|
|
市場調査レポート
商品コード
1734253
高リン血症の世界市場:地域別の分析・予測 (2025~2035年)Hyperphosphatemia Market - A Global and Regional Analysis: Analysis and Forecast, 2025-2035 |
||||||
カスタマイズ可能
|
|||||||
| 高リン血症の世界市場:地域別の分析・予測 (2025~2035年) |
|
出版日: 2025年05月28日
発行: BIS Research
ページ情報: 英文 100 Pages
納期: 1~5営業日
|
全表示
- 概要
- 図表
- 目次
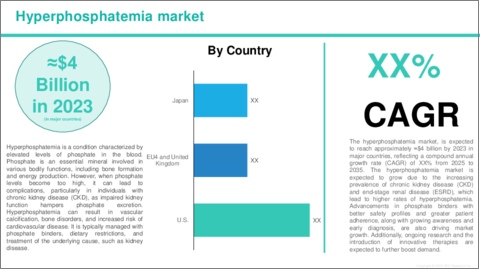
世界の高リン血症の市場は現在、ライフサイクルの成長期にあり、需要の拡大と腎疾患治療の選択肢の増加がその特徴となっています。
この成長は主に、慢性腎疾患 (CKD) の有病率が特に高齢者を中心に増加していること、高リン血症がCKD患者、特に透析を受けている患者にとって重要な疾患として認識されるようになってきたことによって促進されています。さらに、FDAなどの規制当局が新たな治療法の承認を迅速化していることも、市場拡大を後押ししています。CKD患者数が増加し、より多くの治療薬が市場に登場する中で、各企業は製品ポートフォリオの強化や、先進国および新興国の双方における市場アクセスの強化に注力しています。これらの進展を背景に、市場は今後も成長軌道を維持すると予想されますが、競争の激化、ジェネリック医薬品の増加、コスト抑制圧力といった要因が、市場の成熟に伴う課題として浮上する可能性があります。
影響
- 高リン血症治療に対する需要の増加が予測期間中の世界の高リン血症市場の成長を後押しすると見込まれています。
- 診断技術の進歩、革新的な治療法の開発、患者および医療従事者の間での認知度の向上により、世界の高リン血症市場は今後大きな成長が期待されています。
北米は、先進的な医療インフラ、高い有病率、疾患に対する認知度の高さにより、予測期間中の市場をリードすると予想されています。また、同地域には強力な製薬企業の存在があり、効果的な治療法の早期提供を可能にしており、これが世界の高リン血症市場の成長をさらに促進しています。
当レポートでは、世界の高リン血症の市場を調査し、主要動向、市場影響因子の分析、法規制環境、臨床試験の動向、市場規模の推移・予測、各種区分・地域/主要国別の詳細分析、競合情勢、主要企業のプロファイルなどをまとめています。
目次
エグゼクティブサマリー
第1章 世界の高リン血症市場:業界の展望
- 市場概要とエコシステム
- 市場動向
- 高リン血症の疫学的分析
- 臨床試験
- フェーズ別
- スポンサータイプ別
- 規制状況/コンプライアンス
- 米国の法的要件と枠組み
- EUにおける法的要件と枠組み
- アジア太平洋における法的要件と枠組み
- 市場力学
- 影響分析
- 市場促進要因
- 市場抑制要因
- 市場機会
第2章 世界の高リン血症市場:地域別
- 北米
- 欧州
- アジア太平洋
- 市場力学
- 市場規模・予測
第3章 世界の高リン血症市場:競合ベンチマーキング・企業プロファイル
- 競合情勢
- 各社の主要戦略と展開
- 主な展開の分析
- 企業プロファイル
- Kyowa Kirin, Inc.
- Bayer
- Unicycive Therapeutics
- Sanofi
- Shanghai Alebund Pharmaceuticals Limited
- Ardelyx
- Kissei Pharmaceutical Co. Ltd.
- Astellas Pharma Inc.
- Mitsubishi Tanabe Pharma Corporation
第4章 調査手法
List of Figures
- Figure: Global Hyperphosphatemia Market, Market Overview
- Figure: Global Hyperphosphatemia Market, Epidemiological Analysis, U.S.
- Figure: Global Hyperphosphatemia Market Coverage
- Figure: Global Hyperphosphatemia Market Key Trends, Impact Analysis, 2023-2035
- Figure: Global Hyperphosphatemia Market, Competitive Landscape, January 2022-April 2025
List of Tables
- Table: Global Hyperphosphatemia Market, Regulatory Scenario
- Table: Global Hyperphosphatemia Market Dynamics, Impact Analysis
Market Lifecycle Stage
The global hyperphosphatemia market is currently in the growth stage of its lifecycle, marked by expanding demand and increasing renal treatment options. This growth is primarily driven by the rising prevalence of chronic kidney disease (CKD), particularly among aging populations, and the growing recognition of hyperphosphatemia as a critical condition in CKD patients, especially those on dialysis. Additionally, regulatory bodies such as the FDA are fast-tracking approvals for new treatments, which is fueling further market expansion. As the number of patients with CKD increases and more treatments enter the market, companies are focusing on enhancing their product portfolios and strengthening market access, both in developed and emerging markets. With these advancements, the market is expected to maintain its growth trajectory, although increased competition, rising generic alternatives, and cost containment pressures may pose challenges as the market matures.
Impact
- Increasing demand for hyperphosphatemia therapies is anticipated to support the growth of the global hyperphosphatemia market during the forecast period 2025-2035.
- The global hyperphosphatemia market is expected to grow at a significant rate due to advancements in diagnostic technologies, the development of innovative therapies, and increasing awareness among patients and healthcare providers.
North America is expected to dominate the global hyperphosphatemia market during the forecast period due to its advanced healthcare infrastructure, high prevalence, and increased awareness of the disease. The region also benefits from a strong pharmaceutical presence, which accelerates the availability of effective treatments and drives the growth of the global hyperphosphatemia market.
Recent Developments
- Regulatory Activities: In September 2024, Unicycive Therapeutics announced the submission of an NDA to the U.S. FDA for Oxylanthanum Carbonate, intended for the treatment of hyperphosphatemia in patients with chronic kidney disease undergoing dialysis.
- Regulatory Activities: In July 2024, the U.S. FDA expanded the approval of Velphoro (sucroferric oxyhydroxide) for use in patients as young as 9 years old. The drug, previously approved only for adults, is used to control serum phosphorus levels in patients with chronic kidney disease (CKD) undergoing dialysis.
Demand - Drivers and Limitations
Drivers:
- Rising Prevalence of Chronic Kidney Disease
- Continuous Advancements in Treatment Options
Challenges:
- Economic Barriers in Emerging Markets
How Can This Report Add Value to an Organization?
Product/Innovation Strategy: Product launches and innovations in the global hyperphosphatemia market are focused on advancing treatment options to improve patient care. These innovations aim to enhance the efficacy of therapies and streamline the detection and management of the disease. Key players in the market, such as Unicycive Therapeutics, have been involved in the development of therapies for hyperphosphatemia.
Competitive Strategy: Enterprises led by market leaders in the global hyperphosphatemia market are continuously working on updating their product portfolios with innovative treatments to maintain competitiveness. A detailed competitive benchmarking of the key players has been conducted, providing insights into how these companies compare in terms of product offerings, market share, and innovation. This benchmarking provides readers with a clear understanding of the market landscape and the positions of the leading players. Additionally, comprehensive competitive strategies, such as partnerships, agreements, and collaborations, will help readers identify untapped revenue opportunities in the market.
Key Market Players and Competition Synopsis
The companies that are profiled have been selected based on inputs gathered from primary experts and by analyzing company coverage, product portfolio, and market penetration.
Some of the prominent names established in this market are:
- Kyowa Kirin, Inc.
- Bayer
- Unicycive Therapeutics
- Sanofi
- Shanghai Alebund Pharmaceuticals Limited
- Ardelyx
- Kissei Pharmaceutical Co. Ltd.
- Astellas Pharma Inc.
- Mitsubishi Tanabe Pharma Corporation
Table of Contents
Executive Summary
Scope of Study
1. Global Hyperphosphatemia Market: Industry Outlook
- 1.1 Market Overview and Ecosystem
- 1.2 Market Trends
- 1.3 Epidemiological Analysis of Hyperphosphatemia
- 1.3.1 By Region
- 1.4 Clinical Trials
- 1.4.1 By Phase
- 1.4.2 By Sponsor Type
- 1.5 Regulatory Landscape / Compliance
- 1.5.1 Legal Requirement and Framework in the U.S.
- 1.5.2 Legal Requirement and Framework in the E.U.
- 1.5.3 Legal Requirement and Framework in Asia-Pacific
- 1.6 Market Dynamics
- 1.6.1 Impact Analysis
- 1.6.2 Market Drivers
- 1.6.3 Market Restraints
- 1.6.4 Market Opportunities
2. Global Hyperphosphatemia Market, By Region, $Million, 2023-2035
- 2.1 North America
- 2.1.1 Market Dynamics
- 2.1.2 Market Sizing and Forecast
- 2.1.2.1 North America Hyperphosphatemia Market (by Country)
- 2.1.2.1.1 U.S.
- 2.1.2.1 North America Hyperphosphatemia Market (by Country)
- 2.2 Europe
- 2.2.1 Market Dynamics
- 2.2.2 Market Sizing and Forecast
- 2.2.2.1 Europe Hyperphosphatemia Market (by Country)
- 2.2.2.1.1 U.K.
- 2.2.2.1.2 Germany
- 2.2.2.1.3 France
- 2.2.2.1.4 Italy
- 2.2.2.1 Europe Hyperphosphatemia Market (by Country)
- 2.3 Asia-Pacific
- 2.3.1 Market Dynamics
- 2.3.2 Market Sizing and Forecast
- 2.3.2.1 Asia-Pacific Hyperphosphatemia Market (by Country)
- 2.3.2.1.1 Japan
- 2.3.2.1 Asia-Pacific Hyperphosphatemia Market (by Country)
3. Global Hyperphosphatemia Market - Competitive Benchmarking and Company Profiles
- 3.1 Competitive Landscape
- 3.1.1 Key Strategies and Developments by Company
- 3.1.1.1 Funding Activities
- 3.1.1.2 Mergers and Acquisitions
- 3.1.1.3 Regulatory Approvals
- 3.1.1.4 Partnerships, Collaborations and Business Expansions
- 3.1.2 Key Developments Analysis
- 3.1.1 Key Strategies and Developments by Company
- 3.2 Company Profiles
- 3.2.1 Kyowa Kirin, Inc.
- 3.2.1.1 Company Overview
- 3.2.1.2 Product Portfolio
- 3.2.1.3 Target Customers/End Users
- 3.2.1.4 Analyst View
- 3.2.2 Bayer
- 3.2.2.1 Company Overview
- 3.2.2.2 Product Portfolio
- 3.2.2.3 Target Customers/End Users
- 3.2.2.4 Analyst View
- 3.2.3 Unicycive Therapeutics
- 3.2.3.1 Company Overview
- 3.2.3.2 Product Portfolio
- 3.2.3.3 Target Customers/End Users
- 3.2.3.4 Analyst View
- 3.2.4 Sanofi
- 3.2.4.1 Company Overview
- 3.2.4.2 Product Portfolio
- 3.2.4.3 Target Customers/End Users
- 3.2.4.4 Analyst View
- 3.2.5 Shanghai Alebund Pharmaceuticals Limited
- 3.2.5.1 Company Overview
- 3.2.5.2 Product Portfolio
- 3.2.5.3 Target Customers/End Users
- 3.2.5.4 Analyst View
- 3.2.6 Ardelyx
- 3.2.6.1 Company Overview
- 3.2.6.2 Product Portfolio
- 3.2.6.3 Target Customers/End Users
- 3.2.6.4 Analyst View
- 3.2.7 Kissei Pharmaceutical Co. Ltd.
- 3.2.7.1 Company Overview
- 3.2.7.2 Product Portfolio
- 3.2.7.3 Target Customers/End Users
- 3.2.7.4 Analyst View
- 3.2.8 Astellas Pharma Inc.
- 3.2.8.1 Company Overview
- 3.2.8.2 Product Portfolio
- 3.2.8.3 Target Customers/End Users
- 3.2.8.4 Analyst View
- 3.2.9 Mitsubishi Tanabe Pharma Corporation
- 3.2.9.1 Company Overview
- 3.2.9.2 Product Portfolio
- 3.2.9.3 Target Customers/End Users
- 3.2.9.4 Analyst View
- 3.2.1 Kyowa Kirin, Inc.