|
|
市場調査レポート
商品コード
1759275
デスモイド腫瘍の世界市場:地域・国別の分析・予測 (2025-2035年)Desmoid Tumors Market - A Global and Regional Analysis: Focus on Country and Region - Analysis and Forecast, 2025-2035 |
||||||
カスタマイズ可能
|
|||||||
| デスモイド腫瘍の世界市場:地域・国別の分析・予測 (2025-2035年) |
|
出版日: 2025年06月30日
発行: BIS Research
ページ情報: 英文 100 Pages
納期: 1~5営業日
|
全表示
- 概要
- 図表
- 目次
デスモイド腫瘍市場の主要な成長要因の一つは、標的療法の進展です。
デスモイド腫瘍の分子的・遺伝的要因の解明が進むにつれて、企業はEZH2阻害薬であるTazemetostat (EPZ-6438) や、Notchシグナル経路を標的とするAL102など、腫瘍の成長に関与する特定の経路を標的とした、より正確で個別化された治療法を開発しています。これらの治療法は、従来の化学療法に比べて副作用が少なく、高い有効性が期待できるため、患者や医療提供者にとって非常に魅力的な選択肢となっています。
また、希少疾患に対する規制当局の支援も市場成長を後押ししています。希少疾病用医薬品指定や迅速承認制度などの規制上の優遇措置が、新たな治療法の開発を促進しており、市場成長をさらに加速させています。より効果的な標的治療が普及することで、患者は早期診断や専門的ケアを求める傾向が高まり、これにより治療への需要も増加ししています。デスモイド腫瘍に対する認知の高まりと個別化医療の進展により、市場は今後も成長軌道を維持すると見込まれており、この希少かつ困難な疾患を抱える患者に希望を与えると予想されています。
一方で、いくつかの課題も存在し、その成長を妨げています。最大の課題の一つは、デスモイド腫瘍に特化したFDA承認の治療法が依然として限られている点です。Tazemetostat (EPZ-6438) やAL102といった薬剤で一定の進展はあるものの、多くの患者が依然として適応外使用の薬剤や、手術や放射線療法といった侵襲的治療に頼っているのが現状です。承認された標準治療が不足しているため、特に臨床試験や専門医療へのアクセスが困難な地域では、効果的で個別化された治療を受ける機会が限られています。
もう一つの大きな課題は、デスモイド腫瘍の高い再発率です。手術や放射線療法によって一時的に腫瘍が取り除かれても再発することが多く、場合によってはより攻撃的な形で戻ってくることもあります。この予測困難な再発傾向により、長期的な管理が難しくなり、患者は繰り返しの治療を余儀なくされ、身体的にも精神的にも大きな負担を抱えることになります。
さらに、この疾患の稀少性と複雑さが診断や治療を一層難しくしています。特に初期段階においては、多くの医師がデスモイド腫瘍についての知識を持っていない可能性があり、診断の遅れや治療への不安につながることがあります。一般市民や医療従事者の間でも十分な認知が進んでおらず、早期介入の機会を制限しています。
最後に、新しい治療法のコストやアクセスの問題も障壁となっています。Tazemetostatのような標的治療薬は非常に高額であり、継続的な治療や臨床試験への参加にかかる費用は、十分な保険や経済的支援のない患者にとって大きな負担となります。
当レポートでは、世界のデスモイド腫瘍の市場を調査し、主要動向、市場影響因子の分析、法規制環境、臨床試験の動向、市場規模の推移・予測、各種区分・地域/主要国別の詳細分析、競合情勢、主要企業のプロファイルなどをまとめています。
目次
エグゼクティブサマリー
第1章 世界のデスモイド腫瘍市場:業界展望
- 市場動向
- 規制の枠組み
- 疫学分析
- 臨床試験分析
- 市場力学
- 影響分析
- 市場促進要因
- 市場の課題
- 市場機会
第2章 世界のデスモイド腫瘍市場:地域別
- 北米
- 主な調査結果
- 市場力学
- 市場規模と予測
- 欧州
- 主な調査結果
- 市場力学
- 市場規模と予測
- アジア太平洋
- 主な調査結果
- 市場力学
- 市場規模と予測
第3章 世界のデスモイド腫瘍市場:競合情勢と企業プロファイル
- 主な戦略・展開
- M&A
- 相乗効果のある活動
- 事業拡大と資金調達
- 製品の発売と承認
- その他の活動
- 企業プロファイル
- SpringWorks Therapeutics
- Ayala Pharmaceuticals
- Puma Biotechnology
- Bristol-Myers Squibb
- Eli Lilly and Company
- Ipsen
- Deciphera Pharmaceuticals
第4章 調査手法
List of Figures
- Figure: Global Desmoid Tumors Market (by Region), $Billion, 2024 and 2035
- Figure: Global Desmoid Tumors Market Key Trends, Analysis
List of Tables
- Table Global Desmoid Tumors Market Dynamics, Impact Analysis
- Table Global Desmoid Tumors Market (by Region), $Billion, 2024-2035
Global Desmoid Tumors Market, Analysis and Forecast: 2025-2035
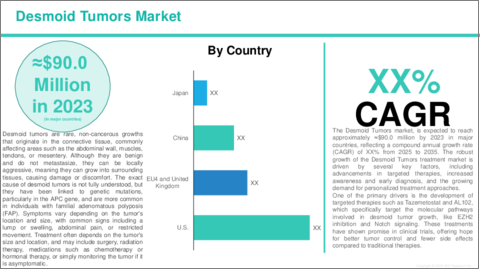
Desmoid Tumors are non-cancerous (benign) fibrous growths that arise from connective tissue, often in the muscles, tendons, or abdominal wall. Despite being benign, they can be locally aggressive, meaning they may grow and invade nearby tissues, causing significant health issues. These tumors are characterized by the abnormal growth of fibroblasts, the cells that produce collagen, which leads to the formation of dense, fibrous masses.
Desmoid tumors are rare and account for less than 3% of all soft tissue tumors. They can develop in any part of the body but are most found in the abdomen, muscles, and limbs. Although they do not metastasize (spread to other parts of the body), their growth can cause pain, discomfort, and, in some cases, can impact the function of nearby organs or muscles.
The exact cause of Desmoid Tumors is not fully understood, but they are often associated with genetic mutations, especially in the APC gene that is involved in regulating cell growth. They are also more commonly found in individuals with Familial Adenomatous Polyposis (FAP), a hereditary condition that increases the risk of various tumors.
Symptoms of Desmoid Tumors vary depending on their size and location and can include pain, swelling, or restricted movement. Treatment options include surgery, radiation therapy, and targeted drug therapies, with the goal of controlling tumor growth and preventing recurrence, as these tumors often have high recurrence rates.
One of the key drivers of the Desmoid Tumors market is the advancement of targeted therapies. As the understanding of the molecular and genetic drivers of Desmoid Tumors improves, companies are developing more precise, personalized treatments that target the specific pathways involved in tumor growth, such as EZH2 inhibition with drugs like Tazemetostat (EPZ-6438) and Notch signaling with therapies like AL102. These treatments offer the potential for greater efficacy with fewer side effects compared to traditional approaches like chemotherapy, making them a highly attractive option for patients and healthcare providers alike.
Additionally, regulatory support for rare diseases, such as Orphan Drug Designation and accelerated approval processes, is encouraging the development of new therapies, further propelling Desmoid tumors market growth. As more effective, targeted treatment options become available, patients are more likely to seek early diagnosis and specialized care, increasing the demand for these therapies. Combined with the growing awareness of Desmoid Tumors and the rise in personalized medicine, the Desmoid Tumors market is expected to continue its growth trajectory, offering hope to patients with this rare and challenging condition.
Despite the growth of the Desmoid Tumors market, several challenges continue to hinder its progress. One of the primary challenges is the limited number of FDA-approved treatments specifically for Desmoid Tumors. While there has been progress with drugs like Tazemetostat (EPZ-6438) and AL102, the treatment landscape remains relatively underdeveloped, as many patients still rely on off-label medications or invasive treatments like surgery or radiation therapy. This lack of approved, standardized therapies means that many patients may not have access to effective, tailored treatments, especially in areas where clinical trials or specialized care are not readily available.
Another significant challenge is the high recurrence rate of Desmoid Tumors. Even after surgical removal or radiation therapy, Desmoid Tumors often return, sometimes in a more aggressive form. This unpredictability makes long-term management difficult and can lead to repeated treatments, causing both physical and emotional strain on patients.
Additionally, the rarity of the condition and its complex nature can make diagnosis and treatment challenging for healthcare providers. Many doctors may be unfamiliar with Desmoid Tumors, especially in the early stages, leading to potential delays in diagnosis and a lack of confidence in managing the disease. This is compounded by insufficient awareness among the general public and healthcare professionals, which further limits early intervention opportunities.
Finally, cost and accessibility of emerging treatments are also obstacles. Targeted therapies like Tazemetostat are expensive, and the costs associated with ongoing treatment, including clinical trial participation, may be prohibitive for some patients, particularly those without adequate insurance coverage or financial support.
These challenges present significant barriers to the Desmoid tumors market's full growth potential, requiring continued research, regulatory support, and advancements in treatment options to overcome them.
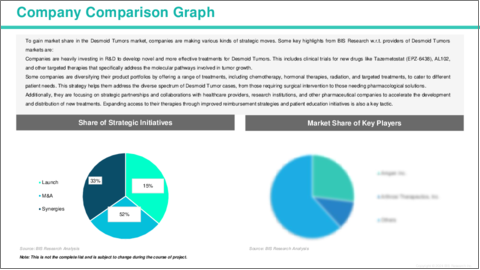
The global Desmoid Tumors market is highly competitive, with several leading companies driving innovation and market growth, such as SpringWorks Therapeutics, Ayala Pharmaceuticals, Ipsen, Deciphera Pharmaceuticals, and Bristol-Myers Squibb. These companies are at the forefront of developing novel therapies to address the unmet needs of patients with this rare condition. SpringWorks Therapeutics is advancing Tazemetostat (EPZ-6438), an EZH2 inhibitor, which is showing promise in treating Desmoid Tumors by targeting the molecular pathways that drive tumor growth. Similarly, Ayala Pharmaceuticals is focusing on AL102, a gamma-secretase inhibitor that targets the Notch signaling pathway, a key driver in Desmoid Tumor formation. Ipsen is investigating Cabometyx (cabozantinib), a multi-kinase inhibitor, for its potential to treat Desmoid Tumors, while Deciphera Pharmaceuticals is developing Ripretinib, a kinase inhibitor that targets resistant tumors. Additionally, Bristol-Myers Squibb is exploring the use of Opdivo (nivolumab), an immune checkpoint inhibitor, for Desmoid Tumors, aiming to harness the power of immunotherapy. These innovations are paving the way for more targeted, effective treatments that offer hope for better management and outcomes for patients with Desmoid Tumors.
Desmoid Tumors Market Segmentation:
Segmentation 1: by Region
- North America
- Europe
- Asia-Pacific
The global Desmoid Tumors market is undergoing significant transformation, fueled by emerging trends that are reshaping the treatment landscape and expanding patient access. One key trend is the advancement of targeted therapies, such as Tazemetostat (EPZ-6438) and AL102, which focus on specific molecular pathways like EZH2 inhibition and Notch signaling. These therapies offer a more precise approach to treating Desmoid Tumors, reducing the side effects typically associated with traditional treatments like chemotherapy. Additionally, personalized medicine is gaining momentum, with therapies being tailored to the unique genetic profiles of patients, improving treatment efficacy and minimizing adverse effects.
Another important trend is the increased participation in clinical trials. As awareness of Desmoid Tumors grows, more patients are enrolling in clinical trials, which accelerates the development and approval of new therapies. Regulatory support, such as Orphan Drug Designation, is also fueling Desmoid tumors market growth by providing incentives for companies to develop treatments for rare diseases like Desmoid Tumors.
The rise of digital health tools and telemedicine is further transforming the landscape, making treatment and monitoring more accessible, especially in underserved areas. These technologies are improving patient engagement and allowing for more efficient management of the condition. Overall, these emerging trends are driving the growth of the Desmoid Tumors market, improving outcomes for patients, and providing new opportunities for pharmaceutical companies and researchers to address this rare and complex disease.
Table of Contents
Executive Summary
Scope and Definition
Market/Product Definition
Inclusion and Exclusion
Key Questions Answered
Analysis and Forecast Note
1. Global Desmoid Tumors Market: Industry Outlook
- 1.1 Introduction
- 1.2 Market Trends
- 1.3 Regulatory Framework
- 1.4 Epidemiology Analysis
- 1.5 Clinical Trial Analysis
- 1.6 Market Dynamics
- 1.6.1 Impact Analysis
- 1.6.2 Market Drivers
- 1.6.3 Market Challenges
- 1.6.4 Market Opportunities
2. Global Desmoid Tumors Market (Region), ($Billion), 2023-2035
- 2.1 North America
- 2.1.1 Key Findings
- 2.1.2 Market Dynamics
- 2.1.3 Market Sizing and Forecast
- 2.1.3.1 North America Desmoid Tumors Market, by Country
- 2.1.3.1.1 U.S.
- 2.1.3.1 North America Desmoid Tumors Market, by Country
- 2.2 Europe
- 2.2.1 Key Findings
- 2.2.2 Market Dynamics
- 2.2.3 Market Sizing and Forecast
- 2.2.3.1 Europe Desmoid Tumors Market, by Country
- 2.2.3.1.1 Germany
- 2.2.3.1.2 U.K.
- 2.2.3.1.3 France
- 2.2.3.1.4 Italy
- 2.2.3.1 Europe Desmoid Tumors Market, by Country
- 2.3 Asia Pacific
- 2.3.1 Key Findings
- 2.3.2 Market Dynamics
- 2.3.3 Market Sizing and Forecast
- 2.3.3.1 Asia Pacific Desmoid Tumors Market, by Country
- 2.3.3.1.1 China
- 2.3.3.1.2 Japan
- 2.3.3.1 Asia Pacific Desmoid Tumors Market, by Country
3. Global Desmoid Tumors Market: Competitive Landscape and Company Profiles
- 3.1 Key Strategies and Development
- 3.1.1 Mergers and Acquisitions
- 3.1.2 Synergistic Activities
- 3.1.3 Business Expansions and Funding
- 3.1.4 Product Launches and Approvals
- 3.1.5 Other Activities
- 3.2 Company Profiles
- 3.2.1 SpringWorks Therapeutics
- 3.2.1.1 Overview
- 3.2.1.2 Top Products / Product Portfolio
- 3.2.1.3 Top Competitors
- 3.2.1.4 Target Customers/End-Users
- 3.2.1.5 Key Personnel
- 3.2.1.6 Analyst View
- 3.2.2 Ayala Pharmaceuticals
- 3.2.2.1 Overview
- 3.2.2.2 Top Products / Product Portfolio
- 3.2.2.3 Top Competitors
- 3.2.2.4 Target Customers/End-Users
- 3.2.2.5 Key Personnel
- 3.2.2.6 Analyst View
- 3.2.3 Puma Biotechnology
- 3.2.3.1 Overview
- 3.2.3.2 Top Products / Product Portfolio
- 3.2.3.3 Top Competitors
- 3.2.3.4 Target Customers/End-Users
- 3.2.3.5 Key Personnel
- 3.2.3.6 Analyst View
- 3.2.4 Bristol-Myers Squibb
- 3.2.4.1 Overview
- 3.2.4.2 Top Products / Product Portfolio
- 3.2.4.3 Top Competitors
- 3.2.4.4 Target Customers/End-Users
- 3.2.4.5 Key Personnel
- 3.2.4.6 Analyst View
- 3.2.5 Eli Lilly and Company
- 3.2.5.1 Overview
- 3.2.5.2 Top Products / Product Portfolio
- 3.2.5.3 Top Competitors
- 3.2.5.4 Target Customers/End-Users
- 3.2.5.5 Key Personnel
- 3.2.5.6 Analyst View
- 3.2.6 Ipsen
- 3.2.6.1 Overview
- 3.2.6.2 Top Products / Product Portfolio
- 3.2.6.3 Top Competitors
- 3.2.6.4 Target Customers/End-Users
- 3.2.6.5 Key Personnel
- 3.2.6.6 Analyst View
- 3.2.7 Deciphera Pharmaceuticals
- 3.2.7.1 Overview
- 3.2.7.2 Top Products / Product Portfolio
- 3.2.7.3 Top Competitors
- 3.2.7.4 Target Customers/End-Users
- 3.2.7.5 Key Personnel
- 3.2.7.6 Analyst View
- 3.2.1 SpringWorks Therapeutics