|
|
市場調査レポート
商品コード
1226675
原発性胆汁性胆管炎(PBC) - 市場考察、疫学、市場予測(2032年)Primary Biliary Cholangitis - Market Insight, Epidemiology And Market Forecast - 2032 |
||||||
|
● お客様のご希望に応じて、既存データの加工や未掲載情報(例:国別セグメント)の追加などの対応が可能です。 詳細はお問い合わせください。 |
|||||||
| 原発性胆汁性胆管炎(PBC) - 市場考察、疫学、市場予測(2032年) |
|
出版日: 受注後更新
発行: DelveInsight
ページ情報: 英文 195 Pages
納期: 2~10営業日
|
更新情報の反映作業にあたり納期もレポート毎に異なりますので、ご検討の際はお問い合わせください。
- 全表示
- 概要
- 図表
- 目次
当レポートでは、原発性胆汁性胆管炎(PBC)の主要7市場(米国・ドイツ・フランス・イタリア・スペイン・英国・日本)と中国市場について調査分析し、市場規模と予測、現在の治療法と新薬の情報などを提供しています。
目次
第1章 主要考察
第2章 レポートのイントロダクション
第3章 原発性胆汁性胆管炎(PBC)市場の概要
- PBCの市場シェア分布(2019年)
- PBCの市場シェア分布(2032年)
第4章 エグゼクティブサマリー
第5章 主な出来事
第6章 疫学と市場調査手法
第7章 疾患の背景と概要
- イントロダクション
- 症状
- 病因学
- 病因
- PBCの合併症
- 診断
- 治療
- 治療ガイドライン
第8章 疫学と患者数
- 主な調査結果
- 前提条件と根拠
- 主要7市場と中国市場のPBCの総診断症例数
- 米国
- 欧州4ヶ国・英国
- 日本
- 中国
第9章 ペイシェントジャーニー
第10章 上市済みの製品
第11章 新たな治療法
第12章 PBC:主要7市場と中国市場の分析
- 主な調査結果
- 主要7市場と中国市場のPBCの総市場規模
- 市場の見通し
- 属性分析
- 主な市場予測の前提条件
- 米国の市場規模
- 米国のPBCの市場規模
- 米国のPBCの市場規模:治療別
- 欧州4ヶ国・英国の市場規模
- 欧州4ヶ国・英国のPBCの総市場規模
- 欧州4ヶ国・英国のPBCの市場規模:治療法別
- 日本の市場規模
- 日本のPBCの市場規模
- 日本のPBCの市場規模:治療法別
- 中国の市場規模
- 中国のPBCの市場規模
- 中国のPBCの市場規模:治療別
第13章 SWOT分析
第14章 KOLの見解
第15章 アンメットニーズ
第16章 市場参入
- 疾病負荷
- 米国
- 欧州
- 日本
- 中国
第17章 付録
第18章 DelveInsightのサービス内容
第19章 免責事項
第20章 DelveInsightについて
List of Tables
- Table 1: Summary of PBC, Market, and Epidemiology (2019-2032)
- Table 2: Key Events
- Table 3: Clinical Features of Primary Biliary Cirrhosis
- Table 4: Currently Known Epigenetic Modifications Described in PBC
- Table 5: Grading evidence and recommendations (adapted from GRADE system)
- Table 6: EASL Clinical Practice Guidelines: The diagnosis and Management of Patients With PBC
- Table 7: Overview of the Utility of Investigation in PBC
- Table 8: The British Society of Gastroenterology/UK recommendations
- Table 9: Scoring Systems Used to Assess UDCA Treatment Response
- Table 10: 2018 Practice Guidance from the American Association for the Study of Liver Diseases
- Table 11: EASL Clinical Practice Guidelines
- Table 12: The British Society of Gastroenterology/UK recommendations
- Table 13: Chinese Recommendations for Liver Cirrhosis
- Table 14: Total Diagnosed Prevalent Cases of PBC in the 7MM + China (2019-2032)
- Table 15: Total Diagnosed Prevalent Cases of PBC in the US (2019-2032)
- Table 16: Gender-specific Cases of PBC in the US (2019-2032)
- Table 17: Age-specific Cases of PBC in the US (2019-2032)
- Table 18: Total Diagnosed Prevalent Cases of PBC in EU4 and the UK (2019-2032)
- Table 19: Gender-specific Cases of PBC in EU4 and the UK (2019-2032)
- Table 20: Age-specific Cases of PBC in EU4 and the UK (2019-2032)
- Table 21: Total Diagnosed Prevalent Cases of PBC in Japan (2019-2032)
- Table 22: Gender-specific Cases of PBC in Japan (2019-2032)
- Table 23: Age-specific Cases of PBC in Japan (2019-2032)
- Table 24: Total Diagnosed Prevalent Cases of PBC in China (2019-2032)
- Table 25: Gender-specific Cases of PBC in China (2019-2032)
- Table 26: Age-specific Cases of PBC in China (2019-2032)
- Table 27: Key cross of the key emerging drugs
- Table 28: Key cross of the key emerging drugs
- Table 29: Seladelpar, Clinical Trial Description, 2022
- Table 30: Saroglitazar Magnesium, Clinical Trial Description, 2022
- Table 31: Elafibranor, Clinical Trial Description, 2022
- Table 32: Linerixibat, Clinical Trial Description, 2022
- Table 33: Setanaxib, Clinical Trial Description, 2022
- Table 34: Bezafibrate + obeticholic acid, Clinical Trial Description, 2022
- Table 35: Volixibat, Clinical Trial Description, 2022
- Table 36: EP547 Clinical Trial Description, 2022
- Table 37: ASC42, Clinical Trial Description, 2022
- Table 38: Obeticholic Acid, Clinical Trial Description, 2022
- Table 39: Total Market Size of PBC in the 7MM + China in USD million (2019-2032)
- Table 40: Key Market Forecast Assumptions for Seladelpar
- Table 41: Key Market Forecast Assumptions for Saroglitazar mg
- Table 42: Key Market Forecast Assumptions for Elafibranor
- Table 43: Key Market Forecast Assumptions for Linerixibat
- Table 44: Key Market Forecast Assumptions for Setanaxib
- Table 45: Market Size of PBC in the United States in USD million (2019-2032)
- Table 46: Market Size of PBC in the United States by Therapies in USD million (2019-2032)
- Table 47: Total Market Size of PBC in EU4 and the UK in USD million (2019-2032)
- Table 48: Market Size of PBC in EU4 and the UK in USD million (2019-2032)
- Table 49: Market Size of PBC in Japan in USD million (2019-2032)
- Table 50: Market Size of PBC in Japan by Therapies in USD million (2019-2032)
- Table 51: Market Size of PBC in China in USD million (2019-2032)
- Table 52: Market Size of PBC in China by Therapies in USD million (2019-2032)
List of Figures
- Figure 1: Symptoms of Primary Biliary Cholangitis
- Figure 2: The Pathogenesis of PBC
- Figure 3: Histology of PBC
- Figure 4: EASL Clinical Practice Guideline in PBC Consensus Management Flow Chart
- Figure 5: EASL The BSG/UK-PBC Consensus Care Pathway for Patients With PBC
- Figure 6: Total Diagnosed Prevalent Cases of PBC in the 7MM + China (2019-2032)
- Figure 7: Total Diagnosed Prevalent Cases of PBC in the US (2019-2032)
- Figure 8: Gender-specific Cases of PBC in the US (2019-2032)
- Figure 9: Age-specific Cases of PBC in the US (2019-2032)
- Figure 10: Total Diagnosed Prevalent Cases of PBC in EU4 and the UK (2019-2032)
- Figure 11: Gender-specific Cases of PBC in EU4 and the UK (2019-2032)
- Figure 12: Age-specific Cases of PBC in EU4 and the UK (2019-2032)
- Figure 13: Total Diagnosed Prevalent Cases of PBC in Japan (2019-2032)
- Figure 14: Gender-specific Cases of PBC in Japan (2019-2032)
- Figure 15: Age-specific Cases of PBC in Japan (2019-2032)
- Figure 16: Total Diagnosed Prevalent Cases of PBC in China (2019-2032)
- Figure 17: Gender-specific Cases of PBC in China (2019-2032)
- Figure 18: Age-specific Cases of PBC in China (2019-2032)
- Figure 19: Total Market Size of PBC in the 7MM + China (2019-2032)
- Figure 20: Market Size of PBC in the United States (2019-2032)
- Figure 21: Market Size of PBC in the United States by Therapies (2019-2032)
- Figure 22: Total Market Size of PBC in EU4 and the UK (2019-2032)
- Figure 23: Market Size of PBC in EU4 and the UK by Therapies (2019-2032)
- Figure 24: Market Size of PBC in Japan (2019-2032)
- Figure 25: Market Size of PBC in Japan by Therapies (2019-2032)
- Figure 26: Market Size of PBC in China (2019-2032)
- Figure 27: Market Size of PBC in China by Therapies (2019-2032)
DelveInsight's 'Primary Biliary Cholangitis (PBC) - Market Insights, Epidemiology, and Market Forecast - 2032' report delivers an in-depth understanding of the historical and forecasted epidemiology as well as the market trends of primary biliary cholangitis in the United States, EU4 (Germany, Spain, Italy, France) and the United Kingdom, Japan, and China.
The primary biliary cholangitis market report provides current treatment practices, emerging drugs, market share of individual therapies, and current and forecasted primary biliary cholangitis market size from 2019 to 2032, segmented by the 7MM + China. The report also covers the current primary biliary cholangitis treatment practice/algorithm, market drivers, market barriers, and unmet medical needs to curate the best opportunities and assesses the underlying market potential.
Geographies Covered
- The United States
- EU4 and the United Kingdom
- Japan
- China
Study period: 2019-2032.
Primary Biliary Cholangitis: Disease Understanding and Treatment Algorithm
Primary Biliary Cholangitis Overview
According to the American Liver Foundation, primary biliary cholangitis (PBC), formerly known as primary biliary cirrhosis, is a chronic liver disease resulting from the progressive destruction of the bile ducts in the liver, called the intrahepatic bile ducts. Prolonged hepatic cholestasis subsequently leads to cirrhosis and portal hypertension.
Patients with PBC can be asymptomatic or may present with jaundice, pruritus, and fatigue. Other symptoms may include abdominal pain, skin darkening, and xanthomas or xanthelasmas. Individuals may also complain of dry mouth, eyes, bone, muscle, and joint pain. As the disease progresses, symptoms of cirrhosis can develop, including jaundice, edema, ascites, and varices.
Since many people with PBC have no symptoms, the disease is often discovered incidentally due to abnormal results on routine liver blood tests. Once PBC is suspected, a blood test is done to check for anti-mitochondrial antibodies (AMA). This test is positive in nearly all people with PBC. A liver biopsy, where a small sample of liver tissue is removed with a small needle, can help confirm the diagnosis. Imaging studies may rule out other diseases or evaluate patients further once diagnosed with PBC.
There are only two medications approved by the Food and Drug Administration (FDA) to treat PBC; ursodeoxycholic acid (UDCA) and obeticholic acid (OCALIVA). UDCA is a hydrophilic bile salt that stabilizes hepatocyte membranes against toxic bile salts and inhibits apoptosis and fibrosis. While, obeticholic acid is a farnesoid X receptor agonist that helps reduce ALP, GGT, and transaminase levels due to its antifibrotic and choleretic properties. It can be given in combination with UDCA in adults with inadequate response to UDCA or as a single therapy in adults unable to tolerate UDCA.
Primary Biliary Cholangitis: Diagnosis and Treatment
It covers the details of conventional and current medical therapies and diagnoses available in the primary biliary cholangitis market to treat the condition. It also provides country-wise treatment guidelines and algorithms across the United States, EU-4 and the United Kingdom, Japan, and China.
Primary Biliary Cholangitis: Epidemiology
The primary biliary cholangitis epidemiology division provides insights into the historical and current patient pool and the forecasted trend for the 7MM + China. It helps recognize the causes of current and forecasted trends by exploring numerous studies and views of KOL. The report also provides the prevalent patient pool, trends, and assumptions.
Key Findings
The disease epidemiology covered in the report provides historical and forecasted primary biliary cholangitis epidemiology segmented as the Diagnosed Prevalent Cases of Primary Biliary Cholangitis, Gender-specific Cases of Primary Biliary Cholangitis, and Age-specific Cases of Primary Biliary Cholangitis. The report includes the total cases of PBC in the 7MM + China covering the United States, Japan, China, EU4, and the United Kingdom from 2019 to 2032.
Country-wise Primary Biliary Cholangitis Epidemiology
The epidemiology segment also provides the primary biliary cholangitis epidemiology data and findings across the United States, EU4 and the United Kingdom, Japan, and China.
In 2021, the total diagnosed prevalent cases of PBC were more than 1,000,000 in the 7MM + China.
As per the estimates, China had the highest diagnosed prevalent patient population of PBC in 2021. Among EU4 and the UK, Germany had the highest number of diagnosed cases of PBC, with around 32,000 cases, followed by the UK in 2021. On the other hand, Spain had the lowest number of diagnosed cases of PBC, with close to 10,000 cases in 2021.
Primary Biliary Cholangitis: Drug Chapters
The drug chapter segment of the primary biliary cholangitis report encloses a detailed analysis of primary biliary cholangitis-marketed drugs and late-stage (Phase III, Phase II/III, and Phase II) pipeline drugs. It also helps understand the primary biliary cholangitis clinical trial details, expressive pharmacological action, agreements and collaborations, approval and patent details, advantages and disadvantages of each included drug, and the latest news and press releases.
Despite the emergence of competing drugs, UDCA is still recognized as the universal first-line standard of care for PBC. This strong recommendation is based on the long-term efficacy of UDCA and its excellent safety profile and low cost. After UDCA, obeticholic acid (OCA), OCALIVA was the second drug to successfully meet the primary endpoint of a large placebo-controlled Phase III trial in PBC.
Products detail in the report…
Primary Biliary Cholangitis Emerging Drugs
Drug developers are gradually shifting their attention toward primary biliary cholangitis to meet the patient pool's current demands and counter the unmet needs of the therapeutic market.
Many molecules are in the pipeline to treat primary biliary cholangitis patients across many countries to cater to their needs. Some of the major late-stage products are likely to hit the market during our study period 2019-2032 include seladelpar (CymaBay Therapeutics), elafibranor (Genfit), setanaxib (Genkyotex SA), saroglitazar Mg (Zydus), and linerixibat (GlaxoSmithKline).
Seladelpar (MBX-8025) is a potent, selective, orally active PPARd agonist in development for treating patients with PBC and an inadequate response to or intolerance to UDCA. The drug is currently in Phase III trial and has received multiple designations from regulatory authorities, likely to speed up the drug approval process.
Elafibranor is a dual PPARa/d agonist, developed by Genfit. It is currently being evaluated in the Phase III trial. With orphan and breakthrough therapy designation, the drug is expected to enter the market by 2024.
Products detail in the report…
Primary Biliary Cholangitis Market Outlook
The market outlook of the primary biliary cholangitis report builds a detailed comprehension of the historical, current, and forecasted primary biliary cholangitis market trends by analyzing the impact of current therapies on the market, unmet needs, and demand for better technology.
This segment gives a thorough detail of the primary biliary cholangitis market trend of each marketed drug and late-stage pipeline therapy by evaluating their impact based on the annual cost of therapy, inclusion and exclusion criteria, mechanism of action, compliance rate, growing need for the market, increasing patient pool, covered patient segment, expected launch year, competition with other therapies, brand value, their impact on the market, and KOL view. The calculated market data are presented with relevant tables and graphs to give a clear view of the market at first sight.
As per DelveInsight, the primary biliary cholangitis market in the 7MM + China is expected to change in 2019-2032.
Key Findings
This section includes a glimpse of the primary biliary cholangitis market in 7MM + China. The primary biliary cholangitis market size in the seven major markets and China was approximately USD 1,560 million in 2021.
The United States: Market Outlook
This section provides the total primary biliary cholangitis market size, and it also provides the market size of anemia by therapies in the United States.
The United States accounts for the highest market size of primary biliary cholangitis than, EU4 and the United Kingdom, and Japan.
EU4 and the UK: Market Outlook
This section provides the total primary biliary cholangitis market size. It also provides primary biliary cholangitis market size by therapies in Germany, France, Italy, Spain, and the United Kingdom.
Japan: Market Outlook
This section provides the total primary biliary cholangitis market size. It also provides the market size of primary biliary cholangitis by therapies in Japan.
China: Market Outlook
This section provides the total primary biliary cholangitis market size. It also provides the market size of primary biliary cholangitis by therapies in China.
Primary Biliary Cholangitis: Drugs Uptake
This section focuses on the uptake rate of potential drugs launched or expected to be launched in the market during 2019-2032. The analysis covers the primary biliary cholangitis market uptake by drugs, patient uptake by therapies, and sales of each drug.
This helps in understanding the drugs with the most rapid uptake and the reasons behind the maximal use of new drugs and allows the comparison of the drugs based on market share and size, which again will be useful in investigating factors important in the market uptake and making financial and regulatory decisions.
Primary Biliary Cholangitis: Pipeline Development Activities
The report provides insights into different therapeutic candidates in Phase III, Phase II/III, Phase II, and Phase I/II. It also analyses primary biliary cholangitis's key players in developing targeted therapeutics.
Major players include: CymaBay Therapeutics, Genfit, Genkyotex SA, Zydus, and GlaxoSmithKline whose key products are expected to get launched in the US market by 20XX.
Pipeline Development Activities
The report covers collaborations, acquisitions, mergers, licensing, and patent details for emerging primary biliary cholangitis therapies.
KOL Views
To keep up with current market trends, we take KOLs and SMEs' opinions working in the primary biliary cholangitis domain through primary research to fill the data gaps and validate our secondary research. Their opinion helps understand and validate current and emerging treatment patterns or primary biliary cholangitis market trends. This will support the clients in potential novel treatments by identifying the overall scenario of the market and the unmet needs.
Competitive Intelligence Analysis
We perform a competitive and market intelligence analysis of the primary biliary cholangitis market using various competitive intelligence tools: SWOT analysis, PESTLE analysis, Porter's five forces, BCG Matrix, Market entry strategies, etc. The inclusion of the analysis entirely depends upon the data availability.
Scope of the Report:
- The report covers the descriptive overview of primary biliary cholangitis, explaining its causes, signs and symptoms, pathophysiology, and currently available therapies
- Comprehensive insight is provided into the primary biliary cholangitis epidemiology and treatment in the 7MM + China
- Additionally, an all-inclusive account of both the current and emerging therapies for primary biliary cholangitis is provided, along with the assessment of new therapies that will impact the current treatment landscape
- A detailed review of the primary biliary cholangitis market, historical and forecast, is included in the report, covering drug outreach in the 7MM + China
- The report provides an edge while developing business strategies by understanding trends shaping and driving the global primary biliary cholangitis market
Report Highlights:
- Recently, the primary biliary cholangitis market has been set to change due to the increase in investment and research funding for primary biliary cholangitis treatment, and the survival rates of those with primary biliary cholangitis have increased because of advancements in treatment, such as liver transplants; which would expand the size of the market to enable the drug manufacturers to penetrate more into the market.
- The companies and academics are working to assess challenges and seek opportunities that could influence primary biliary cholangitis R&D. The therapies under development are focused on novel approaches to treat/improve the disease condition.
- Major players are involved in developing therapies for primary biliary cholangitis. The launch of emerging therapies will significantly impact the primary biliary cholangitis market.
- For primary biliary cholangitis, a better understanding of disease pathogenesis will also contribute to developing novel therapeutics.
- Our in-depth analysis of the pipeline assets across different stages of development (Phase III and Phase II), different emerging trends, and comparative analysis of pipeline products with detailed clinical profiles, key cross-competitor, launch date along with product development activities will support the clients in the decision-making process regarding their therapeutic portfolio by identifying the overall scenario of the R&D activities.
Primary Biliary Cholangitis: Report Insights
- Patient Population
- Therapeutic Approaches
- Primary Biliary Cholangitis Pipeline Analysis
- Primary Biliary Cholangitis Market Size and Trends
- Market Opportunities
- Impact of upcoming Therapies
Primary Biliary Cholangitis Report Key Strengths
- 11-year Forecast
- The 7MM + China Coverage
- Primary Biliary Cholangitis Epidemiology Segmentation
- Key Competitors
- Highly Analyzed Market
- Drugs Uptake
Primary Biliary Cholangitis: Report Assessment
- Current Treatment Practices
- Unmet Needs
- Pipeline Product Profiles
- Market Attractiveness
Key Questions
Market Insights
- What was the market share percentage distribution, and how would it look in 2032?
- What would be the total market size and market size of primary biliary cholangitis by therapies across the 7MM + China forecast period (2019-2032)?
- What are the key findings about the market across the 7MM + China, and which country will have the largest primary biliary cholangitis market size during the forecast period (2019-2032)?
- At what CAGR is the primary biliary cholangitis market expected to grow in the 7MM + China forecast period (2019-2032)?
- What would be the primary biliary cholangitis market outlook across the 7MM + China forecast period (2019-2032)?
- What would be the primary biliary cholangitis market growth until 2032 and the resultant market size in 2032?
- How would future opportunities affect the market dynamics and subsequent analysis of the associated trends?
Epidemiology Insights
- What are the disease risk, burdens, and unmet needs of primary biliary cholangitis?
- What is the historical patient pool of primary biliary cholangitis covering the United States, EU4 and the UK, Japan, and China?
- What would be the forecasted patient pool of primary biliary cholangitis covering the United States, EU4 and the UK, Japan, and China?
- What will be the growth opportunities in the 7MM + China concerning the patient population of primary biliary cholangitis?
- Out of all the 7MM + China, which country would have the highest prevalence of primary biliary cholangitis during the forecast period (2019-2032)?
- At what CAGR is the population expected to grow in the 7MM + China forecast period (2019-2032)?
Current Treatment Scenario, Marketed Drugs, and Emerging Therapies
- What are the current options to treat primary biliary cholangitis?
- What are the current treatment guidelines for treating primary biliary cholangitis in the US, China, Europe, and Japan?
- What are the primary biliary cholangitis-marketed drugs and their MoA, regulatory milestones, product development activities, advantages, disadvantages, safety, efficacy, etc.?
- How many companies are developing therapies to treat primary biliary cholangitis?
- How many therapies are developed by each company to treat primary biliary cholangitis?
- How many emerging therapies are in the mid-stage and late stages of development to treat primary biliary cholangitis?
- What are the key collaborations (Industry-Industry, Industry-Academia), Mergers and acquisitions, and licensing activities related to primary biliary cholangitis therapies?
- What are the recent novel therapies, targets, mechanisms of action, and technologies developed to overcome the limitation of existing therapies?
- What are the clinical studies for primary biliary cholangitis and its status?
- Which key designations have been granted for the emerging therapies for primary biliary cholangitis?
- What are the global historical and forecasted primary biliary cholangitis markets?
Reasons to buy:
- The report will help in developing business strategies by understanding trends shaping and driving the primary biliary cholangitis market
- To understand the future market competition in the primary biliary cholangitis market and an insightful review of the key market drivers and barriers
- Organize sales and marketing efforts by identifying the best opportunities for primary biliary cholangitis in the US, EU4 (Germany, Spain, Italy, France) and the United Kingdom, Japan, and China
- Identifying upcoming solid players in the market will help devise strategies that will help get ahead of competitors
- Organize sales and marketing efforts by identifying the best opportunities for the primary biliary cholangitis market
- To understand the future market competition in the primary biliary cholangitis market
Table of Contents
1. Key Insights
2. Report Introduction
3. Primary Biliary Cholangitis (PBC) Market Overview at a Glance
- 3.1. Market Share (%) Distribution of PBC in 2019
- 3.2. Market Share (%) Distribution of PBC in 2032
4. Executive Summary of Primary Biliary Cholangitis (PBC)
5. Key Events
6. Epidemiology and Market Methodology
7. Disease Background and Overview
- 7.1. Introduction
- 7.2. Symptoms
- 7.3. Etiology
- 7.4. Pathogenesis
- 7.4.1. Genetics, epigenetics, and environmental factors in PBC pathogenesis
- 7.5. Complications of PBC
- 7.5.1. Hepatic complications of PBC
- 7.5.2. Extrahepatic complications of PBC
- 7.6. Diagnosis
- 7.6.1. Diagnostic Guidelines
- 7.7. Treatment
- 7.7.1. Treatment options
- 7.7.2. Approved treatments for an incomplete response to UDCA
- 7.7.3. Symptomatic treatment of PBC
- 7.7.4. Management of hepatic complications in PBC
- 7.8. Treatment Guidelines
- 7.8.1. American Association for the Study of Liver Diseases (AASLD) 2018 Practice Guidelines
- 7.8.2. European Association for Study of Liver (EASL) Clinical Practice Guidelines
- 7.8.3. The British Society of Gastroenterology/UK PBC Treatment and Management Guidelines
- 7.8.4. Chinese Guidelines on the Management of Liver Cirrhosis
8. Epidemiology and Patient Population
- 8.1. Key Findings
- 8.2. Assumptions and Rationale
- 8.3. Total Diagnosed Prevalent Cases of PBC in the 7MM + China
- 8.4. The US
- 8.4.1. Diagnosed prevalent cases of PBC in the US
- 8.4.2. Gender-specific cases of PBC in the US
- 8.4.3. Age-specific cases of PBC in the US
- 8.5. EU4 and the UK
- 8.5.1. Diagnosed Prevalent Cases of PBC in EU4 and the UK
- 8.5.2. Gender-specific cases of PBC in EU4 and the UK
- 8.5.3. Age-specific cases of PBC in EU4 and the UK
- 8.6. Japan
- 8.6.1. Diagnosed prevalent cases of PBC in Japan
- 8.6.2. Gender-specific cases of PBC in Japan
- 8.6.3. Age-specific cases of PBC in Japan
- 8.7. China
- 8.7.1. Diagnosed prevalent cases of PBC in China
- 8.7.2. Gender-specific cases of PBC in China
- 8.7.3. Age-specific cases of PBC in China
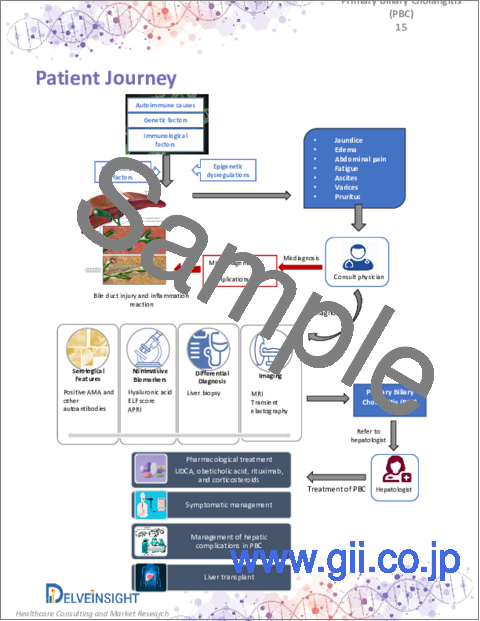
9. Patient Journey
10. Marketed Products
- 10.1. OCALIVA: Intercept Pharmaceuticals
- 10.1.1. Drug description
- 10.1.2. Regulatory milestone
- 10.1.3. Other development activities
- 10.1.4. Safety and efficacy
- 10.1.5. Product profile
11. Emerging Therapies
- 11.1. Key Cross Competition
- 11.2. Seladelpar: CymaBay Therapeutics
- 11.2.1. Drug description
- 11.2.2. Other development activities
- 11.2.3. Clinical development
- 11.2.3.1. Clinical trial information
- 11.2.4. Safety and efficacy
- 11.2.5. Product profile
- 11.2.6. Analyst comment
- 11.3. Saroglitazar Magnesium: Zydus Therapeutics
- 11.3.1. Drug description
- 11.3.2. Other development activities
- 11.3.3. Clinical development
- 11.3.3.1. Clinical trial information
- 11.3.4. Safety and efficacy
- 11.3.5. Product profile
- 11.3.6. Analyst comment
- 11.4. Elafibranor: Genfit
- 11.4.1. Drug description
- 11.4.2. Other development activities
- 11.4.3. Clinical development
- 11.4.3.1. Clinical trial information
- 11.4.4. Safety and efficacy
- 11.4.5. Product profile
- 11.4.6. Analyst comment
- 11.5. Linerixibat: GlaxoSmithKline
- 11.5.1. Drug description
- 11.5.2. Other development activities
- 11.5.3. Clinical development
- 11.5.3.1. Clinical trial information
- 11.5.4. Safety and efficacy
- 11.5.5. Product profile
- 11.5.6. Analyst comment
- 11.6. Setanaxib: Calliditas Therapeutics AB (Calliditas Therapeutics Suisse SA)
- 11.6.1. Drug description
- 11.6.2. Other development activities
- 11.6.3. Clinical development
- 11.6.3.1. Clinical trial information
- 11.6.4. Safety and efficacy
- 11.6.5. Product profile
- 11.6.6. Analyst comment
- 11.7. Bezafibrate + obeticholic acid: Intercept Pharmaceuticals
- 11.7.1. Drug description
- 11.7.2. Clinical development
- 11.7.2.1. Clinical trial information
- 11.7.3. Product profile
- 11.8. Volixibat: Mirum Pharmaceuticals
- 11.8.1. Drug description
- 11.8.2. Other development activities
- 11.8.3. Clinical development
- 11.8.3.1. Clinical trial information
- 11.8.4. Product profile
- 11.9. EP547: Escient Pharmaceuticals
- 11.9.1. Drug description
- 11.9.2. Other development activities
- 11.9.3. Clinical development
- 11.9.3.1. Clinical trial information
- 11.9.4. Product profile
- 11.10. ASC42: Gannex Pharma
- 11.10.1. Drug description
- 11.10.2. Clinical development
- 11.10.2.1. Clinical trial information
- 11.10.3. Product profile
- 11.11. Obeticholic Acid: Nanjing Chia-tai Tianqing Pharmaceutical
- 11.11.1. Drug description
- 11.11.2. Clinical development
- 11.11.3. Product Profile
12. PBC: 7 MM + China Analysis
- 12.1. Key Findings
- 12.2. Total Market Size of PBC in the 7MM + China
- 12.3. Market Outlook
- 12.4. Attribute Analysis
- 12.5. Key Market Forecast Assumptions
- 12.6. The US Market Size
- 12.6.1. Market size of PBC in the United States
- 12.6.2. Market size of PBC by therapies in the United States
- 12.7. EU4 and the UK Market Size
- 12.7.1. Total market size of PBC in EU4 and the UK
- 12.7.2. Market size of PBC by therapies in EU4 and the UK
- 12.8. Japan Market Size
- 12.8.1. Market size of PBC in Japan
- 12.8.2. Market size of PBC by therapies in Japan
- 12.9. China Market Size
- 12.9.1. Market size of PBC in China
- 12.9.2. Market size of PBC by therapies in China
13. SWOT Analysis
14. KOL Views
15. Unmet Needs
16. Market Access
- 16.1. Disease Burden
- 16.2. The US
- 16.2.1. Medicare
- 16.2.2. Liver transplant Medicare coverage
- 16.2.3. Patient assistance program for OCALIVA
- 16.2.4. Cigna coverage policy
- 16.3. Europe
- 16.3.1. The United Kingdom
- 16.3.1.1. The National Institute for Health and Care Excellence (NICE)
- 16.3.1. The United Kingdom
- 16.4. Japan
- 16.5. China
17. Appendix
- 17.1. Bibliography
- 17.2. Acronyms and Abbreviations
- 17.3. Report Methodology