|
|
市場調査レポート
商品コード
1340027
進行性核上性麻痺市場 - 市場の洞察、疫学、市場予測:2032年Progressive Supranuclear Palsy - Market Insight, Epidemiology And Market Forecast - 2032 |
||||||
カスタマイズ可能
適宜更新あり
|
|||||||
| 進行性核上性麻痺市場 - 市場の洞察、疫学、市場予測:2032年 |
|
出版日: 2023年06月01日
発行: DelveInsight
ページ情報: 英文 141 Pages
納期: 2~10営業日
|
- 全表示
- 概要
- 図表
- 目次
主要7ヶ国における進行性核上性麻痺の総市場規模は、2022年に約830万米ドルとなりました。同市場は、予測期間中(2023年~2032年)に増加すると予測されています。米国における進行性核上性麻痺の市場規模は、疾患に対する認知度の向上と新たな治療法の発売により、CAGR 31.2%で拡大します。2022年、米国における進行性核上性麻痺は対症療法が最大の収益を生み出しましたが、新興治療薬の発売により予測期間中に減少すると予測されます。欧州4ヶ国と英国諸国の中では、ドイツが2022年の核上性麻痺の最大市場規模を占め、スペインは最下位でした。日本は主要7ヶ国の中で2番目に大きな核上性麻痺の市場規模を占め、2022年の収益は約230万米ドルで、予測期間中に増加する見込みです。
進行性核上性麻痺の有病率は、人口の増加と認知度の向上により米国で増加しています。認知度の向上と病態生理のより深い理解により、診断基準が強化され、診断が向上しました。現在の治療法は対症療法です。レボドパのような抗パーキンソン薬は、硬直、振戦、徐脈症状の治療に広く使用されています。さらに、抗精神病薬、抗うつ薬などがさまざまな症状のコントロールに役立っています。作業療法、理学療法、認知リハビリテーションなどのその他の薬物療法は、患者の運動能力、平衡感覚、日常生活活動、言語、その他の症状を改善します。
進行性核上性麻痺の市場を理解する上で大きな懸念は、最近の疫学、表現型に特異的な研究の欠如、進行性核上性麻痺の日常管理に用いられる介入を検証するエビデンスの乏しさです。米国、欧州4ヶ国、英国、日本では、進行性核上性麻痺のコンセンサスや臨床ガイドラインはありません。
進行性核上性麻痺に対する生物学的治療法の開発には、さらなる病態研究が必要です。さらに、承認された治療法がなく、現在の治療法の有効性が限られていることから、製薬企業にとっては、治癒可能で、進行性核上性麻痺の症状を効果的に軽減し、進行を遅らせることができる治療法を開発する機会があります。
当レポートでは、主要7ヶ国における進行性核上性麻痺市場について調査し、市場の概要とともに、疫学、患者動向、新たな治療法、2032年までの市場規模予測、および医療のアンメットニーズなどを提供しています。
目次
第1章 重要な洞察
第2章 レポートのイントロダクション
第3章 進行性核上性麻痺市場概要
- 2019年の進行性核上性麻痺の市場シェア(%)分布
- 2032年の進行性核上性麻痺の市場シェア(%)分布
第4章 進行性核上性麻痺の調査手法疫学と市場
第5章 進行性核上性麻痺のエグゼクティブサマリー
第6章 主要な出来事
第7章 疾患の背景と概要
- イントロダクション
- 兆候と症状
- 進行性核上性麻痺の表現型
- 歴史
- 遺伝
- 関連する危険因子
- 病態生理学
- 進行性核上性麻痺に関連する異常
- 診断
- タウオパチーのバイオマーカーとしてのPETイメージング
- 治療
第8章 患者動向
第9章 疫学と患者数
- 主な調査結果
- 仮定と根拠:主要7ヶ国
- 主要7ヶ国における進行性核上性麻痺の有病者数の合計
- 主要7ヶ国における進行性核上性麻痺の診断済み有病症例の総数
- 米国
- 欧州4ヶ国と英国
- 日本
第10章 新興薬剤
第11章 進行性核上性麻痺:市場分析
- 主な調査結果
- 主要な市場予測の前提条件
- 市場の見通し
- コンジョイント分析
- 主要7ヶ国における進行性核上性麻痺の総市場規模
- 主要7ヶ国における進行性核上性麻痺の総市場規模、治療法別
- 米国における進行性核上性麻痺の市場規模
- 欧州4ヶ国および英国における進行性核上性麻痺の市場規模
- 日本における進行性核上性麻痺の市場規模
第12章 主要なオピニオンリーダーの見解
第13章 SWOT
第14章 アンメットニーズ
第15章 市場アクセスと償還
- 米国
- 欧州4ヶ国と英国
- 日本
第16章 付録
第17章 DelveInsightのサービス内容
第18章 免責事項
第19章 DelveInsightについて
List of Tables
- Table 1: Summary of Market and Epidemiology (2019-2032)
- Table 2: Key Events for Progressive Supranuclear Palsy
- Table 3: Clinical Features for NINDS-SPSP Diagnostic Criteria
- Table 4: MDS-PSP Clinical Diagnostic Criteria
- Table 5: Total Prevalent Cases of Progressive Supranuclear Palsy in the 7MM (2019-2032)
- Table 6: Total Diagnosed Prevalent Cases of Progressive Supranuclear Palsy in the 7MM (2019-2032)
- Table 7: Total Prevalent Cases of Progressive Supranuclear Palsy in the US (2019-2032)
- Table 8: Total Diagnosed Prevalent Cases of Progressive Supranuclear Palsy in the US (2019-2032)
- Table 9: Gender-specific Cases of Progressive Supranuclear Palsy in the US (2019-2032)
- Table 10: Phenotype-specific Cases of Progressive Supranuclear Palsy in the US (2019-2032)
- Table 11: Comorbidities Associated With Progressive Supranuclear Palsy in the US (2019-2032)
- Table 12: Total Prevalent Cases of Progressive Supranuclear Palsy in EU4 and the UK (2019-2032)
- Table 13: Total Diagnosed Prevalent Cases of Progressive Supranuclear Palsy in EU4 and the UK (2019-2032)
- Table 14: Gender-specific Cases of Progressive Supranuclear Palsy in EU4 and the UK (2019-2032)
- Table 15: Phenotype-specific Cases of Progressive Supranuclear Palsy in EU4 and the UK (2019-2032)
- Table 16: Comorbidity Associated With Progressive Supranuclear Palsy in EU4 and the UK (2019-2032)
- Table 17: Total Prevalent Cases of Progressive Supranuclear Palsy in Japan (2019-2032)
- Table 18: Total Diagnosed Prevalent Cases of Progressive Supranuclear Palsy in Japan (2019-2032)
- Table 19: Gender-specific Cases of Progressive Supranuclear Palsy in Japan (2019-2032)
- Table 20: Phenotype-specific Cases of Progressive Supranuclear Palsy in Japan (2019-2032)
- Table 21: Comorbidities Associated With Progressive Supranuclear Palsy in Japan (2019-2032)
- Table 22: Comparison of Emerging Drugs for Treatment
- Table 23: BRAVYL (fasudil), Clinical Trial Description, 2023
- Table 24: AZP2006 (ezeprogind), Clinical Trial Description, 2023
- Table 25: TPN-101/OBP-601 (censavudine), Clinical Trial Description, 2023
- Table 26: NIO752, Clinical Trial Description, 2023
- Table 27: UCB0107 (bepranemab), Clinical Trial Description, 2023
- Table 28: Key Market Forecast Assumptions for BRAVYL (fasudil)
- Table 29: Key Market Forecast Assumptions for AZP2006 (ezeprogind)
- Table 30: Key Market Forecast Assumptions for TPN-101/OBP-601 (censavudine)
- Table 31: Total Market Size of Progressive Supranuclear Palsy in the 7MM, in USD million (2019-2032)
- Table 32: Total Market Size of Progressive Supranuclear Palsy by Therapies in the 7MM, in USD million (2019-2032)
- Table 33: Total Market Size of Progressive Supranuclear Palsy in the US, in USD million (2019-2032)
- Table 34: Market Size of Progressive Supranuclear Palsy by Therapies in the US, in USD million (2019-2032)
- Table 35: Total Market Size of Progressive Supranuclear Palsy in EU4 and the UK, in USD million (2019-2032)
- Table 36: Market Size of Progressive Supranuclear Palsy by Therapies in EU4 and the UK, in USD million (2019-2032)
- Table 37: Total Market Size of Progressive Supranuclear Palsy in Japan, in USD million (2019-2032)
- Table 38: Market Size of Progressive Supranuclear Palsy by Therapies in Japan, in USD million (2019-2032)
List of Figures
- Figure 1: Tau Isoforms and Conformations
- Figure 2: Tau Hyperphosphorylation and Aggregation
- Figure 3: Symptomatic Treatment for Progressive Supranuclear Palsy
- Figure 4: Potential Therapeutic Targets for Progressive Supranuclear Palsy
- Figure 5: Progressive Supranuclear Palsy Patient Journey
- Figure 6: Total Prevalent Cases of Progressive Supranuclear Palsy in the 7MM (2019-2032)
- Figure 7: Total Diagnosed Prevalent Cases of Progressive Supranuclear Palsy in the 7MM (2019-2032)
- Figure 8: Total Prevalent Cases of Progressive Supranuclear Palsy in the US (2019-2032)
- Figure 9: Total Diagnosed Prevalent Cases of Progressive Supranuclear Palsy in the US (2019-2032)
- Figure 10: Gender-specific Cases of Progressive Supranuclear Palsy in the US (2019-2032)
- Figure 11: Phenotype-specific Cases of Progressive Supranuclear Palsy in the US (2019-2032)
- Figure 12: Comorbidities Associated With Progressive Supranuclear Palsy in the US (2019-2032)
- Figure 13: Total Prevalent Cases of Progressive Supranuclear Palsy in EU4 and the UK (2019-2032)
- Figure 14: Total Diagnosed Prevalent Cases of Progressive Supranuclear Palsy in EU4 and the UK (2019-2032)
- Figure 15: Gender-specific Cases of Progressive Supranuclear Palsy in EU4 and the UK (2019-2032)
- Figure 16: Phenotype-specific Cases of Progressive Supranuclear Palsy in EU4 and the UK (2019-2032)
- Figure 17: Comorbidities Associated With Progressive Supranuclear Palsy in EU4 and the UK (2019-2032)
- Figure 18: Total Prevalent Cases of Progressive Supranuclear Palsy in Japan (2019-2032)
- Figure 19: Total Diagnosed Prevalent Cases of Progressive Supranuclear Palsy in Japan (2019-2032)
- Figure 20: Gender-specific Cases of Progressive Supranuclear Palsy in Japan (2019-2032)
- Figure 21: Phenotype-specific Cases of Progressive Supranuclear Palsy in Japan (2019-2032)
- Figure 22: Comorbidity Associated With Progressive Supranuclear Palsy in Japan (2019-2032)
- Figure 23: Total Market Size of Progressive Supranuclear Palsy in the 7MM, in USD million (2019-2032)
- Figure 24: Total Market Size of Progressive Supranuclear Palsy by Therapies in the 7MM, in USD million (2019-2032)
- Figure 25: Total Market Size of Progressive Supranuclear Palsy in the US, in USD million (2019-2032)
- Figure 26: Market Size of Progressive Supranuclear Palsy by Therapies in the US, in USD million (2019-2032)
- Figure 27: Total Market Size of Progressive Supranuclear Palsy in EU4 and the UK, in USD million (2019-2032)
- Figure 28: Market Size of Progressive Supranuclear Palsy by Therapies in EU4 and the UK, in USD million (2019-2032)
- Figure 29: Total Market Size of Progressive Supranuclear Palsy in Japan, in USD million (2019-2032)
- Figure 30: Market Size of Progressive Supranuclear Palsy by Therapies in Japan, in USD million (2019-2032)
- Figure 31: SWOT Analysis of Progressive Supranuclear Palsy
- Figure 32: Unmet Needs
- Figure 33: Health Technology Assessment
- Figure 34: Reimbursement Process in Germany
- Figure 35: Reimbursement Process in France
- Figure 36: Reimbursement Process in Italy
- Figure 37: Reimbursement Process in Spain
- Figure 38: Reimbursement Process in the United Kingdom
- Figure 39: Reimbursement Process in Japan
Key Highlights:
- The prevalence of progressive supranuclear palsy has been increasing in the US due to increasing population and awareness.
- Increased awareness and a more in-depth understanding of the disease pathophysiology have led to the development of enhanced diagnostic criteria, improving diagnosis.
- The current treatment regime is symptomatic. Antiparkinsonian medications such as levodopa are widely used to treat rigidity, tremors, and bradykinesia symptoms. Further, antipsychotics, antidepressants, and other agents help control various symptoms.
- Non-pharmacological therapies such as occupational, physical, and cognitive rehabilitation improve patients' mobility, balance, daily life activities, speech, and other symptoms.
- The major concern in understanding the market for progressive supranuclear palsy is a lack of recent epidemiology, phenotype-specific studies, and a paucity of evidence to validate interventions used in daily managing progressive supranuclear palsy. No consensus or clinical guidelines for progressive supranuclear palsy are available in the US, EU4 and the UK, and Japan.
- Recent progress in understanding the mechanisms underlying tau-induced neurodegeneration revealed that the most promising approaches aim to prevent the abnormal aggregation of the microtubule-associated protein tau.
- In 2022, the market size of progressive supranuclear palsy was highest in the US among the 7MM countries, accounting for approximately USD 3.5 million. It is expected to increase by 2032.
- Although levodopa, amantadine, amitriptyline, zolpidem, and botulinum are widely used, they have limited efficacy and are associated with side effects. Further, an attempt to treat one symptom may worsen the others.
- Emerging therapies BRAVYL (fasudil), AZP2006 (ezeprogind), and TPN-101/OBP-601 (censavudine) have the potential to create a positive shift in the market size of progressive supranuclear palsy.
- BRAVYL (fasudil), a small molecule that can inhibit Rho-kinase, is being developed by Woolsey Pharmaceuticals and Asahi Kasei Pharma, targeting progressive supranuclear palsy patients with Richardson syndrome.
- AZP2006, developed by AlzProtect, blocks tau phosphorylation by stabilizing the prosaposin-progranulin complex and can potentially be a disease-modifying therapy for progressive supranuclear palsy.
- The development of biological therapies for progressive supranuclear palsy requires additional pathogenetic studies. Further, with the lack of approved therapy and limited effectiveness of current treatment, there are opportunities for pharma players to develop therapies that are curative and can effectively reduce the symptoms and slow the progression of progressive supranuclear palsy.
DelveInsight's "Progressive Supranuclear Palsy - Market Insights, Epidemiology, and Market Forecast - 2032" report delivers an in-depth understanding of the progressive supranuclear palsy historical and forecasted epidemiology as well as the market trends in the United States, EU4 (Germany, France, Italy, and Spain) and the United Kingdom, and Japan.
The progressive supranuclear palsy market report provides current treatment practices, emerging drugs, market share of individual therapies, and current and forecasted the 7MM progressive supranuclear palsy market size from 2019 to 2032. The report also covers current progressive supranuclear palsy treatment practices/algorithms and unmet medical needs to curate the best opportunities and assess the market's potential.
Geography Covered:
- The United States
- EU4 (Germany, France, Italy, and Spain) and the United Kingdom
- Japan
Study Period: 2019-2032.
Progressive Supranuclear Palsy Understanding and Treatment Algorithm
Progressive Supranuclear Palsy Overview
Progressive supranuclear palsy (PSP), also known as Steele-Richardson-Olszewski and Parkinson-plus syndrome, is a rare neurodegenerative disorder and the most common atypical Parkinsonism. It belongs to a group of diseases called tauopathies. The exact cause of progressive supranuclear palsy is unknown. However, it is believed to involve the abnormal accumulation of tau protein in certain areas of the brain, particularly the basal ganglia and brainstem, forming abnormal tangles, leading to the degeneration of brain cells.
It typically begins around the age of 60, and the symptoms gradually worsen over time. It is often characterized by the gradual deterioration of movement, balance, and coordination, dysphagia, impairment of cognitive functions, the control of eye movements, and others.
Progressive supranuclear palsy encompasses several phenotype variants; Richardson's syndrome is the most common phenotype of progressive supranuclear palsy, accounting for more than 50% of cases. It is characterized by a combination of Parkinsonism (similar to Parkinson's disease) and vertical gaze palsy (difficulty moving the eyes vertically). Other features may include postural instability, falls, early speech and swallowing difficulties, cognitive impairment, and behavioral changes. Progressive supranuclear palsy-Parkinsonism phenotype primarily presents with Parkinsonian features similar to Parkinson's disease. Patients exhibit bradykinesia (slowness of movement), rigidity, and tremor.
Other phenotypes include progressive supranuclear palsy with the predominant frontal presentation, corticobasal syndrome, and pure akinesia with gait freezing. The most frequent comorbidities observed in progressive supranuclear palsy patients are nervous system disorders, connective tissue diseases, eye disorders, and non-traumatic joint disorders.
Progressive supranuclear palsy is a progressive and ultimately debilitating disease. The progression of symptoms varies among individuals but generally worsens over time. The average life expectancy after progressive supranuclear palsy diagnosis is typically around 5-10 years, although survival can vary depending on individual factors and the age at onset.
Progressive Supranuclear Palsy Diagnosis
Diagnosing progressive supranuclear palsy is challenging because its symptoms overlap with other movement disorders like Parkinson's disease, frontotemporal dementia, and corticobasal degeneration. There is no specific test for progressive supranuclear palsy, and diagnosis is usually based on the presence of characteristic symptoms, clinical examination, and ruling out other possible causes. Brain imaging, such as MRI, may support the diagnosis by showing specific patterns of brain atrophy.
Currently, two diagnostic criteria known as the MDS-PSP and NINDS-SPSP are widely used to diagnose progressive supranuclear palsy. MDS-PSP criteria include specific clinical and supportive features that help differentiate progressive supranuclear palsy from other disorders and establish an early diagnosis.
Further details related to country-based variations are provided in the report…
Progressive Supranuclear Palsy Treatment
The current treatment landscape lacks approved products and clinical guidelines. The treatment focuses on managing symptoms, providing supportive care, and enhancing the quality of life. Several medications like levodopa, dopamine agonists, antidepressants, and others used for treating Parkinson's disease and other related disorders may help improve the symptoms of progressive supranuclear palsy.
Levodopa is commonly used to manage the Parkinsonian symptoms of progressive supranuclear palsy, such as bradykinesia, rigidity, and tremors. However, the response is often less favorable, and the benefits may be limited or short-lived. Further, dopamine agonists, such as amantadine and ropinirole, may be prescribed in conjunction with or as an alternative to levodopa.
Selective serotonin reuptake inhibitors (SSRIs) or other antidepressant medications manage depression and mood-related symptoms associated with progressive supranuclear palsy. Several other medications, including hypnotics and anxiolytics, botulinum toxin, anti-inflammatories, and antiepileptics, are also used for different symptoms of progressive supranuclear palsy. Further, physical therapy for mobility and balance, occupational therapy to assist with daily activities, and speech therapy to address speech and swallowing difficulties are also included.
Progressive Supranuclear Palsy Epidemiology
As the market is derived using a patient-based model, the progressive supranuclear palsy epidemiology chapter in the report provides historical as well as forecasted epidemiology segmented by prevalent cases of progressive supranuclear palsy, diagnosed prevalent cases of progressive supranuclear palsy, gender-specific cases of progressive supranuclear palsy, phenotype-specific cases of progressive supranuclear palsy, and comorbidities associated with progressive supranuclear palsy in the 7MM covering the United States, EU4 countries (Germany, France, Italy, and Spain) and the United Kingdom, and Japan from 2019 to 2032.
- In 2022, the total prevalent cases of progressive supranuclear palsy were estimated to be approximately 80,706 cases in the 7MM. These cases are projected to increase during the forecast period.
- In the US, there were approximately 21,087 prevalent cases of progressive supranuclear palsy in 2022. These cases are expected to increase by 2032.
- In 2022, among the 7MM, Japan accounted for the highest prevalent cases of progressive supranuclear palsy, contributing nearly 29%, while Spain accounted for the least with nearly 7% of the total prevalent cases.
- Of the total prevalent cases of progressive supranuclear palsy in the US, around 27% were diagnosed in 2022. The cases are expected to increase at a CAGR of 1.1%.
- Among the 7MM, the US accounted for the largest diagnosed prevalent progressive supranuclear palsy population contributing nearly 33% to the total diagnosed population in 2022. In contrast, Spain accounted for the least, with approximately 5% share of the total population in 2022.
- Among EU4 and the UK, Germany accounted for the highest diagnosed prevalence of progressive supranuclear palsy with approximately 1,646 cases, while with approximately 941 cases, Spain reported the least.
- According to estimates based on DelveInsight's epidemiology model, progressive supranuclear palsy affects more males than females. In EU4 and the UK, there were around 3,655 males and 2,536 females diagnosed with progressive supranuclear palsy in 2022, and the cases are expected to increase during the forecast period.
- According to the epidemiology model, in 2022, the phenotype-specific cases, progressive supranuclear palsy with Richardson's syndrome, progressive supranuclear palsy with Parkinsonism, and progressive supranuclear palsy with other phenotypes accounted for approximately 50%, 33%, and 17%, respectively, in EU4 and the UK.
- In Japan, among the comorbidities associated with progressive supranuclear palsy, nervous system disorders had the highest cases (approximately 5,077), while non-traumatic joint disorders had the least cases (approximately 4,457) in 2022.
Progressive Supranuclear Palsy Drug Chapters
The drug chapter segment of the progressive supranuclear palsy report encloses a detailed analysis of progressive supranuclear palsy, currently used drugs, and mid-stage (Phase II and Phase I) pipeline drugs. It also helps understand the progressive supranuclear palsy clinical trial details, expressive pharmacological action, agreements and collaborations, approval and patent details, advantages and disadvantages of each included drug, and the latest news and press releases.
Emerging Drugs
BRAVYL (fasudil): Woolsey Pharmaceutical/Asahi Kasei Pharma
BRAVYL is a small molecule, repurposed, oral version of fasudil, a potent inhibitor of Rho-kinases (ROCK) that are elevated in progressive supranuclear palsy and are involved in the accumulation of tau aggregates that lead to progressive supranuclear palsy.
ROCK is an enzyme important in mediating vasoconstriction and vascular remodeling in the pathogenesis of pulmonary hypertension. ROCK induces vasoconstriction by phosphorylating the myosin-binding subunit of myosin light chain (MLC) phosphatase, thus decreasing MLC phosphatase activity and enhancing vascular smooth muscle contraction.
ERIL, an IV formulation of fasudil, is approved in Japan for treating cerebral vasospasm and delayed cerebral ischemic symptoms after subarachnoid hemorrhage.
Embark Healthcare launched Woolsey Pharmaceutical, its portfolio company, toward the end of 2019. BRAVYL (fasudil) is currently being tested in Phase IIa clinical studies for progressive supranuclear palsy - Richardson's syndrome and CBS.
AZP2006 (ezeprogind): AlzProtect
AZP2006 (ezeprogind) is an orally available small molecule with a novel mechanism of action and effects. It involves the action of a neurotrophic factor, which combines reinforced neuroprotective efficacy and anti-neuroinflammation activity.
AZP2006 blocks tau phosphorylation by stabilizing the prosaposin-progranulin complex. The stabilization prevents progranulin cleavage and increases progranulin secretion, further protecting the central neurons in progressive supranuclear palsy patients. Moreover, it also inhibits microglial activation and pro-inflammatory cytokine production.
AZP2006 has exceptional therapeutic potential to treat the physiopathological causes of progressive supranuclear palsy and other related tauopathies such as Alzheimer's disease. The company has completed the Phase IIa study to treat progressive supranuclear palsy and showed positive and promising results.
Alzprotect has received approval from the French National Agency for the Safety of Medicines and Health Products (ANSM) and the Ethical Review Board to extend the trial as an open-label for an additional 6 months for all eligible patients. Further, the company plans to initiate a larger pivotal Phase IIb/III by early 2024 in Europe and the US.
Note: Detailed emerging therapies assessment will be provided in the final report.
Drug Class Insights
Progressive supranuclear palsy is a rare neurodegenerative disorder resulting from damage to nerve cell clusters called nuclei (supranuclear) in the brain, deteriorating balance while walking, speech difficulties, trouble in swallowing, changes in mood and behavior, and cognitive impairment. Progressive supranuclear palsy is characterized pathologically by four-repeat (4R) Tau deposition in various cell types and anatomical regions. The mean onset age is 63 years, and the mean survival is 6-9 years. No specific laboratory tests or imaging approaches exist to diagnose progressive supranuclear palsy definitively. The disease is often difficult to diagnose because its symptoms can be similar to those of other movement disorders and because some of the most characteristic symptoms may develop late or not. At present, therapeutic options for progressive supranuclear palsy are symptomatic and insufficient. Progressive supranuclear palsy symptoms usually do not respond to medications. Drugs prescribed to treat Parkinson's disease, such as levodopa, rarely provide additional benefits.
Several drug classes are being used off-label to manage various symptoms experienced by progressive supranuclear palsy patients. These include dopamine agonists, antidepressants, anti-inflammatories, antipsychotics, acetylcholinesterase inhibitors, hypnotics, and anxiolytics.
Selective serotonin reuptake inhibitors (SSRIs) or other antidepressant medications manage depression and mood-related symptoms associated with progressive supranuclear palsy. Several other medications, including hypnotics and anxiolytics, botulinum toxin, anti-inflammatories, and antiepileptics, are also used for different symptoms of progressive supranuclear palsy. Further, physical therapy for mobility and balance, occupational therapy to assist with daily activities, and speech therapy to address speech and swallowing difficulties are also included.
Further, muscle relaxants such as baclofen are primarily used in spasticity treatment. Although no controlled studies have evaluated their efficacy specifically for dystonia, they may be helpful in certain cases of progressive supranuclear palsy. Dosing starts at 5 mg once daily and generally titrates to 10 mg TID. However, baclofen may cause weakness, orthostatic hypotension, sedation, or confusion in patients with marginal gait function. Moreover, treatment with benzodiazepines (most commonly clonazepam starting at 0.25 mg daily, titrating to efficacy, toxicity, or 3 mg/day) may be attempted for dystonia in progressive supranuclear palsy.
Progressive Supranuclear Palsy Market Outlook
Progressive supranuclear palsy, an atypical Parkinsonian condition, is characterized by a range of motor and behavioral syndromes associated with 4-repeat tau neuropathology. Early falls, supranuclear gaze palsy, axial and limb rigidity, and motor eyelid disorders are common disease symptoms. The typical age of disease onset is in the fifth to seventh decade of life. Several phenotypes of progressive supranuclear palsy have been defined over the past few years; PSP-RS, PSP-P, PSP-CBS, and PSP-PGF are a few common among them. Further, due to its complex presentation of phenotype and overlapping symptoms with neurodegenerative disorders, missed and delayed diagnosis is common and an obstacle in managing progressive supranuclear palsy. Similar symptoms in other neurological disorders complicate diagnosis and make differential diagnosis an imperative factor.
Nevertheless, progress has been made in terms of diagnosis; in 2017, the new MDS diagnostic criteria for progressive supranuclear palsy recognized early, suggestive forms of progressive supranuclear palsy and operationalized diagnosis of non-Richardson's progressive supranuclear palsy phenotypes. Earlier, the 1996 NINDS-SPSP criteria did not allow the recognition of variable phenotypic progressive supranuclear palsy presentations and early clinical manifestations.
The current treatment landscape lacks disease-modifying therapies and treatment guidelines. While symptomatic treatments are widely used, they only show mild to moderate efficacy. Although one medication does not treat all the symptoms of progressive supranuclear palsy, specific treatments may be helpful. To improve balance and the flexibility of the muscles, levodopa and amantadine, an antiparkinson medication, are widely used. However, Parkinson's medications are ineffective; only 20-30% respond well to levodopa. Besides antiparkinson drugs, antidepressants, anti-inflammatories, and other agents are used to relieve associated symptoms and conditions. In addition, eye drops are recommended for dry eyes to prevent exposure to keratitis, and botulinum toxin injections may reduce blepharospasm, dystonia, and retrocollis.
Levodopa (with a peripheral decarboxylase inhibitor such as carbidopa or benserazide) is the principal pharmacologic agent used for dopaminergic replacement therapy in progressive supranuclear palsy, with a more robust response generally seen in the PSP-P subtype. Bradykinesia, rigidity, and tremor seen in any phenotype of progressive supranuclear palsy may respond as well as in PSP-P, but postural instability is unlikely to respond. Based on retrospective studies, 20-30% of pathologically confirmed and 20-40% of clinically diagnosed progressive supranuclear palsy patients reported a beneficial response to levodopa alone or combined with another dopaminergic agent (such as amantadine). These responses generally occur early in the disease course and persist for only a few months. Furthermore, levodopa treatments are attempted only in patients with rigidity, bradykinesia, or tremor-impaired daily activities.
For Parkinsonism, a levodopa trial of up to 1,200 mg/d (up to 300 mg per dose if it can be tolerated) for 1 month is recommended to determine responsiveness. Dopamine agonists, however, often provide minimal benefit. In some cases, donepezil is also found to improve cognitive function in progressive supranuclear palsy; however, it has been reported to worsen motor symptoms. Despite several retrospective reports of clinically meaningful improvement in motor function on low doses of amitriptyline, these medications in progressive supranuclear palsy are not recommended due to their anticholinergic side effects and risk of exacerbating falling.
The current market has been covered by the symptomatic treatment that includes different pharmacological agents used across the 7MM, which presents minor variations in the overall prescription pattern. Levodopa, amantadine, amitriptyline, zolpidem, and botulinum toxin A are the major drugs considered for symptomatic treatment in the forecast model.
Key players Woolsey Pharmaceutical/Asahi Kasei Pharma (BRAVYL [fasudil]), AlzProtect (AZP2006 [ezeprogind), Transposon Therapeutics/Oncolys Biopharma (TPN-101/OBP-601 [censavudine]), and others are evaluating their lead candidates in different stages of clinical development. They aim to investigate their products for the treatment of progressive supranuclear palsy.
- The total market size of progressive supranuclear palsy in the 7MM was approximately USD 8.3 million in 2022 and is projected to increase during the forecast period (2023-2032).
- The market size of progressive supranuclear palsy in the US will increase at a CAGR of 31.2% due to increasing awareness of the disease and the launch of the emerging therapy.
- In 2022, the symptomatic treatment generated the maximum revenue in the US for progressive supranuclear palsy, which is anticipated to decrease during the forecast period owing to the launch of emerging therapies.
- Among EU4 and the UK countries, Germany accounted for the maximum market size of progressive supranuclear palsy in 2022, while Spain occupied the bottom of the ladder.
- Japan accounted for the second largest market of progressive supranuclear palsy among the 7MM, with a revenue of approximately USD 2.3 million in 2022, expected to increase during the forecast period.
- In the US, BRAVYL (fasudil), AZP2006 (ezeprogind), and TPN-101/OBP-601 (censavudine) are all expected to enter by 2027. However, AZP2006 (ezeprogind) would have medium uptake, while the other two drugs would have a slow-medium uptake.
- AlzProtect's AZP2006 (ezeprogind) is projected to generate a revenue of approximately USD 0.9 million by 2027 in the US and is expected to attain a peak in its seventh year.
Progressive Supranuclear Palsy Drugs Uptake
This section focuses on the uptake rate of potential drugs expected to be launched in the market during 2019-2032. For example, Woolsey Pharmaceutical/Asahi Kasei Pharma's BRAVYL (fasudil), a potent inhibitor of Rho-kinases (ROCK), with an anticipated entry by 2027 in the US, is predicted to have a slow-medium uptake during the forecast period.
Further detailed analysis of emerging therapies drug uptake in the report…
Progressive Supranuclear Palsy Pipeline Development Activities
The report provides insights into therapeutic candidates in Phase II and Phase I. It also analyzes key players involved in developing targeted therapeutics.
Pipeline Development Activities
The report covers information on collaborations, acquisitions and mergers, licensing, and patent details for emerging therapies for progressive supranuclear palsy.
KOL Views:
To keep up with current market trends, we take KOLs and SMEs' opinions working in the domain through primary research to fill the data gaps and validate our secondary research. Industry Experts contacted for insights on the progressive supranuclear palsy evolving treatment landscape, patient reliance on conventional therapies, patient therapy switching acceptability, and drug uptake, along with challenges related to accessibility, including Medical/scientific writers, Medical Professionals, Professors, Directors, and Others.
DelveInsight's analysts connected with 50+ KOLs to gather insights; however, interviews were conducted with 15+ KOLs in the 7MM. Centers like Southern Illinois University School of Medicine, the University of Washington, the University Hospital of Tours, Navarra Institute for Health Research, the University of Tokyo School of Medicine, and the National Center of Neurology and Psychiatry were contacted. Their opinion helps understand and validate current and emerging therapy treatment patterns or progressive supranuclear palsy market trends. This will support the clients in potential upcoming novel treatments by identifying the overall scenario of the market and the unmet needs.
Physician's View:
According to our primary research analysis, though there is a lack of treatment guidelines, levodopa and amantadine are used to alleviate rigidity and bradykinesia, while amitriptyline and zolpidem were mostly used to manage depression and dystonia, respectively. This botulinum toxin also manages dystonia, including blepharospasm and sialorrhea.
Qualitative Analysis
We perform Qualitative and market Intelligence analysis using various approaches, such as SWOT and Conjoint Analysis. In the SWOT analysis, strengths, weaknesses, opportunities, and threats in terms of disease diagnosis, patient awareness, patient burden, competitive landscape, cost-effectiveness, and geographical accessibility of therapies are provided. These pointers are based on the Analyst's discretion and assessment of the patient burden, cost analysis, and existing and evolving treatment landscape.
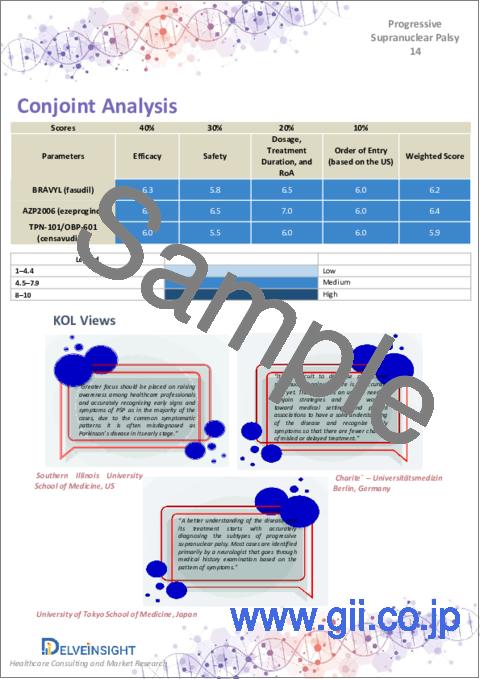
Conjoint Analysis analyzes multiple emerging therapies based on relevant attributes such as safety, efficacy, frequency of administration, route of administration, and order of entry. Scoring is given based on these parameters to analyze the effectiveness of therapy.
The therapies' safety is evaluated wherein the acceptability, tolerability, and adverse events are majorly observed. It sets a clear understanding of the side effects posed by the drug in the trials. In addition, the scoring is also based on the route of administration, order of entry and designation, probability of success, and the addressable patient pool for each therapy. According to these parameters, the final weightage score and the ranking of the emerging therapies are decided.
Market Access and Reimbursement
Reimbursement of rare disease therapies can be limited due to lack of supporting policies and funding, challenges of high prices, lack of specific approaches to evaluating rare disease drugs given limited evidence, and payers' concerns about budget impact. The high cost of rare disease drugs usually has a limited effect on the budget due to the small number of eligible patients being prescribed the drug. The US FDA has approved several rare disease therapies in recent years. From a patient perspective, health insurance and payer coverage guidelines surrounding rare disease treatments restrict broad access to these treatments, leaving only a small number of patients who can bypass insurance and pay for products independently.
The report provides detailed insights on the country-wise accessibility and reimbursement scenarios, cost-effectiveness scenarios, programs making accessibility easier and out-of-pocket costs more affordable, insights on patients insured under federal or state government prescription drug programs, etc.
Scope of the Report:
- The report covers a segment of key events, an executive summary, descriptive overview of progressive supranuclear palsy, explaining its causes, signs and symptoms, pathogenesis, and currently available therapies.
- Comprehensive insight into the epidemiology segments and forecasts, the future growth potential of diagnosis rate, disease progression, and treatment guidelines have been provided.
- Additionally, an all-inclusive account of the current and emerging therapies and the elaborative profiles of late-stage and prominent therapies will impact the current treatment landscape.
- A detailed review of the progressive supranuclear palsy market, historical and forecasted market size, market share by therapies, detailed assumptions, and rationale behind our approach is included in the report, covering the 7MM drug outreach.
- The report provides an edge while developing business strategies, by understanding trends, through SWOT analysis, expert insights/KOL views, patient journey, and treatment preferences that help shape and drive the 7MM progressive supranuclear palsy market.
Progressive Supranuclear Palsy Report Insights
- Patient Population
- Therapeutic Approaches
- Progressive Supranuclear Palsy Pipeline Analysis
- Progressive Supranuclear Palsy Market Size and Trends
- Existing and Future Market Opportunity
Progressive Supranuclear Palsy Report Key Strengths
- 10 years Forecast
- The 7MM Coverage
- Progressive Supranuclear Palsy Epidemiology Segmentation
- Key Cross Competition
- Conjoint Analysis
- Drugs Uptake and Key Market Forecast Assumptions
Progressive Supranuclear Palsy Report Assessment
- Current Treatment Practices
- Unmet Needs
- Pipeline Product Profiles
- Market Attractiveness
- Qualitative Analysis (SWOT and Conjoint Analysis)
Key Questions:
Market Insights
- What was the total market size of progressive supranuclear palsy, the market size of progressive supranuclear palsy by therapies, market share (%) distribution in 2019, and what would it look like by 2032? What are the contributing factors for this growth?
- How will AZP2006 (ezeprogind) and TPN-101/OBP-601 (censavudine) affect the treatment paradigm of progressive supranuclear palsy?
- How will BRAVYL (fasudil) compete with other off-label symptomatic treatments?
- Which drug is going to be the largest contributor by 2032?
- What are the pricing variations among different geographies for off-label therapies?
- How would future opportunities affect the market dynamics and subsequent analysis of the associated trends?
Epidemiology Insights
- What are the disease risk, burdens, and unmet needs of progressive supranuclear palsy? What will be the growth opportunities across the 7MM with respect to the patient population pertaining to progressive supranuclear palsy?
- What is the historical and forecasted patient pool of progressive supranuclear palsy in the United States, EU4 (Germany, France, Italy, and Spain) and the United Kingdom, and Japan?
- Out of the countries mentioned above, which country would have the highest diagnosed progressive supranuclear palsy diagnosed prevalent population during the forecast period (2023-2032)?
- What factors are contributing to the growth of progressive supranuclear palsy cases?
Current Treatment Scenario, Marketed Drugs, and Emerging Therapies
- What are the current options to treat progressive supranuclear palsy?
- How many companies are developing therapies for the treatment of progressive supranuclear palsy?
- How many emerging therapies are in the mid-stage and early stage of development for treating progressive supranuclear palsy?
- What are the recent novel therapies, targets, mechanisms of action, and technologies developed to overcome the limitation of existing therapies?
- What is the cost burden of current treatment on the patient?
- Patient acceptability in terms of preferred treatment options as per real-world scenarios?
- What are the accessibility issues of approved therapy in the US?
- What is the 7MM historical and forecasted market of progressive supranuclear palsy?
Reasons to Buy:
- The report will help develop business strategies by understanding the latest trends and changing treatment dynamics driving the progressive supranuclear palsy market.
- Insights on patient burden/disease prevalence, evolution in diagnosis, and factors contributing to the change in the epidemiology of the disease during the forecast years.
- Understand the existing market opportunity in varying geographies and the growth potential over the coming years.
- Distribution of historical and current patient share based on real-world prescription data in the US, EU4 (Germany, France, Italy, and Spain) and the United Kingdom, and Japan.
- Identifying strong upcoming players in the market will help devise strategies to help get ahead of competitors.
- Detailed analysis and ranking of class-wise potential current and emerging therapies under the conjoint analysis section to provide visibility around leading classes.
- To understand Key Opinion Leaders' perspectives around the accessibility, acceptability, and compliance-related challenges of existing treatment to overcome barriers in the future.
- Detailed insights on the unmet need of the existing market so that the upcoming players can strengthen their development and launch strategy.
Table of Contents
1. Key Insights
2. Report Introduction
3. Progressive Supranuclear Palsy Market Overview at a Glance
- 3.1. Market Share (%) Distribution of Progressive Supranuclear Palsy in 2019
- 3.2. Market Share (%) Distribution of Progressive Supranuclear Palsy in 2032
4. Methodology of progressive supranuclear palsy Epidemiology and Market
5. Executive Summary of progressive supranuclear palsy
6. Key Events
7. Disease Background and Overview
- 7.1. Introduction
- 7.2. Signs and Symptoms
- 7.3. Phenotypes of Progressive Supranuclear Palsy
- 7.4. History
- 7.5. Genetic
- 7.6. Associated Risk Factors
- 7.7. Pathophysiology
- 7.8. progressive Supranuclear Palsy Associated Abnormalities
- 7.9. Diagnosis
- 7.10. PET Imaging as a Biomarker in Tauopathies
- 7.11. Treatment
- 7.11.1. Current Symptomatic Treatment
8. Patient Journey
9. Epidemiology and Patient Population
- 9.1. Key Findings
- 9.2. Assumptions and Rationale: The 7MM
- 9.2.1. Prevalent Cases of Progressive Supranuclear Palsy
- 9.2.2. Diagnosed Prevalent Cases of Progressive Supranuclear Palsy
- 9.2.3. Gender-specific Cases of Progressive Supranuclear Palsy
- 9.2.4. Phenotype-specific Cases of Progressive Supranuclear Palsy
- 9.2.5. Comorbidities Associated With Progressive Supranuclear Palsy
- 9.3. Total Prevalent Cases of Progressive Supranuclear Palsy in the 7MM
- 9.4. Total Diagnosed Prevalent Cases of Progressive Supranuclear Palsy in the 7MM
- 9.5. The US
- 9.5.1. Total Prevalent Cases of Progressive Supranuclear Palsy in the US
- 9.5.2. Total Diagnosed Prevalent Cases of Progressive Supranuclear Palsy in the US
- 9.5.3. Gender-specific Cases of Progressive Supranuclear Palsy in the US
- 9.5.4. Phenotype-specific Cases of Progressive Supranuclear Palsy in the US
- 9.5.5. Comorbidities Associated With Progressive Supranuclear Palsy in the US
- 9.6. EU4 and the UK
- 9.6.1. Total Prevalent Cases of Progressive Supranuclear Palsy in EU4 and the UK
- 9.6.2. Total Diagnosed Prevalent Cases of Progressive Supranuclear Palsy in EU4 and the UK
- 9.6.3. Gender-specific Cases of Progressive supranuclear palsy in EU4 and the UK
- 9.6.4. Phenotype-specific Cases of Progressive Supranuclear Palsy in EU4 and the UK
- 9.6.5. Comorbidities Associated With Progressive Supranuclear Palsy in EU4 and the UK
- 9.7. Japan
- 9.7.1. Total Prevalent Cases of Progressive Supranuclear Palsy in Japan
- 9.7.2. Total Diagnosed Prevalent Cases of Progressive Supranuclear Palsy in Japan
- 9.7.3. Gender-specific Cases of Progressive Supranuclear Palsy in Japan
- 9.7.4. Phenotype-specific Cases of Progressive Supranuclear Palsy in Japan
- 9.7.5. Comorbidities Associated With Progressive Supranuclear Palsy in Japan
10. Emerging Drugs
- 10.1. Key Cross Competition
- 10.2. BRAVYL (fasudil): Woolsey Pharmaceutical/Asahi Kasei Pharma
- 10.2.1. Product Description
- 10.2.2. Other Developmental Activity
- 10.2.3. Clinical Development
- 10.2.4. Clinical Trials Information
- 10.2.5. Product Profile
- 10.2.6. Analysts' View
- 10.3. AZP2006 (ezeprogind): AlzProtect
- 10.3.1. Product Description
- 10.3.2. Other Developmental Activity
- 10.3.3. Clinical Development
- 10.3.4. Clinical Trials Information
- 10.3.5. Safety and Efficacy
- 10.3.6. Product Profile
- 10.3.7. Analysts' Views
- 10.4. TPN-101/OBP-601 (censavudine): Transposon Therapeutics/Oncolys Biopharma
- 10.4.1. Product Description
- 10.4.2. Other Developmental Activity
- 10.4.3. Clinical Development
- 10.4.4. Clinical Trials Information
- 10.4.5. Product Profile
- 10.4.6. Analysts' Views
- 10.5. ASN90: Ferrer/Asceneuron
- 10.5.1. Product Description
- 10.5.2. Other Developmental Activity
- 10.5.3. Product Profile
- 10.6. NIO752: Novartis
- 10.6.1. Product Description
- 10.6.2. Other Developmental Activity
- 10.6.3. Clinical Development
- 10.6.4. Clinical Trials Information
- 10.6.5. Safety and Efficacy
- 10.6.6. Product Profile
- 10.7. UCB0107 (bepranemab): UCB Biopharma
- 10.7.1. Product Description
- 10.7.2. Clinical Development
- 10.7.3. Clinical Trials Information
- 10.7.4. Safety and Efficacy
- 10.7.5. Product Profile
11. Progressive Supranuclear Palsy: Market Analysis
- 11.1. Key Findings
- 11.2. Key Market Forecast Assumptions
- 11.3. Market Outlook
- 11.4. Conjoint Analysis
- 11.5. Total Market Size of Progressive Supranuclear Palsy in the 7MM
- 11.6. Total Market Size of Progressive Supranuclear Palsy by Therapies in the 7MM
- 11.7. Market Size of Progressive Supranuclear Palsy in the US
- 11.7.1. Total Market Size of Progressive Supranuclear Palsy
- 11.7.2. The Market Size of Progressive Supranuclear Palsy by Therapies
- 11.8. Market Size of Progressive Supranuclear Palsy in EU4 and the UK
- 11.8.1. Total Market Size of Progressive Supranuclear Palsy
- 11.8.2. The Market Size of Progressive Supranuclear Palsy by Therapies
- 11.9. Market Size of Progressive Supranuclear Palsy in Japan
- 11.9.1. Total Market Size of Progressive Supranuclear Palsy
- 11.9.2. The Market Size of Progressive Supranuclear Palsy by Therapies
12. Key Opinion Leaders' Views
13. SWOT
14. Unmet Needs
15. Market Access and Reimbursement
- 15.1. The United States
- 15.1.1. Centre for Medicare & Medicaid Services (CMS)
- 15.2. In EU4 and the UK
- 15.2.1. Germany
- 15.2.2. France
- 15.2.3. Italy
- 15.2.4. Spain
- 15.2.5. The United Kingdom
- 15.3. Japan
- 15.3.1. MHLW
16. Appendix
- 16.1. Bibliography
- 16.2. Acronyms and Abbreviations
- 16.3. Report Methodology