|
|
市場調査レポート
商品コード
1729048
非嚢胞性線維症性気管支拡張症の世界市場:薬剤クラス・地域別の分析・予測 (2025-2035年)Non-Cystic Fibrosis Bronchiectasis Market - A Global and Regional Analysis: Focus on Drug Class and Region - Analysis and Forecast, 2025-2035 |
||||||
カスタマイズ可能
|
|||||||
| 非嚢胞性線維症性気管支拡張症の世界市場:薬剤クラス・地域別の分析・予測 (2025-2035年) |
|
出版日: 2025年05月21日
発行: BIS Research
ページ情報: 英文 100 Pages
納期: 1~5営業日
|
全表示
- 概要
- 図表
- 目次
非嚢胞性線維症性気管支拡張症 (NCFB) の診断は高解像度のCTスキャンと肺機能検査によって行われ、治療には通常、感染症を管理するための抗生物質、粘液を除去するための粘液溶解薬、気流を改善するための気管支拡張薬、肺リハビリテーションが含まれます。
感染予防や症状コントロールを含む適切な管理により、患者は生活の質を改善することができるが、この病気は時間とともに肺機能を徐々に低下させる可能性があります。
非嚢胞性線維症性気管支拡張症市場の主な促進要因の一つは、この疾患に対する認知度の向上と診断の改善です。医療従事者がこの疾患とその症状をより深く理解するようになるにつれ、より早期に診断される患者が増加しています。この傾向は、気管支拡張の特徴である気道の拡大を正確に検出できる高解像度CTスキャンなどの診断技術の進歩によって支えられています。認識の向上と診断技術の改善により、非嚢胞性線維症性気管支拡張症は独立した疾患として認識されるようになり、早期治療および症状管理の促進につながっています。その結果、抗生物質、去痰薬、気管支拡張薬、生物学的製剤などの有効な治療法に対する需要が高まり、市場成長を牽引しています。
一方で、この市場の成長にもかかわらず、いくつかの課題が進展を妨げ、治療の有効性を制限しています。主な課題の一つは、非嚢胞性線維症性気管支拡張症に対して米国FDAに承認された特定の治療薬が存在しないことです。現在用いられている治療法の多くは、嚢胞性線維症や慢性閉塞性肺疾患(COPD)など他疾患向けに承認された薬剤の適応外使用であり、標準的な治療指針の確立が困難となっています。さらに、生物学的製剤、長期抗生物質療法、吸入療法などの先進治療の高コストも問題であり、特に低所得地域や保険が不十分な患者にとってはアクセスが制限されます。
また、疾患の多様性があるため、すべての患者に共通する画一的な治療法の開発は困難です。なぜなら、非嚢胞性線維症性気管支拡張症は患者ごとに異なる形で現れ、原因、重症度、治療への反応もそれぞれ異なるからです。 さらに、診断が遅れると重症化することが多く、治療が複雑になり、利用可能な治療法の有効性が制限されます。 また、医療従事者や一般市民の認知度不足も、診断の遅れや見逃しにつながりやすく、特に医療資源の限られた地域では深刻です。これらの課題は、標的研究の推進、特定治療薬の承認、医療アクセスの改善の必要性を強く示しています。
当レポートでは、世界の非嚢胞性線維症性気管支拡張症の市場を調査し、主要動向、市場影響因子の分析、法規制環境、臨床試験の動向、市場規模の推移・予測、各種区分・地域/主要国別の詳細分析、競合情勢、主要企業のプロファイルなどをまとめています。
目次
エグゼクティブサマリー
第1章 世界の非嚢胞性線維症性気管支拡張症市場:業界の展望
- 市場動向
- 規制の枠組み
- 疫学分析
- 臨床試験分析
- 市場力学
- 影響分析
- 市場促進要因
- 市場の課題
- 市場機会
第2章 世界の非嚢胞性線維症性気管支拡張症市場:薬剤クラス別
- 抗生物質
- コルチコステロイド
- 気管支拡張薬
- その他
第3章 世界の非嚢胞性線維症性気管支拡張症市場:地域別
- 北米
- 欧州
- アジア太平洋
- 主な調査結果
- 市場力学
- 市場規模・予測
第4章 世界の非嚢胞性線維症性気管支拡張症市場:競合情勢と企業プロファイル
- 主な戦略・展開
- 企業プロファイル
- AstraZeneca
- Sanofi
- Insmed
- Boehringer Ingelheim
- Zambon
- Chiesi Pharmaceuticals
- Haisco Pharmaceutical Group
- Regeneron Pharmaceuticals
第5章 調査手法
List of Figures
- Figure: Global Non-Cystic Fibrosis Bronchiectasis Market (by Region), $Million, 2024 and 2035
- Figure: Global Non-Cystic Fibrosis Bronchiectasis Market Key Trends, Analysis
List of Tables
- Table: Global Non-Cystic Fibrosis Bronchiectasis Market Dynamics, Impact Analysis
- Table: Global Non-Cystic Fibrosis Bronchiectasis Market (by Drug Class), $Million, 2023-2035
- Table: Global Non-Cystic Fibrosis Bronchiectasis Market (by Region), $Million, 2023-2035
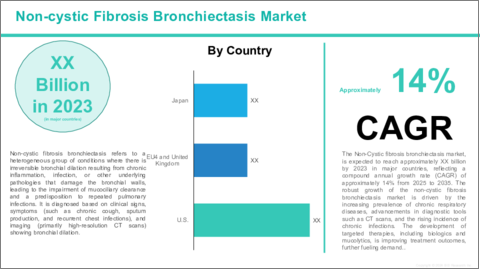
Global Non-Cystic Fibrosis Bronchiectasis Market, Analysis and Forecast: 2025-2035
Non-Cystic fibrosis bronchiectasis (NCFB) is a chronic lung condition characterized by the permanent widening and scarring of the bronchi, the airways in the lungs. Unlike as cystic fibrosis, Non-cystic fibrosis bronchiectasis occurs in individuals without the genetic disorder and is typically caused by recurrent respiratory infections, autoimmune diseases, immunodeficiency disorders, or aspiration of food and liquids into the lungs. The condition leads to the accumulation of mucus in the airways, which makes it difficult for the body to clear bacteria and other pathogens, resulting in frequent infections, chronic cough, sputum production, and shortness of breath. While the exact cause can vary, many cases of non-cystic fibrosis bronchiectasis are associated with chronic inflammation and repeated bacterial infections. Diagnosis is made through high-resolution CT scans and lung function tests, and treatment typically involves antibiotics to manage infections, mucolytics to clear mucus, bronchodilators to improve airflow, and pulmonary rehabilitation. With proper management, including infection prevention and symptom control, patients can improve their quality of life, although the disease may progressively impair lung function over time.
One of the key drivers of the non-cystic fibrosis bronchiectasis market is the increasing awareness and improved diagnosis of the condition. As healthcare professionals gain a better understanding of non-cystic fibrosis bronchiectasis and its symptoms, more patients are being diagnosed at earlier stages.
This has been facilitated by advancements in diagnostic technologies such as high-resolution CT scans, which are able to detect airway dilation characteristic of bronchiectasis more accurately. With greater awareness and better diagnostic capabilities, there is a growing recognition of non-cystic fibrosis bronchiectasis as a distinct disease, leading to earlier treatment and better management of symptoms. As a result, the demand for effective therapies, including antibiotics, mucolytics, bronchodilators, and biologic treatments, is on the rise, driving market growth.
Despite the growth of the non-cystic fibrosis bronchiectasis market, several challenges continue to hinder its progress and the overall effectiveness of treatment. One major issue is the lack of specific FDA-approved therapies for non-cystic fibrosis bronchiectasis, with most treatments being off-label uses of drugs approved for other conditions such as cystic fibrosis or chronic obstructive pulmonary disease (COPD). This limits treatment options and makes it harder to develop standardized treatment protocols. Another challenge is the high cost of advanced therapies, such as biologics, long-term antibiotics, and inhalation treatments, which can be prohibitively expensive, limiting access for patients, especially in low-income regions or for those without comprehensive insurance coverage.
Additionally, disease heterogeneity makes it difficult to develop one-size-fits-all therapies, as Non-cystic fibrosis bronchiectasis presents differently in various patients, with different underlying causes, severity, and response to treatment. Moreover, late-stage diagnosis often results in more severe disease, which complicates treatment and limits the effectiveness of available therapies. There is also a lack of awareness among healthcare providers and the general public, leading to delays in diagnosis and underdiagnosis, particularly in regions with limited access to healthcare resources. These challenges emphasize the need for targeted research, regulatory approval of specific therapies, and improved healthcare access to better manage non-cystic fibrosis bronchiectasis.
The global non-cystic fibrosis bronchiectasis market is highly competitive, with several leading pharmaceutical companies playing a pivotal role in advancing treatments for this chronic respiratory disease. Major players such as AstraZeneca, GlaxoSmithKline (GSK), Novartis, Boehringer Ingelheim, Pfizer, Vertex Pharmaceuticals, Genentech (Roche), Chiesi Pharmaceuticals, and Teva Pharmaceutical Industries are actively developing a range of therapies, including bronchodilators, mucolytics, antibiotics, and biologic treatments. These companies are focused on improving patient outcomes by targeting the underlying causes of non-cystic fibrosis bronchiectasis, such as chronic inflammation and recurrent infections, while also enhancing symptom management. As research in personalized medicine and novel therapies continues to evolve, these companies are positioned to significantly impact the Non-Cystic fibrosis bronchiectasis market, offering better options for those suffering from non-cystic fibrosis bronchiectasis and aiming to improve their quality of life.
Market Segmentation:
Segmentation 1: by Drug Class
- Antibiotics
- Corticosteroids
- Bronchodilators
- Others
Segmentation 2: by Region
- North America
- Europe
- Asia-Pacific
The global non-cystic fibrosis bronchiectasis market is experiencing several key emerging trends that are significantly shaping the future of treatment. One of the most prominent trends is the shift towards personalized medicine, where treatments are becoming more tailored to individual patients based on their specific genetic, microbial, and disease characteristics. This approach aims to improve the effectiveness of therapies while minimizing side effects.
Additionally, there is a growing emphasis on the development of novel biologic therapies and targeted treatments that address the underlying inflammation and infection that drive the progression of non-cystic fibrosis bronchiectasis. The use of advanced diagnostics, such as high-resolution CT scans and microbial profiling, is enabling earlier and more accurate diagnosis, leading to more precise and timely interventions. Another significant trend is the increased focus on combination therapies, where medications such as bronchodilators, antibiotics, and mucolytics are used together to target multiple aspects of the disease. These trends are driving innovation in the market, offering hope for better management and improved quality of life for patients with non-cystic fibrosis bronchiectasis.
Table of Contents
Executive Summary
Scope and Definition
Market/Product Definition
Inclusion and Exclusion
Key Questions Answered
Analysis and Forecast Note
1. Global Non-Cystic Fibrosis Bronchiectasis Market: Industry Outlook
- 1.1 Introduction
- 1.2 Market Trends
- 1.3 Regulatory Framework
- 1.4 Epidemiology Analysis
- 1.5 Clinical Trial Analysis
- 1.6 Market Dynamics
- 1.6.1 Impact Analysis
- 1.6.2 Market Drivers
- 1.6.3 Market Challenges
- 1.6.4 Market Opportunities
2. Global Non-Cystic Fibrosis Bronchiectasis Market, by Drug Class, $Million, 2023-2035
- 2.1 Antibiotics
- 2.2 Corticosteroids
- 2.3 Bronchodilators,
- 2.4 Others
3. Global Non-Cystic Fibrosis Bronchiectasis Market (Region), ($Million), 2023-2035
- 3.1 North America
- 3.1.1 Key Findings
- 3.1.2 Market Dynamics
- 3.1.3 Market Sizing and Forecast
- 3.1.3.1 North America Non-Cystic Fibrosis Bronchiectasis Market, by Country
- 3.1.3.1.1 U.S.
- 3.1.3.1 North America Non-Cystic Fibrosis Bronchiectasis Market, by Country
- 3.2 Europe
- 3.2.1 Key Findings
- 3.2.2 Market Dynamics
- 3.2.3 Market Sizing and Forecast
- 3.2.3.1 Europe Non-Cystic Fibrosis Bronchiectasis Market, by Country
- 3.2.3.1.1 Germany
- 3.2.3.1.2 U.K.
- 3.2.3.1.3 France
- 3.2.3.1.4 Italy
- 3.2.3.1 Europe Non-Cystic Fibrosis Bronchiectasis Market, by Country
- 3.3 Asia Pacific
- 3.3.1 Key Findings
- 3.3.2 Market Dynamics
- 3.3.3 Market Sizing and Forecast
- 3.3.3.1 Asia Pacific Non-Cystic Fibrosis Bronchiectasis Market, by Country
- 3.3.3.1.1 Japan
- 3.3.3.1 Asia Pacific Non-Cystic Fibrosis Bronchiectasis Market, by Country
4. Global Non-Cystic Fibrosis Bronchiectasis Market: Competitive Landscape and Company Profiles
- 4.1 Key Strategies and Development
- 4.1.1 Mergers and Acquisitions
- 4.1.2 Synergistic Activities
- 4.1.3 Business Expansions and Funding
- 4.1.4 Product Launches and Approvals
- 4.1.5 Other Activities
- 4.2 Company Profiles
- 4.2.1 AstraZeneca
- 4.2.1.1 Overview
- 4.2.1.2 Top Products / Product Portfolio
- 4.2.1.3 Top Competitors
- 4.2.1.4 Target Customers/End-Users
- 4.2.1.5 Key Personnel
- 4.2.1.6 Analyst View
- 4.2.2 Sanofi
- 4.2.2.1 Overview
- 4.2.2.2 Top Products / Product Portfolio
- 4.2.2.3 Top Competitors
- 4.2.2.4 Target Customers/End-Users
- 4.2.2.5 Key Personnel
- 4.2.2.6 Analyst View
- 4.2.3 Insmed
- 4.2.3.1 Overview
- 4.2.3.2 Top Products / Product Portfolio
- 4.2.3.3 Top Competitors
- 4.2.3.4 Target Customers/End-Users
- 4.2.3.5 Key Personnel
- 4.2.3.6 Analyst View
- 4.2.4 Boehringer Ingelheim
- 4.2.4.1 Overview
- 4.2.4.2 Top Products / Product Portfolio
- 4.2.4.3 Top Competitors
- 4.2.4.4 Target Customers/End-Users
- 4.2.4.5 Key Personnel
- 4.2.4.6 Analyst View
- 4.2.5 Zambon
- 4.2.5.1 Overview
- 4.2.5.2 Top Products / Product Portfolio
- 4.2.5.3 Top Competitors
- 4.2.5.4 Target Customers/End-Users
- 4.2.5.5 Key Personnel
- 4.2.5.6 Analyst View
- 4.2.6 Chiesi Pharmaceuticals
- 4.2.6.1 Overview
- 4.2.6.2 Top Products / Product Portfolio
- 4.2.6.3 Top Competitors
- 4.2.6.4 Target Customers/End-Users
- 4.2.6.5 Key Personnel
- 4.2.6.6 Analyst View
- 4.2.7 Haisco Pharmaceutical Group
- 4.2.7.1 Overview
- 4.2.7.2 Top Products / Product Portfolio
- 4.2.7.3 Top Competitors
- 4.2.7.4 Target Customers/End-Users
- 4.2.7.5 Key Personnel
- 4.2.7.6 Analyst View
- 4.2.8 Regeneron Pharmaceuticals
- 4.2.8.1 Overview
- 4.2.8.2 Top Products / Product Portfolio
- 4.2.8.3 Top Competitors
- 4.2.8.4 Target Customers/End-Users
- 4.2.8.5 Key Personnel
- 4.2.8.6 Analyst View
- 4.2.1 AstraZeneca