|
|
市場調査レポート
商品コード
1419698
高活性API市場:世界および地域の分析:タイプ別、合成タイプ別、治療領域別、製造タイプ別、エンドユーザー別、国別- 分析と予測、2023-2033年Highly Potent API Market - A Global and Regional Analysis: Focus on Type, Type of Synthesis, Therapeutic Area, Type of Manufacturing, End User, and Country - Analysis and Forecast, 2023-2033 |
||||||
カスタマイズ可能
|
|||||||
| 高活性API市場:世界および地域の分析:タイプ別、合成タイプ別、治療領域別、製造タイプ別、エンドユーザー別、国別- 分析と予測、2023-2033年 |
|
出版日: 2024年02月02日
発行: BIS Research
ページ情報: 英文 116 Pages
納期: 1~5営業日
|
全表示
- 概要
- 図表
- 目次
世界の高活性APIの市場規模は、2023年の274億4,000万米ドルから、予測期間中は11.86%のCAGRで推移し、2033年には842億米ドルに達すると予測されています。
同市場の成長を促進する主な要因は、高活性APIの採用需要につながる癌の発生率の増加、高活性APIの治療用途の拡大などです。
| 主要市場統計 | |
|---|---|
| 予測期間 | 2023-2033年 |
| 2023年評価 | 274億4,000万米ドル |
| 2033年予測 | 842億米ドル |
| CAGR | 11.86% |
市場需要促進要因:
癌罹患率の増加による高活性APIの採用需要:高活性API需要の主な促進要因の一つは、癌領域の活況です。癌の罹患率が上昇を続ける中、より効果的で標的を絞った治療法の必要性が高まっています。高活性APIは、標的ペイロードを腫瘍細胞に直接送達する強力な抗癌剤の一種である抗体薬物複合体 (ADC) の開発において重要な役割を果たしています。高活性APIは、その高い効力と標的作用により、ソリューションを提供します。高活性APIは癌細胞上の特定の分子に結合し、健康な細胞への害を最小限に抑え、従来の薬剤に比べて高い有効性と副作用の軽減をもたらします。
ドラッグデリバリー技術の進歩:高活性APIのドラッグデリバリー技術の進歩には、これらの強力な物質を人体内に効率的かつ安全に送達する革新的な方法の開発が含まれます。これらの技術は、治療効果を高め、副作用を最小限に抑え、患者の治療へのアドヒアランスを向上させることを目的としています。このような進歩の例としては、標的送達システム、ナノテクノロジーに基づく製剤、新規カプセル化技術などが挙げられます。これらの技術革新は薬物の吸収、分布、放出を最適化することに貢献し、それによって強力な原薬を用いた様々な病態の治療に革命をもたらします。
高活性APIの治療用途の拡大:高活性APIの治療用途の拡大は、多様な疾患領域の医療を変革する可能性を秘めた、非常に有望な動向です。このような汎用性の高い化合物の可能性を最大限に引き出し、より広範な病態の患者の転帰を改善するためには、課題に対処し、機会を捉えることが重要です。
当レポートでは、世界の高活性APIの市場を調査し、市場概要、市場成長への各種影響因子の分析、特許動向、法規制環境、市場規模の推移・予測、主要国別の詳細分析、主要企業の分析などをまとめています。
目次
エグゼクティブサマリー
範囲と定義
第1章 市場
- 動向:現在および将来の影響評価
- サプライチェーン/バリューチェーンの概要
- 価格分析
- 特許出願動向 (年・国別)
- 規制状況
- 主要な世界的出来事の影響分析:COVID-19
- 市場力学:概要
- 市場促進要因
- 市場機会
- 市場抑制要因
第2章 世界の高活性API市場:タイプ別
- 画期的高効力API
- ジェネリック高効力API
第3章 世界の高活性API市場:合成タイプ別
- 合成
- バイオテクノロジー
第4章 世界の高活性API市場:製造タイプ別
- 社内
- 外部委託
第5章 世界の高活性API市場:治療領域別
- 腫瘍
- 免疫
- ホルモン障害
- 感染症
- その他
第6章 世界の高活性API市場:エンドユーザー別
- バイオ医薬品およびライフサイエンス企業
- 医薬品受託製造機関
- 研究機関
第7章 地域
- 地域概要
- 北米
- 欧州
- アジア太平洋
- ラテンアメリカ
- 中東・アフリカ
第8章 市場:競合ベンチマーキング・企業プロファイル
- 次のフロンティア
- 地域的評価
- AbbVie Inc.
- Almac Group
- Asymchem Inc.
- Dr. Reddy's Laboratories Ltd.
- Axplora Group GmbH
- BASF SE
- CARBOGEN AMCIS
- CordenPharma International
- Curia Global, Inc.
- Helsinn Healthcare SA.
- ICROM
- Lonza
- Merck KGaA
- PCI Pharma Services
- Pfizer Inc. (Pfizer CentreOne)
- Sterling Pharma Solutions
第9章 調査手法
List of Figures
- Figure 1: Global Highly Potent API Market (by Region), $Billion, 2022-2033
- Figure 2: Global Highly Potent API Market (by Type), $Billion, 2022, 2026, and 2033
- Figure 3: Global Highly Potent API Market (by Therapeutic Area), $Billion, 2022, 2026, and 2033
- Figure 4: Global Highly Potent API Market (by Type of Synthesis), $Billion, 2022, 2026, and 2033
- Figure 5: Global Highly Potent API Market (by Type of Manufacturing), $Billion, 2022, 2026, and 2033
- Figure 6: Global Highly Potent API Market (by End User), $Billion, 2022, 2026, and 2033
- Figure 7: Global Highly Potent API Market, Recent Developments
- Figure 8: Global Highly Potent API Market: Value Chain Analysis
- Figure 9: Global Highly Potent API Market: Pricing Analysis
- Figure 10: Global Highly Potent API Market, Patent Analysis (by Year), January 2018-December 2023
- Figure 11: Global Highly Potent API Market, Patent Analysis (by Country), January 2018-December 2023
- Figure 12: Regulatory Framework for Highly Potent API
- Figure 13: Impact Analysis of Market Navigating Factors, 2022-2033
- Figure 14: Incidence of Cancer (by Region), Million, 2020-2040
- Figure 15: North America Highly Potent API Market, $Billion, 2022-2033
- Figure 16: U.S. Highly Potent API Market, $Billion, 2022-2033
- Figure 17: Canada Highly Potent API Market, $Billion, 2022-2033
- Figure 18: Europe Highly Potent API Market, $Billion, 2022-2033
- Figure 19: France Highly Potent API Market, $Billion, 2022-2033
- Figure 20: Germany Highly Potent API Market, $Billion, 2022-2033
- Figure 21: U.K. Highly Potent API Market, $Billion, 2022-2033
- Figure 22: Spain Highly Potent API Market, $Billion, 2022-2033
- Figure 23: Italy Highly Potent API Market, $Billion, 2022-2033
- Figure 24: Rest-of-Europe Highly Potent API Market, $Billion, 2022-2033
- Figure 25: Asia-Pacific Highly Potent API Market, $Billion, 2022-2033
- Figure 26: China Highly Potent API Market, $Billion, 2022-2033
- Figure 27: India Highly Potent API Market, $Billion, 2022-2033
- Figure 28: Japan Highly Potent API Market, $Billion, 2022-2033
- Figure 29: Australia Highly Potent API Market, $Billion, 2022-2033
- Figure 30: South Korea Highly Potent API Market, $Billion, 2022-2033
- Figure 31: Rest-of-Asia-Pacific Highly Potent API Market, $Billion, 2022-2033
- Figure 32: Latin America Highly Potent API Market, $Billion, 2022-2033
- Figure 33: Brazil Highly Potent API Market, $Billion, 2022-2033
- Figure 34: Mexico Highly Potent API Market, $Billion, 2022-2033
- Figure 35: Rest-of-Latin America Highly Potent API Market, $Billion, 2022-2033
- Figure 36: Middle East and Africa Highly Potent API Market, $Billion, 2022-2033
- Figure 37: U.A.E. Highly Potent API Market, $Billion, 2022-2033
- Figure 38: K.S.A. Highly Potent API Market, $Billion, 2022-2033
- Figure 39: Rest-of-Middle East and Africa Highly Potent API Market, $Billion, 2022-2033
- Figure 40: Some of the Key Innovators in the Highly Potent API Landscape
- Figure 41: Global Highly Potent API Market: Geographical Assessment
- Figure 42: Strategic Initiatives, January 2021-December 2023
- Figure 43: Share of Strategic Initiatives
- Figure 44: Data Triangulation
- Figure 45: Top-Down and Bottom-Up Approach
- Figure 46: Assumptions and Limitations
List of Tables
- Table 1: Global Highly Potent API Market Snapshot
- Table 2: Short-Term and Long-Term Opportunities and Risks (by Region)
- Table 3: Key Trends, Impact Analysis
- Table 4: Global Highly Potent API Market, Key Investment
- Table 5: Recent Innovations for Manufacturing and Handling of Highly Potent Drugs
- Table 6: Global Highly Potent API Market (by Type), $Billion, 2022-2033
- Table 7: Global Highly Potent API Market (Type of Synthesis), $Billion, 2022-2033
- Table 8: Global Highly Potent API Market (by Type of Manufacturing), $Billion, 2022-2033
- Table 9: Global Highly Potent API Market (by Therapeutic Area), $Billion, 2022-2033
- Table 10: Global Highly Potent API Market (by End User), $Billion, 2022-2033
- Table 11: Highly Potent API Market (by Region), $Billion, 2022-2033
- Table 12: Global Highly Potent API Market (by Country), $Billion, 2022-2033
“The Global Highly Potent API Market Expected to Reach $84.20 Billion by 2033.”
Introduction to Highly potent API Market
The global highly potent API market is projected to reach $84.20 billion by 2033 from $27.44 billion in 2023, growing at a CAGR of 11.86% during the forecast period 2023-2033. The key factors driving the growth of the global highly potent API market include increasing incidence of cancer leading to demand in the adoption of highly potent APIs, expanding therapeutic applications of highly potent API.
| KEY MARKET STATISTICS | |
|---|---|
| Forecast Period | 2023 - 2033 |
| 2023 Evaluation | $27.44 Billion |
| 2033 Forecast | $84.20 Billion |
| CAGR | 11.86% |
Market Introduction
The global highly potent API market consists of comprise innovative high-potency APIs and generic high-potency APIs used for therapeutic areas, which include oncology, immunology, hormonal disorders, infectious diseases, and others.
Impact Analysis:
The highly potent API market has made an impact in the following ways:
Advancements in Containment Technologies for Highly Potent APIs: Advancements in containment technologies are key enablers for the safe and sustainable development and production of HPAPIs. Addressing the challenges and seizing the opportunities will be crucial to harnessing the full potential of these potent compounds and shaping a safer, more efficient, and environmentally responsible future for the pharmaceutical industry.
Increasing Investment for Highly Potent API Manufacturing Units: The surge in investments for HPAPI manufacturing units is a highly promising trend with the potential to revolutionize healthcare. Addressing the challenges alongside capitalizing on the opportunities will be key to unlocking the full potential of these potent compounds and transforming patient outcomes across diverse therapeutic areas.
With the increasing demand for targeted therapies and personalized medicine, investments enable companies to align their portfolios with market needs. This strategic alignment can lead to the development of drugs with higher efficacy and fewer side effects.
Market Segmentation:
Segmentation 1: by Type
- Innovative High-Potency APIs
- Generic High-Potency APIs
Innovative High-Potency APIs Segment to Lead the Highly Potent API Market (by Type)
The innovative high-potency APIs segment held the highest share in the global highly potent API market (by type) in 2022 and is anticipated to hold its dominance till the end of the forecast period.
Segmentation 2: by Therapeutic Area
- Oncology
- Immunology
- Hormonal Disorders
- Infectious Diseases
- Others
Oncology Segment to Lead the Highly Potent API Market (by Therapeutic Area)
The oncology segment held the highest share in the global highly potent API market (by therapeutic area) in 2022 and is anticipated to hold its dominance till the end of the forecast period.
Segmentation 3: by Type of Manufacturing
- In-House
- Outsourced
Outsourced Segment to Lead the Highly Potent API Market (by Type of Manufacturing)
The outsourced segment held the highest share in the global highly potent API market (by type of manufacturing) in 2022 and is anticipated to hold its dominance till the end of the forecast period.
Segmentation 4: by Type of Synthesis
- Synthetic
- Biological
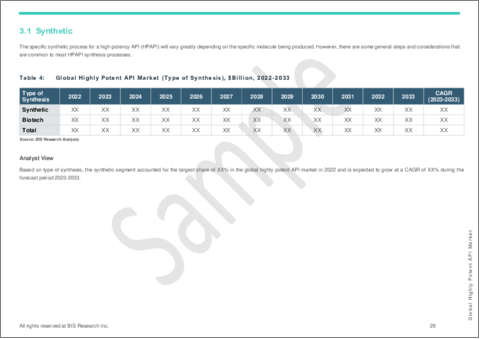
Synthetic Segment to Lead the Highly Potent API Market (by Type of Synthesis)
The synthetic segment held the highest share in the global highly potent API market (by type of synthesis) in 2022 and is anticipated to hold its dominance till the end of the forecast period.
Segmentation 5: by End User
- Biopharmaceutical and Life Science Companies
- Contract Drug Manufacturing Organizations
- Research Institutions
Contract Drug Manufacturing Organizations Segment to Lead the Highly Potent API Market (by End User)
The contract drug manufacturing organizations segment held the highest share in the global highly potent API market (by end user) in 2022 and is anticipated to hold its dominance till the end of the forecast period.
Segmentation 6: by Region
- North America
- U.S.
- Canada
- Europe
- U.K.
- Germany
- France
- Italy
- Spain
- Rest-of-Europe
- Asia-Pacific
- China
- Japan
- India
- South Korea
- Australia
- Rest-of-Asia-Pacific
- Latin America
- Brazil
- Mexico
- Rest-of-Latin America
- Middle East and Africa
- K.S.A.
- U.A.E.
- Rest-of-Middle East and Africa
China dominated the Asia-Pacific highly potent API market in 2022. The country has a growing population with cancer, and hormonal disorder. Highly potent API offers effective and accessible result for these conditions.
The Chinese government recognizes the potential of highly potent API and is actively promoting its development and manufacturing.
Recent Developments in the Highly potent API Market
- In December 2022, Almac concluded the initial phase of its good manufacturing practice (GMP) active pharmaceutical ingredient (API) facility expansion as part of a multi-million-pound investment program.
- In October 2022, Asymchem Inc., a prominent global provider of contract development and manufacturing services, and AUM Biosciences (AUM), a global biotech company in the clinical stage, with a focus on the discovery, acquisition, and development of next-generation targeted oncology therapeutics, jointly declared the successful conclusion of their inaugural GMP production campaign for AUM601.
- In April 2022, Asymchem Inc. announced the construction of a new facility at its Dunhua production site in China. This facility is dedicated to the extensive implementation of continuous flow technology in manufacturing processes.
- In October 2023, Axplora's subsidiary Farmabios secured cGMP approval from AIFA for expanded HPAPI and steroid production capacity.
- In January 2022, Helsinn and Immedica established an exclusive partnership for the commercialization of cancer supportive care products in key European markets.
- In June 2022, Merck KGaA announced that its life science business sector has increased its production capacity for high-potent active pharmaceutical ingredients (HPAPI) twofold through the expansion of its facility in Verona, situated near Madison, Wisconsin, U.S.
- In January 2022, PCI Pharma announced a substantial investment in increased capacity and enhanced capabilities, further augmenting its globally renowned center of excellence dedicated to the manufacturing of highly potent products.
- In March 2022, Pfizer Inc. (Pfizer CentreOne) inaugurated a cutting-edge drug manufacturing facility in Freiburg, Germany, representing a $331 million investment.
Demand - Drivers and Limitations
Market Demand Drivers:
Increasing Incidence of Cancer Leading to Demand in the Adoption of Highly Potent APIs: One of the primary drivers of HPAPI demand is the booming oncology landscape. As cancer rates continue to climb, the need for more effective and targeted therapies intensifies. HPAPIs play a crucial role in the development of antibody-drug conjugates (ADCs), a class of potent cancer drugs that deliver targeted payloads directly to tumor cells. HPAPIs, with their high potency and targeted action, offer a solution. They bind to specific molecules on cancer cells, minimizing harm to healthy cells and leading to higher efficacy and reduced side effects compared to traditional drugs.
Advancements in Drug Delivery Technologies: Advancements in drug delivery technologies for highly potent Active Pharmaceutical Ingredients (APIs) involve the development of innovative methods to deliver these powerful substances efficiently and safely within the human body. These technologies aim to enhance therapeutic efficacy, minimize side effects, and improve patient adherence to treatment. Examples of such advancements include targeted delivery systems, nanotechnology-based formulations, and novel encapsulation techniques. These innovations contribute to optimizing drug absorption, distribution, and release, thereby revolutionizing the treatment of various medical conditions with potent APIs.
Expanding Therapeutic Applications of Highly Potent API: The expanding therapeutic applications of HPAPIs are a highly promising trend with the potential to transform healthcare across diverse disease areas. Addressing the challenges and seizing the opportunities will be crucial to unlocking the full potential of these versatile compounds and improving patient outcomes for a wider range of medical conditions.
Market Restraints:
Regulatory Complexity for Highly Potent APIs' Manufacturing: The production and use of highly potent active pharmaceutical ingredients (HPAPIs) present specific regulatory compliance challenges due to their potent nature. These challenges arise from concerns about worker safety, environmental impact, and potential risks to end users. Stringent regulatory requirements for HPAPIs can contribute to increased costs associated with development, manufacturing, and compliance.
High Development and Production Costs: The significant development and production costs of HPAPIs are a well-recognized hurdle on the path to unlocking their vast therapeutic potential. The shift toward smaller, more targeted HPAPI production leads to higher per-unit costs. Continuous manufacturing and process optimization are seen as potential means to improve efficiency and economies of scale.
Market Opportunities:
Growing Emphasis on Personalized Medicine: HPAPIs are playing a pivotal role in shaping the future of personalized medicine, but challenges remain. By fostering collaboration among researchers, pharmaceutical companies, regulators, and healthcare providers, can overcome these hurdles and unlock the full potential of HPAPIs. This will pave the way for a future where every patient receives treatment tailored to their unique needs, maximizing their chances for a healthy and fulfilling life.
Increasing Opportunities for Pharma Companies in Developing Markets: The opportunity for established companies entering emerging markets for HPAPI production offers a win-win scenario. It can benefit both the companies seeking cost advantages and access to new markets and the emerging countries aiming to develop their pharmaceutical industries and gain valuable expertise. However, careful consideration of potential challenges and ensuring equitable partnerships are crucial for sustainable and mutually beneficial outcomes.
How can this report add value to an organization?
Product/Innovation Strategy: The global highly potent API market has been extensively segmented based on various categories, such as type, therapeutic area, type of manufacturing, type of synthesis, end user, and region. This can help readers get a clear overview of which segments account for the largest share and which ones are well-positioned to grow in the coming years.
Growth/Marketing Strategy: Synergistic activities accounted for the maximum number of key developments, i.e., nearly 87.00% of the total developments in the global highly potent API market were between January 2021 and December 2023.
Competitive Strategy: The global highly potent API market has numerous established players with product and service portfolios. Key players in the global highly potent API market analysed and profiled in the study involve established players offering product and services of highly potent API.
Methodology
Key Considerations and Assumptions in Market Engineering and Validation
- Detailed secondary research was performed to ensure maximum coverage of manufacturers/suppliers operational in a country.
- Exact revenue information, up to a certain extent, was extracted for each company from secondary sources and databases. The revenues specific to the pr type, therapeutic area, type of manufacturing, type of synthesis, end user, and region were then estimated for each market player based on fact-based proxy indicators as well as primary inputs.
- The scope of this report has been carefully derived based on interactions with experts in different companies across the world. This report provides a market study of highly potent API.
- The market contribution of the highly potent API anticipated to be launched in the future has been calculated based on historical analysis. This analysis has been supported by proxy factors such as the innovation scale of the companies, the status of funding, collaborations, customer base, and patent scenario.
- The scope of availability of highly potent API in a particular region has been assessed based on a comprehensive analysis of companies' prospects, regional end-user perception, and other factors impacting the launch of highly potent API in that region.
- The base year considered for the calculation of the market size is 2022. A historical year analysis has been done for the period FY2020-FY2021. The market size has been estimated for FY2022 and projected for the period FY2023-FY2033.
- Revenues of the companies have been referenced from their annual reports for FY2020- FY2022. For private companies, revenues have been estimated based on factors such as inputs obtained from primary research, funding history, product approval status, market collaborations, and operational history.
- Regional distribution of the market revenue has been estimated based on the companies in each region and the adoption rate of highly potent API. All the numbers have been adjusted to a single digit after the decimal for better presentation in the report. However, the real figures have been utilized for compound annual growth rate (CAGR) estimation. The CAGR has been calculated for the period 2023-2033.
- The market has been mapped based on the available highly potent API. All the key companies with significant offerings in this field have been considered and profiled in this report.
- Market strategies and developments of key players have been considered for the calculation of the potential of the market in the forecast period.
Primary Research:
The primary sources involve industry experts in the highly potent API market, including the market players offering highly potent API solutions. Resources such as CXOs, vice presidents, product managers, directors, territory managers, and business development have been interviewed to obtain and verify both qualitative and quantitative aspects of this research study.
The key data points taken from the primary sources include:
- Validation and triangulation of all the numbers and graphs
- Validation of the report's segmentation and key qualitative findings for highly potent API
- Understanding the competitive landscape and business model
- Current and proposed production values of a product by market players
- Validation of the numbers of the different segments of the market in focus
- Percentage split of individual markets for regional analysis
Secondary Research
Open Sources
- European Medicines Agency (EMA), American Chemical Society (ACS), Frontiers, World Health Organization (WHO), and National Center for Biotechnology Information (NCBI), among others
- Annual reports, SEC filings, and investor presentations of the leading market players
- Company websites and detailed study of their portfolios
- Gold standard magazines, journals, whitepapers, press releases, and news articles.
- Databases
The key data points taken from the secondary sources include:
- Segmentation and percentage share estimates
- Company and country understanding, and data for market value estimation.
- Key industry/market trends
- Developments among top players
- Qualitative insights into various aspects of the market, key trends, and emerging areas of innovation
- Quantitative data for mathematical and statistical calculations
Key Market Players and Competition Synopsis
The companies that are profiled have been selected based on inputs gathered from primary experts and analysing company coverage, type portfolio, and market penetration.
Some prominent names in the global highly potent API market include:
|
|
Table of Contents
Executive Summary
Scope and Definition
1. Markets
- 1.1. Trends: Current and Future Impact Assessment
- 1.1.1. Trend 1: Advancements in Containment Technologies for Highly Potent APIs
- 1.1.2. Trend 2: Increasing Investment for Highly Potent API Manufacturing Units
- 1.1.3. Trend 3: Pharmaceutical Companies Outsourcing Highly Potent APIs Production to CDMOs
- 1.2. Supply Chain /Value Overview
- 1.2.1. Supply Chain and Risks within the Supply Chain
- 1.2.2. Value Chain Analysis
- 1.3. Pricing Analysis
- 1.4. Patent Filing Trend (by Year, Country)
- 1.5. Regulatory Landscape
- 1.6. Impact Analysis for Key Global Events - COVID-19
- 1.7. Market Dynamics: Overview
- 1.7.1. Market Drivers
- 1.7.1.1. Increasing Incidence of Cancer Leading to Demand in the Adoption of Highly Potent APIs
- 1.7.1.2. Advancements in Drug Delivery Technologies
- 1.7.1.3. Expanding Therapeutic Applications of Highly Potent API
- 1.7.2. Market Opportunities
- 1.7.2.1. Growing Emphasis on Personalized Medicine
- 1.7.2.2. Increasing Opportunities for Pharma Companies in Developing Markets
- 1.7.3. Market Restraints
- 1.7.3.1. Regulatory Complexity for Highly Potent APIs' Manufacturing
- 1.7.3.2. High Development and Production Costs
- 1.7.1. Market Drivers
2. Global Highly Potent API Market (by Type)
- 2.1. Innovative High-Potency APIs
- 2.2. Generic High-Potency APIs
3. Global Highly Potent API Market (by Type of Synthesis)
- 3.1. Synthetic
- 3.2. Biotech
4. Global Highly Potent API Market (by Type of Manufacturing)
- 4.1. In-House
- 4.2. Outsourced
5. Global Highly Potent API Market (by Therapeutic Area)
- 5.1. Oncology
- 5.2. Immunology
- 5.3. Hormonal Disorders
- 5.4. Infectious Diseases
- 5.5. Others
6. Global Highly Potent API Market (by End User)
- 6.1. Biopharmaceutical and Life Science Companies
- 6.2. Contract Drug Manufacturing Organizations
- 6.3. Research Institutions
7. Regions
- 7.1. Regional Summary
- 7.2. North America
- 7.2.1. Regional Overview
- 7.2.2. Driving Factors for Market Growth
- 7.2.3. Factors Challenging the Market
- 7.2.4. U.S.
- 7.2.5. Canada
- 7.3. Europe
- 7.3.1. Regional Overview
- 7.3.2. Driving Factors for Market Growth
- 7.3.3. Factors Challenging the Market
- 7.3.4. France
- 7.3.5. Germany
- 7.3.6. U.K.
- 7.3.7. Spain
- 7.3.8. Italy
- 7.3.9. Rest-of-Europe
- 7.4. Asia-Pacific
- 7.4.1. Regional Overview
- 7.4.2. Driving Factors for Market Growth
- 7.4.3. Factors Challenging the Market
- 7.4.4. China
- 7.4.5. India
- 7.4.6. Japan
- 7.4.7. Australia
- 7.4.8. South Korea
- 7.4.9. Rest-of-Asia-Pacific
- 7.5. Latin America
- 7.5.1. Regional Overview
- 7.5.2. Driving Factors for Market Growth
- 7.5.3. Factors Challenging the Market
- 7.5.4. Brazil
- 7.5.1. Mexico
- 7.5.2. Rest-of-Latin America
- 7.6. Middle East and Africa
- 7.6.1. Regional Overview
- 7.6.2. Driving Factors for Market Growth
- 7.6.3. Factors Challenging the Market
- 7.6.4. U.A.E.
- 7.6.5. K.S.A.
- 7.6.6. Rest-of-Middle East and Africa
8. Markets -Competitive Benchmarking & Company Profiles
- 8.1. Next Frontiers
- 8.2. Geographic Assessment
- 8.2.1. AbbVie Inc.
- 8.2.1.1. Overview
- 8.2.1.2. Top Products/Product/Service Portfolio
- 8.2.1.3. Top Competitors
- 8.2.1.4. Key Personnel
- 8.2.1.5. Analyst View
- 8.2.2. Almac Group
- 8.2.2.1. Overview
- 8.2.2.2. Key Developments
- 8.2.2.3. Top Products/Product/Service Portfolio
- 8.2.2.4. Top Competitors
- 8.2.2.5. Key Personnel
- 8.2.2.6. Analyst View
- 8.2.3. Asymchem Inc.
- 8.2.3.1. Overview
- 8.2.3.2. Key Developments
- 8.2.3.3. Top Products/Product/Service Portfolio
- 8.2.3.4. Top Competitors
- 8.2.3.5. Key Personnel
- 8.2.3.6. Analyst View
- 8.2.4. Dr. Reddy's Laboratories Ltd.
- 8.2.4.1. Overview
- 8.2.4.2. Key Developments
- 8.2.4.3. Top Products/Product/Service Portfolio
- 8.2.4.4. Top Competitors
- 8.2.4.5. Key Personnel
- 8.2.4.6. Analyst View
- 8.2.5. Axplora Group GmbH
- 8.2.5.1. Overview
- 8.2.5.2. Top Products/Product/Service Portfolio
- 8.2.5.3. Key Developments
- 8.2.5.4. Top Competitors
- 8.2.5.5. Key Personnel
- 8.2.5.6. Analyst View
- 8.2.6. BASF SE
- 8.2.6.1. Overview
- 8.2.6.2. Top Products/Product/Service Portfolio
- 8.2.6.3. Key Developments
- 8.2.6.4. Top Competitors
- 8.2.6.5. Key Personnel
- 8.2.6.6. Analyst View
- 8.2.7. CARBOGEN AMCIS
- 8.2.7.1. Overview
- 8.2.7.2. Top Products/Product/Service Portfolio
- 8.2.7.3. Key Developments
- 8.2.7.4. Top Competitors
- 8.2.7.5. Key Personnel
- 8.2.7.6. Analyst View
- 8.2.8. CordenPharma International
- 8.2.8.1. Overview
- 8.2.8.2. Top Products/Product/Service Portfolio
- 8.2.8.3. Key Developments
- 8.2.8.4. Top Competitors
- 8.2.8.5. Key Personnel
- 8.2.8.6. Analyst View
- 8.2.9. Curia Global, Inc.
- 8.2.9.1. Overview
- 8.2.9.2. Top Products/Product/Service Portfolio
- 8.2.9.3. Key Developments
- 8.2.9.4. Top Competitors
- 8.2.9.5. Key Personnel
- 8.2.9.6. Analyst View
- 8.2.10. Helsinn Healthcare SA.
- 8.2.10.1. Overview
- 8.2.10.2. Top Products/Product/Service Portfolio
- 8.2.10.3. Key Developments
- 8.2.10.4. Top Competitors
- 8.2.10.5. Key Personnel
- 8.2.10.6. Analyst View
- 8.2.11. ICROM
- 8.2.11.1. Overview
- 8.2.11.2. Top Products/Product/Service Portfolio
- 8.2.11.3. Top Competitors
- 8.2.11.4. Key Personnel
- 8.2.11.5. Analyst View
- 8.2.12. Lonza
- 8.2.12.1. Overview
- 8.2.12.2. Top Products/Product/Service Portfolio
- 8.2.12.3. Key Developments
- 8.2.12.4. Top Competitors
- 8.2.12.5. Key Personnel
- 8.2.12.6. Analyst View
- 8.2.13. Merck KGaA
- 8.2.13.1. Overview
- 8.2.13.2. Top Products/Product/Service Portfolio
- 8.2.13.3. Key Developments
- 8.2.13.4. Top Competitors
- 8.2.13.5. Key Personnel
- 8.2.13.6. Analyst View
- 8.2.14. PCI Pharma Services
- 8.2.14.1. Overview
- 8.2.14.2. Top Products/Product/Service Portfolio
- 8.2.14.3. Key Developments
- 8.2.14.4. Top Competitors
- 8.2.14.5. Key Personnel
- 8.2.14.6. Analyst View
- 8.2.15. Pfizer Inc. (Pfizer CentreOne)
- 8.2.15.1. Overview
- 8.2.15.2. Top Products/Product/Service Portfolio
- 8.2.15.3. Key Developments
- 8.2.15.4. Top Competitors
- 8.2.15.5. Key Personnel
- 8.2.15.6. Analyst View
- 8.2.16. Sterling Pharma Solutions
- 8.2.16.1. Overview
- 8.2.16.2. Top Products/Product/Service Portfolio
- 8.2.16.3. Key Developments
- 8.2.16.4. Top Competitors
- 8.2.16.5. Key Personnel
- 8.2.16.6. Analyst View
- 8.2.1. AbbVie Inc.
9. Research Methodology
- 9.1. Data Sources
- 9.1.1. Primary Data Sources
- 9.1.2. Secondary Data Sources
- 9.1.3. Data Triangulation
- 9.2. Market Estimation and Forecast