate)
- Table 54: Key Market Forecast Assumptions for E2023 (lorcaserin)
- Table 55: Key Market Forecast Assumptions for Soticlestat (TAK-935)
- Table 56: Key Market Forecast Assumptions for BHV-7000 (KB-3061)
- Table 57: Key Market Forecast Assumptions for FINTEPLA (fenfluramine)
- Table 58: Key Market Forecast Assumptions for ZTALMY (ganaxolone)
- Table 59: Key Market Forecast Assumptions for XCOPRI/ONTOZRY (cenobamate)
- Table 60: Key Market Forecast Assumptions for FYCOMPA (perampanel)
- Table 61: Key Market Forecast Assumptions for BRIVIACT (brivaracetam)
- Table 62: Key Market Forecast Assumptions for STACCATO alprazolam (benzodiazepine)
- Table 63: Total Market Size of Epilepsy in the 7MM, in USD million (2019-2032)
- Table 64: Total Market Size of Epilepsy by Therapies in the 7MM, in USD million (2019-2032)
- Table 65: Total Market Size of Epilepsy in the US, in USD million (2019-2032)
- Table 66: The Market Size of Epilepsy by Therapies in the US, in USD million (2019-2032)
- Table 67: Total Market Size of Epilepsy in EU4 and the UK, in USD million (2019-2032)
- Table 68: The Market Size of Epilepsy by Therapies in EU4 and the UK, in USD million (2019-2032)
- Table 69: Total Market Size of Epilepsy in Japan, in USD million (2019-2032)
- Table 70: The Market Size of Epilepsy by Therapies in Japan, in USD million (2019-2032)
List of Figures
- Figure 1: Classification of Seizures
- Figure 2: Classification as per International League Against Epilepsy (ILAE)
- Figure 3: Clinical Manifestation of Epilepsy
- Figure 4: Classification of Epilepsy
- Figure 5: Pathophysiology of Epilepsy
- Figure 6: Diagnosis of Epilepsy
- Figure 7: Treatment Algorithm for Epilepsy
- Figure 8: American Epilepsy Society Guidelines 2016 - Status Epilepticus
- Figure 9: ILAE Proposed Diagnostic Framework of Neonatal Seizures
- Figure 10: Relation Between Clinical Trial Ratings, Level Of Evidence, And Conclusions Under ILAE Guidelines
- Figure 11: ILAE Recommendations for use as initial monotherapy
- Figure 12: APLS Algorithm on the Management of the Convulsing Child
- Figure 13: Total Diagnosed Prevalent Cases of Epilepsy in the 7MM (2019-2032)
- Figure 14: Total Diagnosed Prevalent Cases of Epilepsy in the US (2019-2032)
- Figure 15: Gender-specific Diagnosed Prevalent Cases of Epilepsy in the US (2019-2032)
- Figure 16: Diagnosed Prevalent Cases of Epilepsy Based on Seizure Type in the US (2019-2032)
- Figure 17: Diagnosed Cases of Other Types of Epilepsies and Associated Diseases in the US (2019-2032)
- Figure 18: Total Diagnosed Prevalent Cases of Epilepsy in EU4 and the UK (2019-2032)
- Figure 19: Gender-specific Diagnosed Prevalent Cases of Epilepsy in EU4 and the UK (2019-2032)
- Figure 20: Diagnosed Prevalent Cases of Epilepsy Based on Seizure Type in EU4 and the UK (2019-2032)
- Figure 21: Diagnosed Cases of Other Types of Epilepsies and Associated Diseases in EU4 and the UK (2019-2032)
- Figure 22: Total Diagnosed Prevalent Cases of Epilepsy in Japan (2019-2032)
- Figure 23: Gender-specific Diagnosed Prevalent Cases of Epilepsy in Japan (2019-2032)
- Figure 24: Diagnosed Prevalent Cases of Epilepsy Based on Seizure Type in Japan (2019-2032)
- Figure 25: Diagnosed Cases of Other Types of Epilepsies and Associated Diseases in Japan (2019-2032)
- Figure 26: Total Market Size of Epilepsy in the 7MM, in USD million (2019-2032)
- Figure 27: Total Market Size of Epilepsy by Therapies in the 7MM, in USD million (2019-2032)
- Figure 28: Total Market Size of Epilepsy in the US, in USD million (2019-2032)
- Figure 29: The Market Size of Epilepsy by Therapies in the US, in USD million (2019-2032)
- Figure 30: Total Market Size of Epilepsy in EU4 and the UK, in USD million (2019-2032)
- Figure 31: The Market Size of Epilepsy by Therapies in EU4 and the UK, in USD million (2019-2032)
- Figure 32: Total Market Size of Epilepsy in Japan, in USD million (2019-2032)
- Figure 33: The Market Size of Epilepsy by Therapies in Japan, in USD million (2019-2032)
- Figure 34: SWOT Analysis of Epilepsy
- Figure 35: Unmet Needs of Epilepsy
- Figure 36: Health Technology Assessment
- Figure 37: Reimbursement Process in Germany
- Figure 38: Reimbursement Process in France
- Figure 39: Reimbursement Process in Italy
- Figure 40: Reimbursement Process in Spain
- Figure 41: Reimbursement Process in the United Kingdom
- Figure 42: Reimbursement Process in Japan
Key Highlights:
- The prevalence of epilepsy has been increasing in the US due to the increasing population and awareness of the disease. The disease affects individuals of all ages; however, the risk is higher in the very young and the elderly.
- Diagnosing epilepsy can be challenging due to its diverse clinical presentations and seizure types; further identification of biomarkers for diverse subtypes of epilepsy is an ongoing challenge.
- The management of epilepsy involves a comprehensive approach aimed at controlling seizures, improving the individual's quality of life, and addressing any associated issues. Several anti-epileptic drugs (AEDs) have been approved for the treatment of epileptic patients, but none of them is curative. The generics of most of the approved AEDs are already available in the market, including lamotrigine, sodium valproate, carbamazepine, levetiracetam, topiramate, and others.
- Certain recently approved therapies include XCOPRI, FYCOMPA, NAYZILAM, EPIDIOLEX, BRIVIACT, SYMPAZAN, and others.
- The first-line monotherapy of AEDs can be classified as sodium channel modulators (phenytoin, lacosamide, BRIVIACT, OXTELLAR XR), gamma-aminobutyric acid (GABA) receptor modulators (XCOPRI, NAYZILAM, clobazam, and others), synaptic vesicle protein SV2A modulator, Ca2+ channel modulators, other mono or combo therapies. While the second line of therapies includes other mono or combo AEDs.
DelveInsight's "Epilepsy - Market Insights, Epidemiology, and Market Forecast - 2032" report delivers an in-depth understanding of Epilepsy, historical and forecasted epidemiology, as well as the Epilepsy market trends in the United States, EU4 (Germany, France, Italy, and Spain) and the United Kingdom, and Japan.
The Epilepsy market report provides current treatment practices, emerging drugs, market share of individual therapies, and current and forecasted 7MM Epilepsy market size from 2019 to 2032. The report also covers Epilepsy treatment practices/algorithms and unmet medical needs to curate the best opportunities and assess the market's potential.
Geography Covered:
- The United States
- EU4 (Germany, France, Italy, and Spain) and the United Kingdom
- Japan
Study Period: 2019-2032.
Epilepsy Understanding and Treatment Algorithm
Epilepsy Overview
The World Health Organization (WHO) describes epilepsy as a chronic, non-communicable brain disorder that affects individuals of all ages and contributes to a burden worldwide. It is a neurological disorder characterized by recurrent, unprovoked seizures. A seizure is a sudden, uncontrolled electrical disturbance in the brain that can manifest in various ways, such as convulsions, altered consciousness, unusual sensations, or behaviors.
Epilepsy diagnosis
Epilepsy is diagnosed using a combination of clinical examination, physical examination, and various diagnostic tests such as imaging analysis (magnetic resonance imaging (MRI), CT scan), electroencephalogram (EEG), blood tests, and others. Electrical activity and structural abnormalities in the brain can be measured using brain scans such as magnetic resonance imaging (MRI), electroencephalogram, or computed tomography. Blood tests are performed to check for the underlying conditions causing epilepsy.
Further details related to country-based variations are provided in the report…
Epilepsy treatment
The management of epilepsy involves a comprehensive approach aimed at controlling seizures, improving the individual's quality of life, and addressing any associated issues, but none of them is curative. It includes the pharmacological, nonpharmacological (ketogenic diet), and medical device approaches (vagus nerve stimulation [VNS], deep brain stimulation).
Medications are the early treatment choice for almost all patients with multiple seizures. Some patients who only have a single seizure and whose tests do not indicate a high likelihood of seizure recurrence may not need medications. The medications treat the symptoms of epilepsy (seizures) rather than curing the underlying condition. They are highly effective and completely control seizures in most patients (approximately 70%). The drugs prevent seizures from starting by reducing the tendency of brain cells to send excessive and confused electrical signals. The choice of medication depends on a variety of factors, some of which include the type of seizure and type of epilepsy, the likely side effects of the medication, other medical conditions the patient may have, potential interactions with the patient's other medications, age, gender and cost of the medication.
Epilepsy Epidemiology
As the market is derived using a patient-based model, the epilepsy epidemiology chapter in the report provides historical as well as forecasted epidemiology segmented by total diagnosed prevalent cases of epilepsy, gender-specific cases of epilepsy, diagnosed prevalent cases of epilepsy based on seizure type, and diagnosed cases of other types of epilepsies and associated diseases in the 7MM covering the United States, EU4 countries (Germany, France, Italy, and Spain) and the United Kingdom, and Japan from 2019 to 2032.
- In 2022, the total diagnosed prevalent cases of epilepsy were estimated to be approximately 7,001,492 cases in the 7MM, of which nearly 13% were diagnosed in children and 87% in adults. These cases are expected to increase by 2032.
- Among the 7MM, the United States accounted for nearly 48% of the total diagnosed prevalent cases of epilepsy in 2022. These cases are expected to increase during the study period (2019-2032).
- As per DelveInsight analysis, EU4 and the UK accounted for around 2,741,851 diagnosed prevalent cases of epilepsy in 2022. These cases are expected to change during the study period (2019-2032).
- Among the EU4 and the UK, Germany accounted for the highest number of cases of Epilepsy, representing nearly 27% of the cases, followed by France, while Spain had the least cases in 2022.
According to estimates based on DelveInsight's epidemiology model, epilepsy exhibits a female preponderance than males in EU4 and the UK. Of the total cases, nearly 47% were males and 53% were females.
- In 2022, among the other types of epilepsies and associated diseases, the highest cases were found in highly drug-resistant focal epilepsy or drug-resistant epilepsy or refractory cases, i.e., around 267,342, while the least cases were found in CDKL5 deficiency disorder, around 1,671 in the US.
- In 2022, among the 7MM, Japan had the third-highest cases of epilepsy, contributing approximately 13% to the total cases.
- In Japan, of the total diagnosed prevalent cases of epilepsy, the highest number of cases of epilepsy, i.e., nearly 685,000 were classified as focal epileptic seizures, 199,468 cases as generalized epileptic seizures, while around 9,205 cases were other determined or undetermined epileptic seizures.
Epilepsy Drug Chapters
The drug chapter segment of the Epilepsy report encloses a detailed analysis of Epilepsy-marketed drugs and mid to late-stage (Phase III and Phase II) pipeline drugs. It also helps understand the Epilepsy clinical trial details, expressive pharmacological action, agreements and collaborations, approval and patent details, advantages and disadvantages of each included drug, and the latest news and press releases.
Marketed Drugs
EPIDIOLEX/EPIDYOLEX (cannabidiol): Jazz Pharmaceuticals
EPIDIOLEX/EPIDYOLEX (cannabidiol), an oral solution, is a clear, colorless-to-yellow liquid containing cannabidiol at 100 mg/mL concentration. Cannabidiol, the active ingredient in EPIDIOLEX, is a cannabinoid that naturally occurs in the Cannabis sativa L. plant. Inactive ingredients include dehydrated alcohol, sesame seed oil, strawberry flavor, and sucralose. EPIDIOLEX contains no ingredient made from a gluten-containing grain
EPIDIOLEX is approved for the treatment of seizures associated with Lennox-Gastaut syndrome, Dravet syndrome, or tuberous sclerosis complex. Jazz Pharmaceuticals had initiated a Phase III trial to treat children and adolescents living with developmental and epileptic encephalopathy; however, the trial was terminated due to a business decision. The Japanese unit of Jazz Pharmaceuticals has started a Phase III trial of the drug for epilepsy in Japan.
EPIDIOLEX is to be administered orally. The recommended starting dosage is 2.5 mg/kg taken twice daily (5 mg/kg/day). After 1 week, the dosage can be increased to a maintenance dosage of 5 mg/kg twice daily (10 mg/kg/day).
Note: Further marketed drugs and their details will be provided in the report…
Emerging Drugs
XEN1101: Xenon Pharmaceuticals
XEN1101 is a novel, orally administered, potent, selective KCNQ2/3 (Kv7.2/7.3) potassium channel opener being developed to treat focal onset seizures and primary generalized tonic-clonic seizures (PGTCS). It acts as a neuronal Kv7 voltage-gated potassium channel opener and has been developed to stabilize nerve cells, control action potential burst firing, and reduce brain hyperexcitability as a seizure treatment.
The Kv7 potassium channel mechanism has been clinically validated with ezogabine. The FDA approved this earlier generation Kv7 modulator as an adjunctive treatment for adults with focal seizures with or without secondary generalization. XEN1101's unique composition is chemically designed to improve the potency, selectivity, and pharmacokinetics of ezogabine and is not expected to have ezogabine's composition-specific tissue pigmentation effects.
The drug is currently investigated in multiple Phase III clinical trials to treat patients with focal-onset and primary generalized tonic-clonic seizures. The drug is also being developed for major depressive disorder.
Note: Further emerging therapies and their detailed assessment will be provided in the final report.
Drug Class Insights
Epilepsy, as a chronic, non-communicable disease of the brain, affects people of all ages and contributes significantly to the global disease burden. It is a neurological disorder characterized by recurrent, unprovoked seizures. A seizure occurs when the brain has sudden and abnormal electrical activity. These abnormal electrical discharges may cause changes in behavior, consciousness, sensation, or a combination of these factors. Treatment involves pharmacologic (use of anti-epileptic drugs), nonpharmacologic (such as exercise, ketogenic diet), and medical device approaches. Anti-epileptic medications (AEDs) are the most widely prescribed class of medication for epilepsy; however, they do not treat the condition; instead, they alter the chemical balance in the brain to prevent seizures. Several AEDs with different modes of action, some possessing more than one, are available in the market and prescribed as monotherapy or adjunctive therapy in different lines of treatment.
Commonly, initial or first-line treatment with AED monotherapy is recommended for new-onset epilepsy, with subsequent substitution with another AED monotherapy; however, if results are not satisfactory, AED polytherapy or combination therapy is adopted. Further, even after mono and combination therapy in the first line, treatment failure, known as drug-resistant epilepsy, still occurs in epilepsy patients. These patients are further recommended a second-line therapy, where AEDs are usually used as substitution therapy (mono or combination) to control seizures, although international consensus and clarity for the AEDs used in second-line is not available yet. If seizures continue, a third-line therapy is instituted using intravenous sedation (therapeutic coma). Propofol and midazolam are the most commonly used agents, partly because of their short half-life.
The current first-line therapy for epilepsy involves AEDs with following mechanism of action: GABA enhancers/inhibitors (vigabatrin, gabapentin, benzodiazepines, phenobarbital, clonazepam, clobazam, cenobamate, etc.), calcium channel blockers (topiramate, valproate, ethosuximide, etc.), sodium channel blockers (phenytoin, carbamazepine, oxcarbazepine, zonisamide, lamotrigine, etc.) AMPA receptor antagonist (perampanel and topiramate), and SV2a vesicle inhibition (levetiracetam and brivaracetam).
AEDs that are frequently prescribed include lamotrigine, sodium valproate, carbamazepine, levetiracetam, topiramate, and others. Several US FDA-approved medications such as EPIDIOLEX, XCOPRI (cenobamate), FINTEPLA (fenfluramine hydrochloride), NAYZILAM (midazolam) (nasal spray), and others are also being used to treat seizures associated with epilepsy. Few people are also observed with refractory epilepsy (intractable), i.e., seizures that are not controlled by medications. In addition, vagus nerve stimulation (VNS) devices are inserted beneath the skin to activate the vagus nerve and lessen seizures.
Epilepsy Market Outlook
Epilepsy is a disorder of the brain characterized by repeated seizures. A seizure is defined as a sudden alteration of behavior due to a temporary change in the brain's electrical functioning. Usually, the brain continuously generates tiny electrical impulses in an orderly pattern. The epilepsies have many possible causes, and there are several types of seizures. Anything that disturbs the normal neuron activity pattern-from illness to brain damage to abnormal brain development-can lead to seizures. In patients with seizures, the normal electrical pattern is disrupted by sudden and synchronized bursts of electrical energy that may briefly affect their consciousness, movements, or sensations.
The treatment paradigm of epilepsy or its management strategies involves three main categories, i.e., pharmacotherapy, surgery, and alternative treatment strategies, including neurostimulation, ketogenic diet, and lifestyle changes. Medical professionals decide the treatment line according to the patient's condition and the severity of the case.
Continued in report…
The current market segmentation is based on the lines of therapies prescribed and is further sub-segment based on the mechanism of action and their usage as a combo or monotherapy. Additionally, the different epileptic syndromes and associated diseases have also been considered to represent the epilepsy market. First-line therapies (GABA enhancers/Inhibitors, calcium channel blockers, sodium channel blockers, AMPA receptor antagonist, SV2a vesicle inhibition, other mono or combo therapies), second-line (mono AEDs and mono or combo AEDs), mono or adjunctive therapies (EPIDIOLEX/EPIDYOLEX [cannabidiol], FINTEPLA [fenfluramine], DIACOMIT [stiripentol], AFINITOR DISPERZ/VOTUBIA [everolimus], ZTALMY [ganaxolone], TROKENDI XR [topiramate], BANZEL [rufinamide], SYMPAZAN [clobazam]) are the major segmentation covered in the forecast model.
Several key players are evaluating their lead candidates in different stages of clinical development including FINTEPLA (fenfluramine), LIBERVANT (diazepam buccal film), XEN1101, ZTALMY (ganaxolone), XCOPRI/ONTOZRY (cenobamate), lorcaserin (E2023), BHV-7000 (KB-3061), STACCATO alprazolam (benzodiazepine), and others. They aim to investigate their products to treat epilepsy and their types of associated conditions.
- The total market size of Epilepsy in the 7MM was approximately USD 8,528.9 million in 2022 and is projected to increase during the forecast period (2023-2032).
- The market size of Epilepsy in the US was approximately USD 4,122.7 million in 2022, which is anticipated to increase due to the increasing awareness of the disease and the launch of the emerging therapy.
- The total market size of Epilepsy in EU4 and the UK was calculated to be approximately USD 2,423.5 million in 2022, which was nearly 28% of the total market revenue for the 7MM.
- According to DelveInsight's estimates, among EU4 and the UK, Germany accounted for the highest market with approximately USD 649.2 million in 2022, followed by France with approximately USD 612.5 million in the respective year, while Spain accounted for the lowest market in 2022.
- According to DelveInsight's analysis, in the US, sodium channel blockers (including phenytoin, carbamazepine, oxcarbazepine, zonisamide, lamotrigine, lacosamide, valproate, cenobamate, eslicarbazepine, rufinamide, topiramate,etc) had the highest market share among all the therapies, in 2022, with a revenue of approximately USD 1,225.4 million, followed by other mono or combo therapies, and others.
- EPIDIOLEX/EPIDYOLEX (cannabidiol) is an oral solution that exerts anticonvulsant effects and is approved for treating seizures associated with LGS or Dravet syndrome in patients 2 years and older. Among the drugs marketed for different types of epilepsies and associated diseases in the EU4 and the UK, in 2022, EPIDIOLEX/EPIDYOLEX (cannabidiol), with around USD 169.1 million, generated the second-highest revenue, while AFINITOR DISPERZ/VOTUBIA (everolimus) was the highest revenue generating drug.
- In 2022, the market size of Epilepsy in Japan was nearly 23% of the total market size in the 7MM, with a revenue of approximately USD 1,982.7 million.
- Among the emerging therapies, XEN1101, a promising drug in epilepsy treatment, offering a novel approach through its role as a selective KCNQ2/3 potassium channel opener, shows potential for effectively managing focal onset seizures and primary generalized tonic-clonic seizures. The drug is expected to enter the US market by 2026 and is projected to generate a revenue of approximately USD 6.2 million in its launch year; the drug is predicted to peak in the 8th year.
- FINTEPLA (fenfluramine), an approved therapy for treating seizures associated with LGS, is also being developed for CDKL5 deficiency disorder and is anticipated to launch in the US in 2025, for CDKL5 deficiency disorder and generate a revenue of around USD 15.7 million.
Epilepsy Drugs Uptake
This section focuses on the uptake rate of potential drugs expected to be launched in the market during 2019-2032. For example, Marinus Pharmaceuticals, Ovid Therapeutics, and Orion's ZTALMY (ganaxolone), a neuroactive steroid GABAA receptor modulator with a slow-medium uptake, will enter the US market in 2025. On the contrary, UCB and Nippon Shinyaku's FINTEPLA (fenfluramine), which also enters in 2025, and is being investigated for CDKL5 deficiency disorder will grow in the market with fast uptake.
Further detailed analysis of emerging therapies drug uptake in the report…
Epilepsy Pipeline Development Activities
The report provides insights into different therapeutic candidates in Phase III, Phase II, and Phase I. It also analyzes key players involved in developing targeted therapeutics.
Pipeline development activities
The report covers information on collaborations, acquisitions and mergers, licensing, and patent details for emerging therapies for Epilepsy.
KOL Views
To keep up with current market trends, we take KOLs and SMEs' opinions working in the domain through primary research to fill the data gaps and validate our secondary research. Industry Experts contacted for insights on Epilepsy evolving treatment landscape, patient reliance on conventional therapies, patient therapy switching acceptability, and drug uptake, along with challenges related to accessibility, including Medical/scientific writers, Medical Professionals, Professors, Directors, and Others.
DelveInsight's analysts connected with 50+ KOLs to gather insights; however, interviews were conducted with 15+ KOLs in the 7MM. Centers like the American Association of Neurological Surgeons, Johns Hopkins University School of Medicine, Charite University Medicine Berlin, Italian College of General Practitioners, University of Leicester, and the Kanagawa Children's Medical Center were contacted. Their opinion helps understand and validate current and emerging therapy treatment patterns or Epilepsy market trends. This will support the clients in potential upcoming novel treatments by identifying the overall scenario of the market and the unmet needs.
Physician's View
According to our primary research analysis, several AEDs with different modes of action, some possessing more than one, are available in the market and prescribed as monotherapy or adjunctive therapy in different lines of treatment. The specific treatment plan is tailored to each individual based on factors such as the type of seizures, their frequency, the person's age, overall health, and any underlying causes.
Qualitative Analysis
We perform Qualitative and market Intelligence analysis using various approaches, such as SWOT and Conjoint Analysis. In the SWOT analysis, strengths, weaknesses, opportunities, and threats in terms of disease diagnosis, patient awareness, patient burden, competitive landscape, cost-effectiveness, and geographical accessibility of therapies are provided. These pointers are based on the Analyst's discretion and assessment of the patient burden, cost analysis, and existing and evolving treatment landscape.
Conjoint Analysis analyzes multiple emerging therapies based on relevant attributes such as safety, efficacy, frequency of administration, route of administration, and order of entry. Scoring is given based on these parameters to analyze the effectiveness of therapy.
In efficacy, the trial's primary and secondary outcome measures are evaluated; for instance, in Epilepsy trials, efficacy scores are according to seizure response rate, reduction in seizure frequency, reduction in drop seizure, seizure cessation, and others.
Further, the therapies' safety is evaluated wherein the acceptability, tolerability, and adverse events are majorly observed. It sets a clear understanding of the side effects posed by the drug in the trials. In addition, the scoring is also based on the route of administration, order of entry and designation, probability of success, and the addressable patient pool for each therapy. According to these parameters, the final weightage score and the ranking of the emerging therapies are decided.
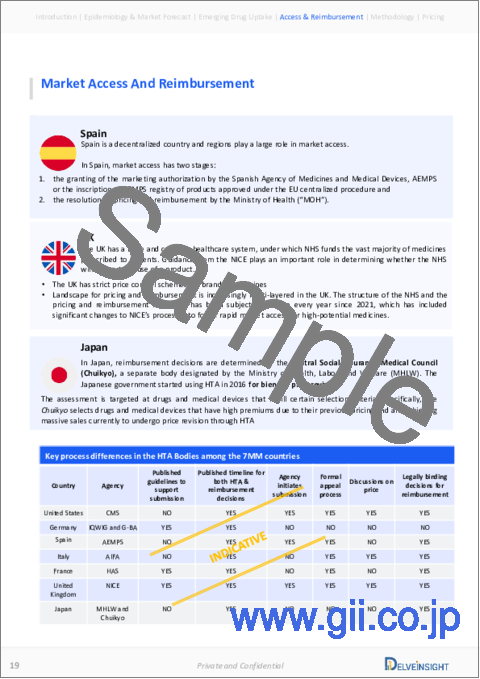
Market Access and Reimbursement
Reimbursement of rare disease therapies can be limited due to lack of supporting policies and funding, challenges of high prices, lack of specific approaches to evaluating rare disease drugs given limited evidence, and payers' concerns about budget impact. The high cost of rare disease drugs usually has a limited effect on the budget due to the small number of eligible patients being prescribed the drug. The US FDA has approved several rare disease therapies in recent years. From a patient perspective, health insurance and payer coverage guidelines surrounding rare disease treatments restrict broad access to these treatments, leaving only a small number of patients who can bypass insurance and pay for products independently.
The reimbursement challenges related to medical care and treatment for individuals with Epilepsy can be significant as it often requires specialized medical attention, covering the costs of diagnosis, treatment, and ongoing care. Health insurance plans may not fully cover limited coverage of some medical treatments, therapies, and devices specific to Epilepsy. This can result in high out-of-pocket expenses for families seeking the best care for their loved ones. Moreover, it requires specialized care from healthcare providers with expertise. Finding and accessing such specialists may be challenging, and the associated costs may not always be fully reimbursed by insurance.
Further details will be provided in the report
The report provides detailed insights on the country-wise accessibility and reimbursement scenarios, cost-effectiveness scenarios, programs making accessibility easier and out-of-pocket costs more affordable, insights on patients insured under federal or state government prescription drug programs, etc.
Scope of the Report:
- The report covers a segment of key events, an executive summary, and a descriptive overview of Epilepsy, explaining its causes, signs and symptoms, pathogenesis, and currently available therapies.
- Comprehensive insight into the epidemiology segments and forecasts, the future growth potential of diagnosis rate, disease progression, and treatment guidelines have been provided.
- Additionally, an all-inclusive account of the current and emerging therapies and the elaborative profiles of late-stage and prominent therapies will impact the current treatment landscape.
- A detailed review of the Epilepsy market, historical and forecasted market size, market share by therapies, detailed assumptions, and rationale behind our approach is included in the report, covering the 7MM drug outreach.
- The report provides an edge while developing business strategies by understanding trends through SWOT analysis and expert insights/KOL views, patient journey, and treatment preferences that help shape and drive the 7MM Epilepsy market.
Epilepsy report insights
- Patient Population
- Therapeutic Approaches
- Epilepsy Pipeline Analysis
- Epilepsy Market Size and Trends
- Existing and Future Market Opportunity
Epilepsy report key strengths
- Ten years Forecast
- The 7MM Coverage
- Epilepsy Epidemiology Segmentation
- Key Cross Competition
- Attribute analysis
- Drugs Uptake and Key Market Forecast Assumptions
Epilepsy report assessment
- Current Treatment Practices
- Unmet Needs
- Pipeline Product Profiles
- Market Attractiveness
- Qualitative Analysis (SWOT and Conjoint Analysis)
Key Questions:
Market Insights
- What was the total market size of Epilepsy, the market size of Epilepsy by therapies, and market share (%) distribution in 2019, and what would it look like by 2032? What are the contributing factors for this growth?
- How will XEN1101 and LIBERVANT (diazepam buccal film) affect the treatment paradigm of Epilepsy?
- How will Soticlestat (TAK-935) compete with upcoming products and marketed therapies?
- Which drug is going to be the largest contributor by 2032?
- What are the pricing variations among different geographies for approved and marketed therapies?
- How would future opportunities affect the market dynamics and subsequent analysis of the associated trends?
Epidemiology Insights
- What are the disease risks, burdens, and unmet needs of Epilepsy? What will be the growth opportunities across the 7MM with respect to the patient population pertaining to Epilepsy?
- What is the historical and forecasted Epilepsy patient pool in the United States, EU4 (Germany, France, Italy, and Spain) and the United Kingdom, and Japan?
- Out of the countries mentioned above, which country would have the highest diagnosed prevalent Epilepsy population during the forecast period (2023-2032)?
- What factors are contributing to the growth of Epilepsy cases?
Current Treatment Scenario, Marketed Drugs, and Emerging Therapies
- What are the current options for the treatment of Epilepsy? What are the current clinical and treatment guidelines for treating Epilepsy?
- How many companies are developing therapies for the treatment of Epilepsy?
- How many emerging therapies are in the mid-stage and late stage of development for treating Epilepsy?
- What are the recent novel therapies, targets, mechanisms of action, and technologies developed to overcome the limitations of existing therapies?
- What is the cost burden of current treatment on the patient?
- Patient acceptability in terms of preferred treatment options as per real-world scenarios?
- What are the accessibility issues of approved therapy in the US?
- What is the 7MM historical and forecasted market of Epilepsy?
Reasons to Buy:
- The report will help develop business strategies by understanding the latest trends and changing treatment dynamics driving the Epilepsy market.
- Insights on patient burden/disease prevalence, evolution in diagnosis, and factors contributing to the change in the epidemiology of the disease during the forecast years.
- Understand the existing market opportunities in varying geographies and the growth potential over the coming years.
- The distribution of historical and current patient share is based on real-world prescription data in the US, EU4 (Germany, France, Italy, and Spain) and the United Kingdom, and Japan.
- Identifying upcoming solid players in the market will help devise strategies to help get ahead of competitors.
- Detailed analysis and ranking of class-wise potential current and emerging therapies under the conjoint analysis section to provide visibility around leading classes.
- Highlights of Access and Reimbursement policies for Epilepsy, barriers to accessibility of approved therapy, and patient assistance programs.
- To understand Key Opinion Leaders' perspectives around the accessibility, acceptability, and compliance-related challenges of existing treatment to overcome barriers in the future.
- Detailed insights on the unmet needs of the existing market so that the upcoming players can strengthen their development and launch strategy.
Table of Contents
1. Key Insights
2. Report Introduction
3. Epilepsy Market Overview at a Glance
- 3.1. Market Share (%) Distribution of Epilepsy in 2019
- 3.2. Market Share (%) Distribution of Epilepsy in 2032
4. Methodology of Epilepsy Epidemiology and Market
5. Executive Summary of Epilepsy
6. Key Events
7. Disease Background and Overview
- 7.1. Introduction
- 7.2. Seizure Types
- 7.2.1. Generalized Seizures
- 7.2.1.1. Generalized tonic-clonic seizures
- 7.2.1.2. Myoclonic Seizures
- 7.2.1.3. Atonic Seizures
- 7.2.1.4. Absence of Seizures (petit mal)
- 7.2.2. Focal Seizure
- 7.2.2.1. Simple Partial Seizures
- 7.2.2.2. Complex Partial Seizures
- 7.2.3. Unknown Seizures
- 7.2.1. Generalized Seizures
- 7.3. Clinical Manifestations
- 7.4. Causes
- 7.5. Types of Epilepsies
- 7.5.1. West Syndrome
- 7.5.2. Dravet syndrome
- 7.5.3. Lennox-Gastaut syndrome
- 7.5.4. Landau-Kleffner syndrome
- 7.5.5. Epilepsy with continuous spike-and-waves during slow-wave sleep (ECSWS)
- 7.5.6. CDKL5 deficiency disorder (CDD)
- 7.6. Risk Factors
- 7.7. Pathophysiology
- 7.8. Diagnosis
- 7.8.1. Diagnostic Guidelines
- 7.8.1.1. NICE: Epilepsies in Children, Young People, and Adults (2022)
- 7.8.1.2. American Family Physician: Diagnostic Evaluation (2017)
- 7.8.1.3. The French National Authority for Health (HAS): 2020
- 7.8.1.4. German Society for Neurology (DGN) Guidelines: Diagnostics And Therapy In Neurology (2023)
- 7.8.1. Diagnostic Guidelines
- 7.9. Treatment
- 7.9.1. Antiepileptic Medications (AEDs)
- 7.9.2. Receptor Blockers
- 7.9.3. Others
- 7.9.4. Diet Therapy
- 7.9.5. Surgery
- 7.9.5.1. Phase I Evaluation (Noninvasive Tests)
- 7.9.5.2. Phase II Evaluation (Invasive Monitoring)
- 7.9.6. Treatment Algorithm
- 7.9.7. Treatment Guidelines
- 7.9.7.1. American Epilepsy Society
- 7.9.7.1.1. Practice Guideline Update: Efficacy and Tolerability of the New Anti-epileptic Drugs I: Treatment of new-onset epilepsy
- 7.9.7.1.2. Practice Guideline Update: Efficacy and Tolerability of the New Anti-epileptic Drugs II: Treatment-resistant Epilepsy
- 7.9.7.2. American Family Physician: Epilepsy Treatment Options (2017)
- 7.9.7.3. The International League Against Epilepsy (ILAE) Epilepsy Guidelines
- 7.9.7.4. NICE Guidelines: (2022)
- 7.9.7.4.1. Epilepsies in children, young and adults
- 7.9.7.4.2. Treating childhood-onset epilepsies
- 7.9.7.4.3. Treating Status Epilepticus, Repeated Or Cluster Seizures, And Prolonged Seizures
- 7.9.7.4.4. Nonpharmacological Treatments as per NICE Guidelines
- 7.9.7.5. Psychological, Neurobehavioral, Cognitive, and Developmental Comorbidities in Epilepsy as per NICE guidelines
- 7.9.7.6. Advanced Pediatric Life Support (APLS) guideline (2021): Management of the Convulsing Child
- 7.9.8. Living and Coping with Epilepsy
8. Patient Journey
9. Epidemiology and Patient Population
- 9.1. Key Findings
- 9.2. Assumptions and Rationale: The 7MM
- 9.2.1. Diagnosed Prevalent Cases of Epilepsy
- 9.2.2. Gender-specific Cases of Epilepsy
- 9.2.3. Type of Seizure-specific Cases of Epilepsy
- 9.2.4. Diagnosed Cases of Other Types of Epilepsies and Associated Diseases
- 9.3. Total Diagnosed Prevalent Cases of Epilepsy in the 7MM
- 9.4. The US
- 9.4.1. Total Diagnosed Prevalent Cases of Epilepsy in the US
- 9.4.2. Gender-specific Diagnosed Prevalent Cases of Epilepsy in the US
- 9.4.3. Diagnosed Prevalent Cases of Epilepsy Based on Seizure Type in the US
- 9.4.4. Diagnosed Cases of Other Types of Epilepsies and Associated Diseases in the US
- 9.5. EU4 and the UK
- 9.5.1. Total Diagnosed Prevalent Cases of Epilepsy in EU4 and the UK
- 9.5.2. Gender-specific Diagnosed Prevalent Cases of Epilepsy in EU4 and the UK
- 9.5.3. Diagnosed Prevalent Cases of Epilepsy Based on Seizure Type in EU4 and the UK
- 9.5.4. Diagnosed Cases of Other Types of Epilepsies and Associated Diseases in EU4 and the UK
- 9.6. Japan
- 9.6.1. Total Diagnosed Prevalent Cases of Epilepsy in Japan
- 9.6.2. Gender-specific Diagnosed Prevalent Cases of Epilepsy in Japan
- 9.6.3. Diagnosed Prevalent Cases of Epilepsy Based on Seizure Type in Japan
- 9.6.4. Diagnosed Cases of Other Types of Epilepsies and Associated Diseases in Japan
10. Marketed Drugs
- 10.1. Key Cross Competition
- 10.2. EPIDIOLEX/EPIDYOLEX (cannabidiol): Jazz Pharmaceuticals
- 10.2.1. Product Description
- 10.2.2. Regulatory Milestones
- 10.2.3. Other Developmental Activities
- 10.2.4. Clinical Development
- 10.2.5. Clinical Trials Information
- 10.2.6. Safety and Efficacy
- 10.2.7. Product Profile
- 10.3. XCOPRI/ONTOZRY (cenobamate): SK Biopharmaceutical/Angelini Pharma/Ono Pharmaceutical
- 10.3.1. Product Description
- 10.3.2. Regulatory Milestones
- 10.3.3. Other Developmental Activities
- 10.3.4. Clinical Development
- 10.3.5. Clinical Trials Information
- 10.3.6. Safety and Efficacy
- 10.3.7. Product Profile
- 10.4. FINTEPLA (fenfluramine): UCB/Nippon Shinyaku
- 10.4.1. Product Description
- 10.4.2. Regulatory Milestones
- 10.4.3. Other Developmental Activities
- 10.4.4. Clinical Development
- 10.4.5. Clinical Trial Information
- 10.4.6. Safety and Efficacy
- 10.4.7. Product Profile
- 10.5. NAYZILAM (midazolam) nasal spray: UCB Pharma
- 10.5.1. Product Description
- 10.5.2. Regulatory Milestone
- 10.5.3. Other Developmental Activities
- 10.5.4. Clinical Development
- 10.5.5. Clinical Trials Information
- 10.5.6. Safety and Efficacy
- 10.5.7. Product Profile
- 10.6. VALTOCO (diazepam nasal spray): Neurelis/Aculys Pharma
- 10.6.1. Product Description
- 10.6.2. Regulatory Milestones
- 10.6.3. Other Developmental Activities
- 10.6.4. Clinical Development
- 10.6.5. Clinical Trials Information
- 10.6.6. Safety and Efficacy
- 10.6.7. Product Profile
- 10.7. ZTALMY (ganaxolone): Marinus Pharmaceuticals/Ovid Therapeutics/Orion
- 10.7.1. Product Description
- 10.7.2. Regulatory Milestones
- 10.7.3. Other Developmental Activities
- 10.7.4. Clinical Development
- 10.7.5. Clinical Trials Information
- 10.7.6. Safety and Efficacy
- 10.7.7. Product Profile
- 10.8. BRIVIACT/NUBRIVEO (brivaracetam): UCB Pharma
- 10.8.1. Product Description
- 10.8.2. Regulatory Milestones
- 10.8.3. Other Developmental Activities
- 10.8.4. Clinical Development
- 10.8.5. Clinical Trials Information
- 10.8.6. Safety and Efficacy
- 10.8.7. Product Profile
- 10.9. FYCOMPA (perampanel): Eisai/Catalyst Pharmaceutical
- 10.9.1. Product Description
- 10.9.2. Regulatory Milestones
- 10.9.3. Other Developmental Activities
- 10.9.4. Clinical Development
- 10.9.5. Clinical Trials Information
- 10.9.6. Safety and Efficacy
- 10.9.7. Product Profile
- 10.10. OXTELLAR XR (oxcarbazepine): Supernus Pharmaceuticals
- 10.10.1. Product Description
- 10.10.2. Regulatory Milestones
- 10.10.3. Other Development Activities
- 10.10.4. Clinical Development
- 10.10.5. Clinical Trials Information
- 10.10.6. Safety and Efficacy
- 10.10.7. Product Profile
- 10.11. VIMPAT (lacosamide): UCB Pharma/Daiichi Sankyo
- 10.11.1. Product Description
- 10.11.2. Regulatory Milestones
- 10.11.3. Other Developmental Activities
- 10.11.4. Clinical Development
- 10.11.5. Clinical Trials Information
- 10.11.6. Safety and Efficacy
- 10.11.7. Product Profile
- 10.12. DIACOMIT (stiripentol): Biocodex/Meiji Seika Pharma
- 10.12.1. Product Description
- 10.12.2. Regulatory Milestones
- 10.12.3. Other Developmental Activities
- 10.12.4. Clinical Development
- 10.12.5. Clinical Trials Information
- 10.12.6. Safety and Efficacy
- 10.12.7. Product Profile
- 10.13. AFINITOR DISPERZ/VOTUBIA (everolimus): Novartis
- 10.13.1. Product Information
- 10.13.2. Regulatory Milestones
- 10.13.3. Other Developmental Activities
- 10.13.4. Clinical Development
- 10.13.5. Clinical Trials Information
- 10.13.6. Safety and Efficacy
- 10.13.7. Product Profile
- 10.14. EPRONTIA (topiramate): Azurity Pharmaceuticals/Eton Pharmaceuticals
- 10.14.1. Product Description
- 10.14.2. Regulatory Milestones
- 10.14.3. Other Developmental Activities
- 10.14.4. Safety and Efficacy
- 10.14.5. Product Profile
- 10.15. TROKENDI XR (topiramate): Supernus Pharmaceutical
- 10.15.1. Product Description
- 10.15.2. Regulatory Milestones
- 10.15.3. Other Developmental Activities
- 10.15.4. Safety and Efficacy
- 10.15.5. Product Profile
- 10.16. MOTPOLY XR (lacosamide): Aucta Pharmaceuticals
- 10.16.1. Product Description
- 10.16.2. Regulatory Milestones
- 10.16.3. Other Developmental Activities
- 10.16.4. Clinical Development
- 10.16.5. Clinical Trials Information
- 10.16.6. Safety and Efficacy
- 10.16.7. Product Profile
- 10.17. KIGABEQ (vigabatrin): ORPHELIA Pharma/Veriton Pharma/ Biocodex
- 10.17.1. Product Description
- 10.17.2. Regulatory Milestones
- 10.17.3. Other Developmental Activities
- 10.17.4. Clinical Development
- 10.17.5. Clinical Trials Information
- 10.17.6. Safety and Efficacy
- 10.17.7. Product Profile
- 10.18. APTIOM/ZEBINIX (eslicarbazepine acetate): Bial/Sunovion Pharmaceuticals (Sumitomo Pharma)
- 10.18.1. Product Description
- 10.18.2. Regulatory Milestones
- 10.18.3. Other Developmental Activities
- 10.18.4. Clinical Development
- 10.18.5. Clinical Trials Information
- 10.18.6. Safety and Efficacy
- 10.18.7. Product Profile
11. Emerging Drugs
- 11.1. Key Cross Competition
- 11.2. XEN1101: Xenon Pharmaceuticals
- 11.2.1. Drug Description
- 11.2.2. Other Developmental Activities
- 11.2.3. Clinical Development
- 11.2.4. Clinical Trials Information
- 11.2.5. Safety and Efficacy
- 11.2.6. Product Profile
- 11.2.7. Analysts' Views
- 11.3. LIBERVANT (diazepam buccal film): Aquestive Therapeutics/Atnahs Pharma (Pharmanovia)
- 11.3.1. Drug Description
- 11.3.2. Other Developmental Activities
- 11.3.3. Clinical Development
- 11.3.4. Clinical Trials Information
- 11.3.5. Safety and Efficacy
- 11.3.6. Product Profile
- 11.3.7. Analysts' Views
- 11.4. Soticlestat (TAK-935): Takeda/Ovid Therapeutics
- 11.4.1. Drug Description
- 11.4.2. Other Developmental Activities
- 11.4.3. Clinical Development
- 11.4.4. Clinical Trials Information
- 11.4.5. Safety and Efficacy
- 11.4.6. Product Profile
- 11.4.7. Analysts' Views
- 11.5. COMFYDE (carisbamate): SK Biopharmaceuticals (SK Life Science)
- 11.5.1. Drug Description
- 11.5.2. Other Developmental Activities
- 11.5.3. Clinical Development
- 11.5.4. Clinical Trials Information
- 11.5.5. Product Profile
- 11.5.6. Analysts' Views
- 11.6. Lorcaserin (E2023): Eisai
- 11.6.1. Drug Description
- 11.6.2. Other Developmental Activities
- 11.6.3. Clinical Development
- 11.6.4. Clinical Trials Information
- 11.6.5. Safety and Efficacy
- 11.6.6. Product Profile
- 11.6.7. Analysts' Views
- 11.7. BHV-7000 (KB-3061): Biohaven Pharmaceuticals/Knopp Biosciences
- 11.7.1. Drug Description
- 11.7.2. Other Developmental Activities
- 11.7.3. Clinical Development
- 11.7.4. Clinical Trials Information
- 11.7.5. Safety and Efficacy
- 11.7.6. Product Profile
- 11.7.7. Analysts' Views
- 11.8. STACCATO alprazolam (benzodiazepine): UCB Pharma/Alexza Pharmaceuticals
- 11.8.1. Drug Description
- 11.8.2. Other Developmental Activities
- 11.8.3. Clinical Development
- 11.8.4. Clinical Trials Information
- 11.8.5. Safety and Efficacy
- 11.8.6. Product Profile
- 11.8.7. Analysts' Views
- 11.9. NBI-827104 (ACT-709478): Neurocrine Biosciences/Idorsia Pharmaceuticals
- 11.9.1. Drug Description
- 11.9.2. Other Developmental Activities
- 11.9.3. Clinical Development
- 11.9.4. Clinical Trials Information
- 11.9.5. Safety and Efficacy
- 11.9.6. Product Profile
- 11.9.7. Analysts' Views
- 11.10. NBI-921352: Neurocrine Biosciences/Xenon Pharmaceuticals
- 11.10.1. Drug Description
- 11.10.2. Other Developmental Activities
- 11.10.3. Clinical Development
- 11.10.4. Clinical Trials Information
- 11.10.5. Product Profile
- 11.10.6. Analysts' Views
- 11.11. Ivermectin (EQU-001): Equilibre Biopharmaceuticals
- 11.11.1. Drug Description
- 11.11.2. Clinical Development
- 11.11.3. Safety and Efficacy
- 11.11.4. Product Profile
- 11.11.5. Analysts' Views
12. Epilepsy: Market Analysis
- 12.1. Key Findings
- 12.2. Key Market Forecast Assumptions
- 12.3. Market Outlook
- 12.4. Conjoint Analysis
- 12.5. Total Market Size of Epilepsy in the 7MM
- 12.6. Total Market Size of Epilepsy by Therapies in the 7MM
- 12.7. Market Size of Epilepsy in the US
- 12.7.1. Total Market Size of Epilepsy in the US
- 12.7.2. The Market Size of Epilepsy by Therapies
- 12.8. Market Size of Epilepsy in EU4 and the UK
- 12.8.1. Total Market Size of Epilepsy in EU4 and the UK
- 12.8.2. The Market Size of Epilepsy by Therapies
- 12.9. Market Size of Epilepsy in Japan
- 12.9.1. Total Market Size of Epilepsy in Japan
- 12.9.2. The Market Size of Epilepsy by Therapies
13. Key Opinion Leaders' Views
14. SWOT Analysis
15. Unmet Needs
16. Market Access and Reimbursement
- 16.1. The United States
- 16.1.1. Center for Medicare & Medicaid Services (CMS)
- 16.2. In EU4 and the UK
- 16.2.1. Germany
- 16.2.2. France
- 16.2.3. Italy
- 16.2.4. Spain
- 16.2.5. The United Kingdom
- 16.3. Japan
- 16.3.1. MHLW
17. Appendix
- 17.1. Bibliography
- 17.2. Acronyms and Abbreviations
- 17.3. Report Methodology