|
|
市場調査レポート
商品コード
1376837
トリプルネガティブ乳がん(TNBC)市場 - 市場洞察、疫学、市場予測:2032年Triple Negative Breast Cancer (TNBC) - Market Insight, Epidemiology And Market Forecast - 2032 |
||||||
カスタマイズ可能
|
|||||||
| トリプルネガティブ乳がん(TNBC)市場 - 市場洞察、疫学、市場予測:2032年 |
|
出版日: 2023年11月01日
発行: DelveInsight
ページ情報: 英文 200 Pages
納期: 1~3営業日
|
- 全表示
- 概要
- 図表
- 目次
乳がんは遺伝的にも臨床的にも不均一な疾患であり、複数のサブタイプが存在します。これらのサブタイプの分類は長年にわたって進化してきました。最も一般的で広く受け入れられている乳がんの分類は、以下のホルモン受容体(エストロゲン(ER)、プロゲステロン(PR)、ヒト上皮成長因子(HER2))の発現に基づく免疫組織化学的観点からのものです。
TNBCの特徴的な免疫微小環境は、高レベルの血管内皮増殖因子、腫瘍浸潤リンパ球(TIL)、腫瘍関連マクロファージ(TAM)、および腫瘍細胞の増殖と移動を促進する他の分子を含み、この疾患の発生、増殖、転移において二重の役割を果たしています。
TNBCのリスク因子は、非修飾可能なものと修飾可能なものに分類されています。年齢、性別、遺伝子変異、遺伝歴、乳房組織密度、その他は非修飾性のリスク変数です。修正可能な危険因子には、医薬品、BMI、不十分なビタミン補給、化学物質や薬物への曝露などが含まれます。
TNBCは、他の種類の乳がんと比較して、最初に悪性度の高い悪性腫瘍として現れ、より攻撃的な臨床経過をとることがあります。さらに、遠隔転移の割合が高く、遠隔転移までの平均期間が短いです。内分泌受容体の発現がないため、介入は制限されます。TNBCは非TNBCよりも予後が悪く、残遺率を伴う死亡率が高いが、これはその進行性、再発率の高さ、および代替療法が限られているためです。
TNBCの疫学に関しては、米国が主要7ヶ国におけるTNBCの総症例の約42%を占めています。米国の市場規模は、主要7ヶ国諸国の中で最大であることがわかっています。米国は、主要7ヶ国地域の市場規模全体の約61%を占めています。
2022年には、米国が主要7ヶ国諸国の中で最大の市場規模を占め、主要7ヶ国全体の市場規模の61%を占めました。主要7ヶ国諸国の中で2022年の市場規模が最も小さいのはスペインで、主要7ヶ国地域の市場規模の3%を占めています。EU4ヶ国地域では、ドイツが最大の市場規模を占め、EU4ヶ国地域全体の市場規模の~29%を占めました。
当レポートでは、主要7ヶ国におけるトリプルネガティブ乳がん(TNBC)市場について調査し、市場の概要とともに、疫学、患者動向、新たな治療法、2032年までの市場規模予測、および医療のアンメットニーズなどを提供しています。
目次
第1章 重要な洞察
第2章 レポートのイントロダクション
第3章 トリプルネガティブ乳がん(TNBC)市場概要
第4章 TNBCのエグゼクティブサマリー
第5章 疾患の背景と概要
- イントロダクション
- 免疫組織化学的発現に基づく乳がんのさまざまなサブタイプ
- トリプルネガティブ乳がん(TNBC)の概要
- 診断
- 病期
- 診断アルゴリズム
第6章 治療と管理
第7章 調査手法
第8章 疫学と患者数
- 主な調査結果
- 仮定と根拠:主要7ヶ国
- 主要7ヶ国における乳がんの総発生件数
- 主要7ヶ国におけるトリプルネガティブ乳がん(TNBC)の総発生件数
- 米国における疫学シナリオ
- EU4ヶ国と英国における疫学シナリオ
- 日本における疫学シナリオ
第9章 患者動向
第10章 上市済み製品
第11章 新たな治療法
第12章 TNBC- 主要7ヶ国市場分析
- 主な調査結果
- 市場の見通し
- コンジョイント分析
- 主要な市場予測の前提条件
- 主要7ヶ国市場規模
- 米国の市場規模
- EU4ヶ国と英国の市場規模
- 日本の市場規模
第13章 市場アクセスと償還
- 米国
- EU4ヶ国と英国
- 日本
第14章 KOLの見解
第15章 アンメットニーズ
第16章 SWOT分析
第17章 付録
第18章 DelveInsightのサービス内容
第19章 免責事項
第20章 DelveInsightについて
List of Tables
- Table 1: Summary of TNBC Market, Epidemiology (2019-2032)
- Table 2: Key Events
- Table 3: Definitions for T, N, and M
- Table 4: Clinical Prognostic Staging
- Table 5: Pathological Prognostic Staging
- Table 6: Total Incident Cases of Breast Cancer in the 7MM (2019-2032)
- Table 7: Total Incident Cases of Triple Negative Breast Cancer (TNBC) in the 7MM (2019-2032)
- Table 8: Total Incident Cases of TNBC in the United States (2019-2032)
- Table 9: Subtype-specific Cases of TNBC in the United States (2019-2032)
- Table 10: Gene Mutation-specific Cases of TNBC in the United States (2019-2032)
- Table 11: Stage-specific Cases of TNBC in the United States (2019-2032)
- Table 12: Age-specific Cases of TNBC in the United States (2019-2032)
- Table 13: Line wise Treated Cases of TNBC in the US (2019-2032)
- Table 14: Total Incident Cases of TNBC in EU4 and the UK (2019-2032)
- Table 15: Subtype-specific Cases of TNBC in EU4 and the UK (2019-2032)
- Table 16: Gene Mutation-specific Cases of TNBC in EU4 and the UK (2019-2032)
- Table 17: Stage-specific Cases of TNBC in EU4 and the UK (2019-2032)
- Table 18: Age-specific cases of TNBC in EU4 and the UK (2019-2032)
- Table 19: Line wise Treated Cases of TNBC in EU4 and the UK (2019-2032)
- Table 20: Total Incident Cases of TNBC in Japan (2019-2032)
- Table 21: Subtype-specific Cases of TNBC in Japan (2019-2032)
- Table 22: Gene Mutation-specific Cases of TNBC in Japan (2019-2032)
- Table 23: Stage-specific Cases of TNBC in Japan (2019-2032)
- Table 24: Age-specific Cases of TNBC in Japan (2019-2032)
- Table 25: Line wise Treated Cases of TNBC in Japan (2019-2032)
- Table 27: Comparison of Marketed Drugs of TNBC
- Table 28: LYNPARZA, Clinical Trial Description, 2023
- Table 29: Early Breast Cancer Stage, Receptor Status, and Grade Scoring Requirements for Study Enrollment
- Table 30: Efficacy Results - OlympiA
- Table 31: Efficacy Results - OlympiAD (BICR-assessed)
- Table 32: TALZENNA, Clinical Trial Description, 2023
- Table 33: Summary of Efficacy Results - EMBRACA Study
- Table 34: TECENTRIQ, Clinical Trial Description, 2023
- Table 35: Efficacy Results from IMpassion130 in Patients with PD-L1 Expression = 1%
- Table 36: TRODELVY, Clinical Trial Description, 2023
- Table 37: Efficacy Results From ASCENT
- Table 38: Efficacy results for patients with mTNBC in IMMU-132-01
- Table 39: KEYTRUDA, Clinical Trial Description, 2023
- Table 40: Efficacy Results - KEYNOTE-522
- Table 41: Efficacy Results in KEYNOTE-355 (CPS =10)
- Table 42: Comparison of Emerging drugs of TNBC
- Table 43: COSELA (trilacicilib), Clinical Trial Description, 2023
- Table 44: Datopotamab Deruxtecan (Dato-DXd), Clinical Trial Description, 2023
- Table 45: Capivasertib, Clinical Trial Description, 2023
- Table 46: ZEN003694, Clinical Trial Description, 2023
- Table 47: TAVO, Clinical Trial Description, 2023
- Table 48: PVX-410, Clinical Trial Description, 2023
- Table 49: IPI-549, Clinical Trial Description, 2023
- Table 50: Key Market Forecast Assumptions for COSELA + gemcitabine + carboplatin
- Table 51: Key Market Forecast Assumptions for COSELA + TRODELVY
- Table 52: Key Market Forecast Assumptions for Datopotamab deruxtecan (Dato-DXd)
- Table 53: Key Market Forecast Assumptions for Capivasertib
- Table 54: Key Market Forecast Assumptions for ZEN003694
- Table 55: Key Market Forecast Assumptions for TAVO
- Table 56: Key Market Forecast Assumptions for IPI-549 (eganelisib)
- Table 57: Key Market Forecast Assumptions for TRODELVY (sactizumab govtecan-hziy)
- Table 58: Key Market Forecast Assumptions for KEYTRUDA (pembrolizumab)
- Table 59: Total Market Size of TNBC in the 7MM, in USD million (2019-2032)
- Table 60: Market Size of TNBC by Therapies (First-line) in the 7MM, in USD million (2019-2032)
- Table 61: Market Size of TNBC by Therapies (Second-line) in the 7MM, in USD million (2019-2032)
- Table 62: Market Size of TNBC by Therapies (Third-line) in the 7MM, in USD million (2019-2032)
- Table 63: Total Market Size of TNBC in the United States, in USD million (2019-2032)
- Table 64: Market Size of TNBC by Therapies (First-line) in the United States, in USD million (2019-2032)
- Table 65: Market Size of TNBC by Therapies (Second-line) in the United States, in USD million (2019-2032)
- Table 66: Market Size of TNBC by Therapies (Third-line) in the United States, in USD million (2019-2032)
- Table 67: Total Market Size of TNBC in EU4 and the UK, in USD million (2019-2032)
- Table 68: Market Size of TNBC by Therapies (First-line) in EU4 and the UK, in USD million (2019-2032)
- Table 69: Market Size of TNBC by Therapies (Second-line) in EU4 and the UK, in USD million (2019-2032)
- Table 70: Market Size of TNBC by Therapies (Third-line) in EU4 and the UK, in USD million (2019-2032)
- Table 71: Total Market Size of TNBC in Japan, in USD million (2019-2032)
- Table 72: Market Size of TNBC by Therapies (First-line) in Japan, in USD million (2019-2032)
- Table 73: Market Size of TNBC by Therapies (Second-line) in Japan, in USD million (2019-2032)
- Table 74: Market Size of TNBC by Therapies (Third-line) in Japan, in USD million (2019-2032)
List of Figures
- Figure 1: Intrinsic Molecular Subtypes of Breast Cancer
- Figure 2: Classification of Neoplastic Infiltrating Lymphocytes
- Figure 3: Type of Tumor-associated Macrophages
- Figure 4: Diagnostic Work-up and Staging of Metastatic Breast Cancer
- Figure 5: Line-wise Treatment Recommendations of NCCN for TNBC
- Figure 6: Treatment Algorithm of Metastatic TNBC
- Figure 7: Total Incident Cases of Breast Cancer in the 7MM (2019-2032)
- Figure 8: Total Incident Cases of Triple Negative Breast Cancer (TNBC) in the 7MM (2019-2032)
- Figure 9: Total Incident Cases of TNBC in the United States (2019-2032)
- Figure 10: Subtype-specific Cases of TNBC in the United States (2019-2032)
- Figure 11: Gene Mutation-specific Cases of TNBC in the United States (2019-2032)
- Figure 12: Stage-specific Cases of TNBC in the United States (2019-2032)
- Figure 13: Age-specific Cases of TNBC in the United States (2019-2032)
- Figure 14: Line wise Treated Cases of TNBC in the US (2019-2032)
- Figure 15: Total Incident Cases of TNBC in EU4 and the UK (2019-2032)
- Figure 16: Subtype-specific Cases of TNBC in EU4 and the UK (2019-2032)
- Figure 17: Gene Mutation-specific Cases of TNBC in EU4 and the UK (2019-2032)
- Figure 18: Stage-specific Cases of TNBC in EU4 and the UK (2019-2032)
- Figure 19: Age-specific Cases of TNBC in EU4 and the UK (2019-2032)
- Figure 20: Line wise Treated Cases of TNBC in EU4 and the UK (2019-2032)
- Figure 21: Total Incident Cases of TNBC in Japan (2019-2032)
- Figure 22: Subtype-specific Cases of TNBC in Japan (2019-2032)
- Figure 23: Gene Mutation-specific Cases of TNBC in Japan (2019-2032)
- Figure 24: Stage-specific Cases of TNBC in Japan (2019-2032)
- Figure 25: Age-specific Cases of TNBC in Japan (2019-2032)
- Figure 26: Line wise Treated cases of TNBC in Japan (2019-2032)
- Figure 27: Market Size of TNBC by Therapies (First-line) in the 7MM, in USD million (2019-2032)
- Figure 28: Market Size of TNBC by Therapies (Second-line) in the 7MM, in USD million (2019-2032)
- Figure 29: Market Size of TNBC by Therapies (Third-line) in the 7MM, in USD million (2019-2032)
- Figure 30: Total Market Size of TNBC in the United States, in USD million (2019-2032)
- Figure 31: Market Size of TNBC by Therapies (First-line) in the United States, in USD million (2019-2032)
- Figure 32: Market Size of TNBC by Therapies (Second-line) in the United States, in USD million (2019-2032)
- Figure 33: Market Size of TNBC by Therapies (Third-line) in the United States, in USD million (2019-2032)
- Figure 34: Total Market Size of TNBC in EU4 and the UK, in USD million (2019-2032)
- Figure 35: Market Size of TNBC by Therapies (First-line) in EU4 and the UK, in USD million (2019-2032)
- Figure 36: Market Size of TNBC by Therapies (Second-line) in EU4 and the UK, in USD million (2019-2032)
- Figure 37: Market Size of TNBC by Therapies (Third-line) in EU4 and the UK, in USD million (2019-2032)
- Figure 38: Total Market Size of TNBC in Japan, in USD million (2019-2032)
- Figure 39: Market Size of TNBC by Therapies (First-line) in Japan, in USD million (2019-2032)
- Figure 40: Market Size of TNBC by Therapies (Second-line) in Japan, in USD million (2019-2032)
- Figure 41: Market Size of TNBC by Therapies (Third-line) in Japan, in USD million (2019-2032)
- Figure 42: Health Technology Assessment
- Figure 43: Reimbursement Process in Germany
- Figure 44: Reimbursement Process in France
- Figure 45: Reimbursement Process in Italy
- Figure 46: Reimbursement Process in Spain
- Figure 47: Reimbursement Process in the United Kingdom
- Figure 48: Reimbursement Process in Japan
- Figure 49: Unmet Needs
Key Highlights:
- Breast cancer is a genetically and clinically heterogeneous disease with multiple subtypes. The classification of these subtypes has evolved over the years. The most common and widely accepted classification of breast cancer is from an immunohistochemical perspective, based on the expression of the following hormone receptors: estrogen (ER), progesterone (PR), and human epidermal growth factor (HER2)
- TNBC's distinct immune microenvironment, which includes high levels of vascular endothelial growth factors, tumor-infiltrating lymphocytes (TILs), tumor-associated macrophages (TAMs), and other molecules that promote tumor cell growth and migration, plays a dual role in the occurrence, growth, and metastasis of the disease.
- TNBC risk factors have been classified as either non-modifiable or modifiable. Age, sex, genetic mutations, genetic history, breast tissue density, and others are non-modifiable risk variables. Modifiable risk factors include medicines, BMI, inadequate vitamin supplements, chemical and drug exposure, and so on.
- TNBC can manifest as a higher-grade malignancy at first and have a more aggressive clinical course than other kinds of breast cancer. In addition, the rate of distant metastatic disease is greater, and the mean time to distant metastatic disease is shorter. Because of the absence of endocrine receptor expression, interventions are restricted. TNBC has a worse prognosis and greater mortality with residual illness than non-TNBC because of its aggressive course, high rates of recurrence, and limited therapy alternatives.
- Approximately half of the breast cancers, historically categorized as human epidermal growth factor receptor 2 (HER2)-negative, have low expression of HER2, defined as an immunohistochemical (IHC) score of 1+ or 2+ with negative in situ hybridization. The current clinical definition of HER2-low BC used in clinical practice and ongoing clinical trials relies on the standard IHC and ISH approach; thus, tumors with a low level of HER2 expression (defined as a HER2 IHC score of 1+ or 2+) and no detectable ERBB2 amplification fall into this category.
- Regarding the epidemiology of TNBC, it was found that the United States accounted for ~42% of the total cases of TNBC in the 7MM.
- The market size captured by the United States was found to be the largest among the 7MM countries. It was found that the United States accounted for ~61% of the total market size captured by the 7MM region.
- The term "triple negative breast cancer diagnosis" is somewhat misnomer. Rather than being an official medical diagnosis, TNBC refers to the cellular makeup of certain breast cancers, which can influence a patient's prognosis. The pathology for these cancers usually shows that the cancer cells tested negatively for three specific hormone receptors: estrogen, progesterone, and HER-2/neu.
- In contrast to hormone receptor (ER/PR)-positive and HER2-positive breast cancers, there are few FDA-approved targeted therapies for triple-negative breast cancer. Historically, chemotherapy has been the main systemic treatment, especially for patients with metastatic disease; although TNBC tends to respond well to chemo initially, recurrences are frequent.
- The recent approval of various immune checkpoint inhibitors (like pembrolizumab) and targeted therapies (like olaparib and talazoparib) has led to a dynamic shift in the treatment landscape, with their increased usage across various lines of therapy, leading to significant improvement in treatment outcomes and patient prognosis.
- The emerging pipeline for TNBC patients consists of early-stage and late-stage drugs in various lines of therapy. As per DelveInsight's estimates, the potential drugs that can significantly change in the market during the forecast period include COSELA, Capivasertib, Datopotamab Deruxtecan (Dato-DXd), ZEN003694 and TAVO (tavokinogene telseplasmid) being administered in various combinations. These drugs are in different stages of clinical development.
Report Summary
- The report covers a segment of key events describing the latest developments from a treatment point of view, including designations, collaboration, and agreements, etc., as well as a descriptive overview of Triple Negative Breast Cancer (TNBC), explaining the definition of Triple Negative Breast Cancer (TNBC), types and risk factors involved in Triple Negative Breast Cancer (TNBC), pathophysiology, and the treatment of Triple Negative Breast Cancer (TNBC).
- The report offers extensive knowledge regarding the epidemiology segments and predictions, presenting a deep understanding of the potential future growth in diagnosis rates, disease progression, and treatment guidelines. It provides comprehensive insights into these aspects, enabling a thorough assessment of the subject matter.
- Additionally, an all-inclusive account of the current and emerging therapies and the elaborative profiles of late-stage (Phase III and Phase II) and prominent therapies that would impact the current treatment landscape and result in an overall market shift has been provided in the report.
- The report also encompasses a comprehensive analysis of the Triple Negative Breast Cancer (TNBC) market, thoroughly examining its historical and projected market size (2019-2032). It also includes the market share of therapies, along with detailed assumptions and the underlying rationale for methodology. The report also includes the drug outreach coverage in the 7MM region.
- The report includes qualitative insights that provide an edge while developing business strategies by understanding trends through SWOT analysis and expert insights/KOL views, including experts from various hospitals and prominent universities, patient journey, and treatment preferences that help shape and drive the 7MM Triple Negative Breast Cancer (TNBC) market.
Market
Various key players are currently leading the treatment landscape of TNBC, such as G1 Therapeutics, AstraZeneca, Zenith Epigenetics, etc. The details of the country and therapywise market size have been provided below.
- In 2022, the United States accounted for the largest market size among the 7MM countries, making ~61% of the total market size of the 7MM.
- Among the 7MM countries, Spain had the least market size in 2022, accounting for ~3% of the market size in the 7MM region.
- In the EU4 region, Germany accounted for the largest market size, making up ~29% of the total market size of the EU4 region.
- The market size has also been segregated linewise. In 2022, among the therapies being administered to first-line patients, KEYTRUDA (pembrolizumab) + paclitaxel/nab-paclitaxel/gemcitabine + carboplatin combination garnered the largest market share in the 7MM region.
Triple Negative Breast Cancer (TNBC) Drug Chapters
The section dedicated to drugs in the Triple Negative Breast Cancer (TNBC) report provides an in-depth evaluation of both the marketed drugs and late-stage pipeline drugs (Phase III and Phase II) related to TNBC. Among the FDA-approved treatments are LYNPARZA (olaparib), TALZENNA (talazoparib), KEYTRUDA (pembrolizumab), and others. There are several emerging therapies, and detailed coverage of the same has been provided in the report.
The drug chapters section provides valuable information on various aspects related to clinical trials of TNBC, including specific details, such as the pharmacological mechanisms of the drugs involved, agreements and partnerships, approval status, patent information, and a comprehensive analysis of the pros and cons associated with each drug. Furthermore, it presents the most recent news updates and press releases related to drugs targeting TNBC.
Marketed Therapies
LYNPARZA (olaparib)
LYNPARZA (olaparib) is a poly (ADP-ribose) polymerase (PARP) inhibitor. LYNPARZA is indicated for the treatment of adult patients with deleterious or suspected deleterious gBRCAm, HER2-negative metastatic breast cancer, who have been treated with chemotherapy in the neoadjuvant, adjuvant, or metastatic setting. Patients with hormone receptor (HR)-positive breast cancer should have been treated with prior endocrine therapy or be considered inappropriate for endocrine therapy.
TALZENNA (talazoparib)
Talazoparib is an inhibitor of poly (ADP-ribose) polymerase (PARP) enzymes, including PARP1 and PARP2, which play a role in DNA repair. TALZENNA is indicated as a single agent for the treatment of adult patients with deleterious or suspected deleterious germline breast cancer susceptibility gene (BRCA)-mutated (gBRCAm) human epidermal growth factor receptor 2 (HER2)-negative locally advanced or metastatic breast cancer. Select patients for therapy based on an FDA-approved companion diagnostic for TALZENNA.
Triple Negative Breast Cancer (TNBC) Market Outlook
Unlike other forms of breast cancer, TNBC does not respond with hormonal or HER2-targeted treatment. Hence, for a long time, systemic chemotherapy has remained the mainstay treatment for metastatic TNBC. Even for resectable/non-metastatic disease, chemotherapy and surgery in neoadjuvant or adjuvant therapy have been widely used.
But the recent approval of various immune checkpoint inhibitors (like pembrolizumab) and targeted therapies (like olaparib and talazoparib) has led to a dynamic shift in the treatment landscape, with their increased usage across various lines of therapy, leading to significant improvement in treatment outcomes and patient prognosis.
In patients with localized TNBC, dose-dense doxorubicin-cyclophosphamide, and paclitaxel were the standard neoadjuvant chemotherapy backbone until 2021. With the approval of neoadjuvant pembrolizumab, chemotherapy with immunotherapy is the new standard of care for localized TNBC.
Before these recent developments, taxane-based chemotherapies have remained the standard of care for metastatic disease, specifically in first-line treatment. However, with their recent approvals, immune checkpoint inhibitors like pembrolizumab and atezolizumab have established their effectiveness when combined with taxane-based chemotherapies; however, their usage is limited to patients with high levels of PD-L1 expression. Due to their superior results, they remain the most preferred form of treatment in the first-line setting.
The TNBC market is witnessing significant growth and evolution due to increased awareness, technological advancements, therapeutic advancements, and integration into clinical trials. However, challenges related to cost, access, and market competition remain important considerations for TNBC, keeping the dynamic market scenario in mind.
Drug Class Insights
Treatment for triple-negative breast cancer (TNBC) is more difficult since there is no apparent therapeutic target, as there is for ER/PR-positive or HER2-positive breast cancer. TNBC is treated with surgery, chemotherapy, and radiation.
The second most preferred form of treatment are poly (adenosine diphosphate-ribose) polymerase (PARP) inhibitors LYNPARZA (olaparib) and TALZENNA (talazoparib); their usage is preferred in patients with germline BRCA1/2 mutations. Platinum-based chemotherapies are also used at times in the first-line treatment setting, but their usage is highly debated among physicians, as they tend to have very severe adverse effects and do not have that good of treatment outcome (PFS) either.
For patients with recurrent/refractory metastatic TNBC (mTNBC), the antibody-drug conjugate TRODELVY (sacituzumab govitecan-hziy) has emerged as an effective treatment option, in the second-line of the treatment. It targets Trop-2, which is present in most patients with triple-negative disease.
Triple Negative Breast Cancer (TNBC) Disease Understanding and Treatment
Triple Negative Breast Cancer (TNBC) Overview
Triple-negative breast cancer (TNBC) encompasses a heterogeneous group of fundamentally different diseases with different histologic, genomic, and immunologic profiles, which are aggregated under this term because of their lack of estrogen receptor, progesterone receptor, and human epidermal growth factor receptor 2 expressions. Massively parallel sequencing and other omics technologies have demonstrated the level of heterogeneity in TNBCs and shed light on the pathogenesis of this therapeutically challenging entity in breast cancer.
Triple Negative Breast Cancer (TNBC) diagnosis
The phrase "triple negative breast cancer (TNBC) diagnosis" is a bit misleading. TNBC refers to the cellular composition of some breast tumors, which might impact a patient's prognosis rather than an official medical diagnosis. Pathology for these malignancies typically reveals that the cancer cells tested negative for three hormone receptors: estrogen, progesterone, and HER-2/neu. The first step in developing an effective treatment strategy is to confirm any breast cancer diagnosis. A triple negative test result means these hormones will not promote cancer development. Another problem is that standard breast cancer hormone treatment, such as tamoxifen or aromatase inhibitors, is unsuccessful in treating these tumors.
Further details related to country-based variations are provided in the report…
Triple Negative Breast Cancer (TNBC) Treatment
Triple-negative breast cancer (TNBC) represents a small but aggressive subtype of breast cancer with high mortality, partly because many cancers become metastatic. Relapse is common in TNBC, including for women with localized disease. TNBCs test negative for progesterone, estrogen receptors, and excess HER2 protein. For these reasons, this cancer type is not responsive to hormonal or HER2-targeted therapies.
In contrast to hormone receptor (ER/PR)-positive and HER2-positive breast cancers, there are few FDA-approved targeted therapies for triple-negative breast cancer. Historically, chemotherapy has been the main systemic treatment, especially for patients with metastatic disease; although TNBC tends to respond well to chemo initially, recurrences are frequent.
Patients have a worse prognosis with fewer treatment options than other breast cancer subtypes, and TNBC is more resistant to conventional treatment. Overall survival rates for TNBC patients with advanced or Stage IV disease is approximately 12 months compared to 36 months for those with ER-positive/PR-positive/HER2-negative disease.
But, of late, this pattern of treatment and survival is changing, as with an increased understanding of the disease's pathophysiology, TNBC is classified into several distinct molecular subtypes based on gene expression profiles rather than being considered a single disease. Following these developments, accompanied by newer therapies being recently approved, there has been improvement in the prognosis of patients.
Further details related to treatment and management are provided in the report…
Triple Negative Breast Cancer (TNBC) Epidemiology
The Triple Negative Breast Cancer (TNBC) epidemiology chapter in the report provides historical as well as forecasted epidemiology segmented by Total Incident Cases of Triple Negative Breast Cancer (TNBC), Subtype-specific Cases of Triple Negative Breast Cancer (TNBC), Gene Mutation-specific Cases of Triple Negative Breast Cancer (TNBC), Stage-specific Cases of Triple Negative Breast Cancer (TNBC), Age-specific Cases of Triple Negative Breast Cancer (TNBC) and Line wise Treated Cases of Triple Negative Breast Cancer (TNBC) in the 7MM covering the United States, EU4 countries (Germany, France, Italy, Spain) and the United Kingdom, and Japan from 2019 to 2032.
- The total incident cases of TNBC in the United States comprised ~43,000 cases in 2022. These cases are expected to increase by 2032.
- Among the EU4 countries, Germany accounted for the highest number of incident cases of TNBC, i.e., ~10,750 cases in 2022. Whereas Spain accounted for the least number of cases, i.e., ~5,200 cases in 2022.
- In Japan, as per subtype-specific classification, the HER2-negative cases were found to be the highest in number, ~8,400 cases, in 2022.
- As per gene mutation-specific cases, the BRCA1 cases were found to be the highest in number, i.e., ~730 cases in 2022, in the UK.
KOL Views
To stay abreast of the latest trends in the market, we conduct primary research by seeking the opinions of Key Opinion Leaders (KOLs) and Subject Matter Experts (SMEs) who work in the relevant field. This helps us fill any gaps in data and validate our secondary research.
We have reached out to industry experts to gather insights on various aspects related to Triple Negative Breast Cancer (TNBC), including the evolving treatment landscape, patients' reliance on conventional therapies, their acceptance of therapy switching, drug uptake, and challenges related to accessibility. The experts we contacted included medical/scientific writers, professors, and researchers from prestigious universities in the US, Europe, the UK, and Japan.
Our team of analysts at DelveInsight connected with more than 10 KOLs across the 7MM. We contacted institutions such as the National Cancer Center Hospital, University of Messina, Icahn School of Medicine, and Tufts Medical Center, among others. By obtaining the opinions of these experts, we gained a better understanding of the current and emerging treatment patterns in the Triple Negative Breast Cancer (TNBC) market, which will assist our clients in analyzing the overall epidemiology and market scenario.
Qualitative Analysis
We conduct qualitative and market intelligence analysis by employing the SWOT analysis approach. Within the SWOT analysis framework, we assess the strengths, weaknesses, opportunities, and threats pertaining to various aspects such as disease diagnosis, patient awareness, patient burden, competitive landscape, cost-effectiveness, and geographical accessibility of therapies. This analysis provides a comprehensive evaluation of the current situation and helps identify areas of advantage, areas for improvement, potential opportunities, and potential challenges in the specified domains.
Market Access and Reimbursement
Cancer treatments are funded in several countries through public reimbursement programs. This type of support promotes equitable access for people by removing direct fees from patients. The escalating prices of cancer medications have compelled several nations to seek cost-cutting measures.
Cancer drugs are highly specialized medicines with limited authorized indications, frequently specific to one or a combination of tumor sites, chemotherapy regimens, and treatment sequences. Typically, public payers limit reimbursement to certain specific conditions.
Patients with Medicare may or may not have to pay a portion of the cost of KEYTRUDA (pembrolizumab) based on their insurance plan. For example, with a Medicare Advantage plan, 41% of patients had no out-of-pocket costs for the 200 mg dose of KEYTRUDA. Roughly 80% of patients are responsible for a portion of the cost paid between USD 0 and USD 925 per infusion after meeting their deductible. Most patients with Medicaid typically pay USD 4-8 per KEYTRUDA infusion. The copay part, which needs to be paid by the patient, ranges from USD 12,045.
Further details related to reimbursement will be provided in the report….
Triple Negative Breast Cancer (TNBC) Report Insights
- Patient Population
- Therapeutic Approaches
- Triple Negative Breast Cancer (TNBC) Market Size and Trends
- Existing Market Opportunity
Triple Negative Breast Cancer (TNBC) Report Key Strengths
- Ten-year Forecast
- The 7MM Coverage
- Triple Negative Breast Cancer (TNBC) Epidemiology Segmentation
- Key Cross Competition
Triple Negative Breast Cancer (TNBC) Report Assessment
- Current Treatment Practices
- Unmet Needs
- Market Attractiveness
- Qualitative Analysis (SWOT)
Key Questions
- Would there be any changes observed in the current treatment approach?
- Will there be any improvements in Triple Negative Breast Cancer (TNBC) management recommendations?
- Would technical advances pave the way for future therapies for Triple Negative Breast Cancer (TNBC)?
- How would the emerging therapies fare against the already established approved treatment regimens?
Table of Contents
1. Key Insights
2. Report Introduction
3. Triple Negative Breast Cancer (TNBC) Market Overview at a Glance
- 3.1. Market Share (%) Distribution of TNBC by Therapies in the 7MM in 2019
- 3.2. Market Share (%) Distribution of TNBC by Therapies in the 7MM in 2032
4. Executive Summary of TNBC
- 4.1. Key Events
5. Disease Background and Overview
- 5.1. Introduction
- 5.2. Various Subtypes of Breast Cancer Based on Immunohistochemical Expression
- 5.3. Triple Negative Breast Cancer (TNBC) Overview
- 5.3.1. Intrinsic Molecular Subtypes in TNBC
- 5.3.2. Characteristics of Tumor Microenvironment in TNBC
- 5.3.3. Potential Risk Factors
- 5.3.4. Clinical Presentation
- 5.3.5. Characterization of HER2-low Breast Cancers
- 5.4. Diagnosis
- 5.5. Staging
- 5.5.1. Details of the TNM Staging System
- 5.5.2. Grading
- 5.5.3. Clinical Prognostic Stage
- 5.5.4. Pathological Prognostic Stage
- 5.6. Diagnostic Algorithm
6. Treatment and Management
- 6.1. NCCN Clinical Practice Guidelines in Oncology for Breast Cancer
- 6.2. ESMO Clinical Practice Guidelines for Diagnosis, Treatment, and Follow-up of Early Breast Cancer
- 6.3. ESMO Clinical Practice Guideline for the Diagnosis, Staging, and Treatment of Patients With Metastatic Breast Cancer
- 6.4. Treatment Algorithm
7. Methodology
8. Epidemiology and Patient Population
- 8.1. Key Findings
- 8.2. Assumptions and Rationale: 7MM
- 8.3. Total Incident Cases of Breast Cancer in the 7MM
- 8.4. Total Incident Cases of Triple Negative Breast Cancer (TNBC) in the 7MM
- 8.5. Epidemiology Scenario in the United States
- 8.5.1. Total Incident Cases of TNBC in the United States
- 8.5.2. Subtype-specific Cases of TNBC in the United States
- 8.5.3. Gene Mutation-specific Cases of TNBC in the United States
- 8.5.4. Stage-specific Cases of TNBC in the United States
- 8.5.5. Age-specific Cases of TNBC in the United States
- 8.5.6. Line wise Treated Cases of TNBC in the United States
- 8.6. Epidemiology Scenario in EU4 and the UK
- 8.6.1. Total Incident Cases of TNBC in EU4 and the UK
- 8.6.2. Subtype-specific Cases of TNBC in EU4 and the UK
- 8.6.3. Gene Mutation-specific Cases of TNBC in EU4 and the UK
- 8.6.4. Stage-specific Cases of TNBC in EU4 and the UK
- 8.6.5. Age-specific Cases of TNBC in EU4 and the UK
- 8.6.6. Line wise Treated cases of TNBC in EU4 and the UK
- 8.7. Epidemiology Scenario in Japan
- 8.7.1. Total Incident Cases of TNBC in Japan
- 8.7.2. Subtype-specific Cases of TNBC in Japan
- 8.7.3. Gene Mutation-specific Cases of TNBC in Japan
- 8.7.4. Stage-specific Cases of TNBC in Japan
- 8.7.5. Age-specific Cases of TNBC in Japan
- 8.7.6. Line wise Treated Cases of TNBC in Japan
9. Patient Journey
10. Marketed Products
- 10.1. Key-cross
- 10.2. LYNPARZA (olaparib): Astra Zeneca
- 10.2.1. Product Description
- 10.2.2. Regulatory Milestones
- 10.2.3. Clinical Developmental Activities
- 10.2.4. Safety and Efficacy
- 10.3. TALZENNA (talazoparib): Pfizer
- 10.3.1. Product Description
- 10.3.2. Regulatory Milestones
- 10.3.3. Clinical Developmental Activities
- 10.3.4. Safety and Efficacy
- 10.4. TECENTRIQ (atezolizumab): Hoffman-la Roche/Genentech Inc.
- 10.4.1. Product Description
- 10.4.2. Regulatory Milestones
- 10.4.3. Clinical Developmental Activities
- 10.4.4. Safety and Efficacy
- 10.5. TRODELVY (sacitzumab govitecan-hziy): Gilead Sciences
- 10.5.1. Product Description
- 10.5.2. Regulatory Milestones
- 10.5.3. Clinical Developmental Activities
- 10.5.4. Safety and Efficacy
- 10.6. KEYTRUDA (perbrolizumab): Merck Sharp & Dohme
- 10.6.1. Product Description
- 10.6.2. Regulatory Milestones
- 10.6.3. Clinical Developmental Activities
- 10.6.4. Safety and Efficacy
11. Emerging Therapies
- 11.1. Key-cross
- 11.2. COSELA (trilacicilib): G1 Therapeutics
- 11.2.1. Product Description
- 11.2.2. Other Developmental Activities
- 11.2.3. Clinical Developmental Activities
- 11.2.4. Safety and Efficacy
- 11.3. Datopotamab Deruxtecan (Dato-DXd): AstraZeneca/Daiichi Sankyo
- 11.3.1. Product Description
- 11.3.2. Other Developmental Activities
- 11.3.3. Clinical Developmental Activities
- 11.3.4. Safety and Efficacy
- 11.4. Capivasertib: AstraZeneca
- 11.4.1. Product Description
- 11.4.2. Other Developmental Activities
- 11.4.3. Clinical Developmental Activities
- 11.4.4. Safety and Efficacy
- 11.5. ZEN003694: Zenith Epigenetics/Pfizer
- 11.5.1. Product Description
- 11.5.2. Other Developmental Activities
- 11.5.3. Clinical Developmental Activities
- 11.5.4. Safety and Efficacy
- 11.6. TAVO (Tavokinogene Telseplasmid): OncoSec Medical Incorporated/Merck Sharp & Dohme LLC
- 11.6.1. Product Description
- 11.6.2. Other Developmental Activities
- 11.6.3. Clinical Developmental Activities
- 11.6.4. Safety and Efficacy
- 11.7. PVX-410: OncoPep
- 11.7.1. Product Description
- 11.7.2. Other Developmental Activities
- 11.7.3. Clinical Developmental Activities
- 11.7.4. Safety and Efficacy
- 11.8. IPI-549 (Eganelisib): Infinity Pharmaceuticals/Roche Pharma AG
- 11.8.1. Product Description
- 11.8.2. Other Developmental Activities
- 11.8.3. Clinical Developmental Activities
- 11.8.4. Safety and Efficacy
12. TNBC-Seven Major Market Analysis
- 12.1. Key Findings
- 12.2. Market Outlook
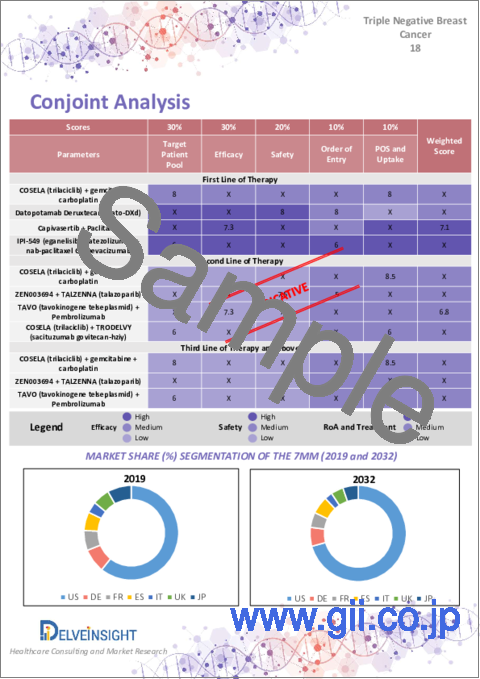
- 12.3. Conjoint Analysis
- 12.4. Key Market Forecast Assumptions
- 12.5. Seven Major Market Size
- 12.5.1. Total Market Size of TNBC in the 7MM
- 12.5.2. Market Size of TNBC by Therapies (First-line) in the 7MM
- 12.5.3. Market Size of TNBC by Therapies (Second-line) in the 7MM
- 12.5.4. Market Size of TNBC by Therapies (Third-line) in the 7MM
- 12.6. United States Market Size
- 12.6.1. Total Market Size of TNBC in the United States
- 12.6.2. Market Size of TNBC by Therapies (First-line) in the United States
- 12.6.3. Market Size of TNBC by Therapies (Second-line) in the United States
- 12.6.4. Market Size of TNBC by Therapies (Third-line) in the United States
- 12.7. EU4 and the UK Market Size
- 12.7.1. Total Market size of TNBC in EU4 and the UK
- 12.7.2. Market Size of TNBC by Therapies (First-line) in EU4 and the UK
- 12.7.3. Market Size of TNBC by Therapies (Second-line) in EU4 and the UK
- 12.7.4. Market Size of TNBC by Therapies (Third-line) in EU4 and the UK
- 12.8. Japan Market Size
- 12.8.1. Total Market size of TNBC in Japan
- 12.8.2. Market Size of TNBC by Therapies (First-line) in Japan
- 12.8.3. Market Size of TNBC by Therapies (Second-line) in Japan
- 12.8.4. Market Size of TNBC by Therapies (Third-line) in Japan
13. Market Access and Reimbursement
- 13.1. The United States
- 13.1.1. Centre for Medicare & Medicaid Services (CMS)
- 13.2. EU4 and the UK
- 13.2.1. Germany
- 13.2.2. France
- 13.2.3. Italy
- 13.2.4. Spain
- 13.2.5. United Kingdom
- 13.3. Japan
- 13.3.1. MHLW
14. KOL Views
15. Unmet Needs
16. SWOT Analysis
17. Appendix
- 17.1. Report Methodology
- 17.2. Bibliography