|
|
市場調査レポート
商品コード
1076957
肺がんゲノム検査の世界市場の分析と予測:製品タイプ別、技術別、パネルタイプ別、サンプルタイプ別、エンドユーザー別、地域別分析(2021年~2031年)Lung Cancer Genomic Testing Market - A Global and Regional Analysis: Focus on Product, Technology, Panel Type, Sample Type, and End User - Analysis and Forecast, 2021-2031 |
||||||
|
● お客様のご希望に応じて、既存データの加工や未掲載情報(例:国別セグメント)の追加などの対応が可能です。 詳細はお問い合わせください。 |
|||||||
| 肺がんゲノム検査の世界市場の分析と予測:製品タイプ別、技術別、パネルタイプ別、サンプルタイプ別、エンドユーザー別、地域別分析(2021年~2031年) |
|
出版日: 2022年05月12日
発行: BIS Research
ページ情報: 英文 244 Pages
納期: 1~5営業日
|
- 全表示
- 概要
- 図表
- 目次
世界の肺がんゲノム検査の市場規模は、2020年の12億6,200万米ドルから、2031年までに32億7,980万米ドルに達し、2021年~2031年の予測期間中にCAGRで8.97%の成長が予測されています。
市場の成長促進要因として、肺がんゲノム検査市場の認知度と採用の高まり、最近の新製品の上市、ファーマコゲノミクスやコンパニオン診断薬の開発に重点を置いた肺がん分野の研究の拡大などが挙げられます。
当レポートでは、世界の肺がんゲノム検査市場について調査分析し、市場概要、業界分析、市場力学、競合情勢、セグメント別の市場分析、主要企業などについて、最新の情報を提供しています。
目次
第1章 市場
- 製品定義
- 調査手法
- 市場推定モデル
- 企業プロファイリングの基準
第2章 市場概要
- イントロダクション
- 肺がんの世界の有病率
- 肺がんにおけるゲノム検査の重要性
- 肺がんゲノム検査市場に対するCOVID-19の影響
第3章 業界分析
- 世界の法的要件と規制
- 米国の法的要件と枠組み
- 欧州の法的要件と枠組み
- アジア太平洋地域の法的要件と枠組み
- ラテンアメリカ(ブラジルとメキシコ)
- 特許情勢
- 特許出願動向
- 特許分析:国別
- 特許分析:技術別
第4章 市場力学
- 概要
- 影響分析
- 市場促進要因
- 高い肺がん死亡率
- ゲノム検査における次世代技術の進歩
- 標的治療薬の増加
- 世界的な遺伝子検査コストの低下
- 市場抑制要因
- ゲノム検査に対する不確かな規制シナリオ
- 有効な組織生検サンプルの欠如
- ゲノム検査に対する不均等な償還のシナリオ
- 市場機会
- 肺がんゲノム検査に対する世界的な推奨事項の向上
- 研究開発投資の増加
- 新興経済国
第5章 競合情勢
- 主要戦略と発展
第6章 世界の肺がんゲノム検査市場:製品タイプ別(2020年~2031年)
- 概要
- 製品
- サービス
第7章 世界の肺がんゲノム検査市場:技術別(2020年~2031年)
- 概要
- 次世代シーケンシング
- ポリメラーゼ連鎖反応(PCR)
- 蛍光in situハイブリダイゼーション(FISH)/in situハイブリダイゼーション(ISH)
- その他
第8章 世界の肺がんゲノム検査市場:パネルタイプ別(2020年~2031年)
- 概要
- 単一遺伝子
- マルチジーンパネル
第9章 世界の肺がんゲノム検査市場:サンプルタイプ別(2020年~2031年)
- 概要
- 組織生検
- 液体生検
- cfDNA
- ctDNA
第10章 世界の肺がんゲノム検査市場:エンドユーザー別(2020年~2031年)
- 概要
- 研究機関
- 病院/クリニック
- 診断研究所
- その他
第11章 世界の肺がんゲノム検査市場:地域別(2020年~2031年)
- 概要
- 北米
- 米国
- カナダ
- ラテンアメリカ
- ブラジル
- メキシコ
- その他
- 欧州
- 英国
- ドイツ
- イタリア
- スペイン
- フランス
- その他
- アジア太平洋地域
- 日本
- 中国
- 韓国
- シンガポール
- オーストラリア
- インド
- その他
- その他の地域
第12章 企業プロファイル
- QIAGEN N.V.
- Agilent Technologies, Inc.
- Thermo Fisher Scientific, Inc.
- Quest Diagnostics Incorporated
- Laboratory Corporation of America Holdings
- CENTOGENE N.V.
- BGI
- CeGaT GmbH
- Illumina, Inc.
- F. Hoffmann-La Roche Ltd.
- Abbott Laboratories
- CD Genomics
- NeoGenomics Laboratories
- Admera Health
- OncoDNA
- OPKO Health, Inc.
- Emerging Companies
List of Figures
- Figure 1: Types of Lung Cancer
- Figure 2: Incidence and Mortality of Lung Cancer (in Thousands), 2018 and 2020
- Figure 3: Global Lung Cancer Genomic Testing Market, $Million, 2020-2031
- Figure 4: Global Lung Cancer Genomic Testing Market, Market Dynamics
- Figure 5: Share of Key Developments and Strategies, January 2017-February 2022
- Figure 6: Global Lung Cancer Genomic Testing Market (by Product Type), $Million, 2020 vs. 2031
- Figure 7: Global Lung Cancer Genomic Testing Market (by Technology), $Million, 2020 vs. 2031
- Figure 8: Global Lung Cancer Genomic Testing Market (by Sample Type), $Million, 2020 vs. 2031
- Figure 9: Global Lung Cancer Genomic Testing Market (by Panel Type), $Million, 2020 vs. 2031
- Figure 10: Global Lung Cancer Genomic Testing Market (by End User), $Million, 2020 vs. 2031
- Figure 11: Global Lung Cancer Genomic Testing Market (by Region), $Million, 2020 vs. 2031
- Figure 12: Global Lung Cancer Genomic Testing Market: Segmentation
- Figure 14: Primary Research Methodology
- Figure 15: Bottom-Up Approach (Segment-Wise Analysis)
- Figure 16: Top-Down Approach (Segment-Wise Analysis)
- Figure 17: Global Five-Year Prevalence of Lung Cancer, 2015-2020 (in Thousands)
- Figure 18: Global Lung Cancer Genomics Testing Market: COVID-19 Impact Analysis
- Figure 19: COVID-19 Impact (March-June 2020)
- Figure 20: Biomarker Testing Reduction in the U.S.: February-March 2020
- Figure 21: Recommendations to Navigate COVID-19 Crisis
- Figure 22: FDA Guidelines for Companion Diagnostics Approval
- Figure 23: Criteria for CMS Coverage/Reimbursement
- Figure 24: Components Considered for Clinical Actionability as per the In-Vitro Diagnostic Medical Devices Regulation
- Figure 25: Europe In-Vitro Diagnostic Devices Regulation Regulatory Process
- Figure 26: China NMPA Regulatory Approval Process
- Figure 27: Steps to Register a Medical Device in Japan
- Figure 28: Regulatory Approval for Medical Devices in Latin-America
- Figure 29: Country-Wise Analysis (January 2019-January 2022) of Patents Related to Lung Cancer Genomic Testing
- Figure 30: Technology Wise Analysis (January 2019-January 2022) of Patents Related to Lung Cancer Genomic Testing
- Figure 31: Market Dynamics
- Figure 32: Likert Scale
- Figure 33: Impact Analysis
- Figure 34: Global Cancer Incidence (2020)
- Figure 35: Global Cancer Mortality (2020)
- Figure 36: Estimated Lung Cancer New Cases and Death in the U.S. in 2030 (in Thousands)
- Figure 37: Cost of Genome Sequencing (2014-2021)
- Figure 38: In-Vitro Diagnostics and Laboratory Developed Test Regulatory in the U.S., Europe, and Japan
- Figure 39: Reimbursement Barriers in U.S., Europe, and APAC regions
- Figure 40: ASCO Non-Small Cell Lung Cancer Biomarker Test Recommendations
- Figure 41: ESMO Recommendations as per ESMO Scale for Clinical Actionability of Molecular Targets (ESCAT) Level for NGS Panel Test
- Figure 42: Pan-Asia NSCLC Clinical Recommendations
- Figure 43: Number of Cancer Research Funders Globally
- Figure 44: Share of Key Developments and Strategies, January 2017-February 2022
- Figure 45: Synergistic Activities Share (by Company), January 2017-January 2022
- Figure 46: Product and Regulatory Approvals (by Company), January 2017-January 2022
- Figure 47: Product Launches and Upgradations (by Company), January 2017-February 2022
- Figure 48: Share of Acquisitions (by Company), January 2017-February 2022
- Figure 49: Share of Business Expansion (by Company), January 2017-February 2022
- Figure 50: Market Share Analysis for Global Lung Cancer Genomics Testing Market, $Million, 2019 and 2020
- Figure 51: Growth-Share Analysis of Global Lung Cancer Genomics Testing Market (by Company) 2019-2020
- Figure 52: Global Lung Cancer Genomic Testing Market (by Product Type)
- Figure 53: Global Lung Cancer Genomic Testing Market (by Product Type), 2020 and 2031
- Figure 54: Global Lung Cancer Genomic Testing Market (by Product Type), $Million, 2020-2031
- Figure 55: Global Lung Cancer Genomic Testing Market (Services), $Million, 2020-2031
- Figure 56: Global Lung Cancer Genomic Testing Market (Technology), 2020 and 2031
- Figure 57: Global Lung Cancer Genomic Testing Market (Next-Generation Sequencing), $Million, 2020-2031
- Figure 58: Global Lung Cancer Genomic Testing Market (Polymerase Chain Reaction), $Million, 2020-2031
- Figure 59: Global Lung Cancer Genomic Testing Market (Fluorescence In-Situ Hybridization), $Million, 2020-2031
- Figure 60: Global Lung Cancer Genomic Testing Market (Other), $Million, 2020-2031
- Figure 61: Global Lung Cancer Genomic Testing Market (by Panel Type), 2020 and 2031
- Figure 62: Global Lung Cancer Genomic Testing Market (Single-Gene Panel Type), $Million, 2020-2031
- Figure 63: Global Lung Cancer Genomic Testing Market (Multi-Gene Panel Type), $Million, 2020-2031
- Figure 64: Global Lung Cancer Genomic Testing Market (by Sample Type), $Million, 2020 and 2031
- Figure 65: Global Lung Cancer Genomic Testing Market (Tissue Biopsy), $Million, 2020-2031
- Figure 66: Global Lung Cancer Genomic Testing Market (Liquid Biopsy), $Million, 2020-2031
- Figure 67: Global Lung Cancer Genomic Testing Market (Liquid Biopsy), $Million, 2020-2031
- Figure 68: Global Lung Cancer Genomic Testing Market (cfDNA), $Million, 2020-2031
- Figure 69: Global Lung Cancer Genomic Testing Market (ctDNA), $Million, 2020-2031
- Figure 71: Global Lung Cancer Genomic Testing Market (by End User), $Million, 2020 and 2031
- Figure 72: Global Lung Cancer Genomic Testing Market (Research Organization), $Million, 2020-2031
- Figure 73: Global Lung Cancer Genomic Testing Market (Hospitals/Clinics), $Million, 2020-2031
- Figure 74: Global Lung Cancer Genomic Testing Market (Diagnostic Laboratories), $Million, 2020-2031
- Figure 75: Global Lung Cancer Genomic Testing Market (Other End Users), $Million, 2020-2031
- Figure 76: Market Share of North America Lung Cancer Genomic Testing Market Revenue (by Country), $Million, 2020 and 2031
- Figure 77: North America: Market Dynamics
- Figure 78: North America: Key Trends
- Figure 79: U.S. Lung Cancer Genomic Testing Market, $Million, 2020-2031
- Figure 80: Estimated Lung Cancer Cases in the U.S. (in Thousands), 2018-2022
- Figure 81: Canada Lung Cancer Genomic Testing Market, $Million, 2020-2031
- Figure 82: Latin America Lung Cancer Genomic Testing Market, $Million, 2020-2031
- Figure 83: Latin America: Market Dynamics
- Figure 84: Market Share of Latin America Lung Cancer Genomic Testing Market Revenue (by Country), $Million, 2020 and 2031
- Figure 85: Brazil Lung Cancer Genomic Testing Market, $Million, 2020-2031
- Figure 86: Mexico Lung Cancer Genomic Testing Market, $Million, 2020-2031
- Figure 87: Rest-of-Latin America (RoLA) Lung Cancer Genomic Testing Market, $Million, 2020-2031
- Figure 88: Market Share of Europe Lung Cancer Genomic Testing Market Revenue (by Country), $Million, 2020 and 2031
- Figure 89: Europe: Market Dynamics
- Figure 90: Europe: Key Trends
- Figure 91: U.K. Lung Cancer Genomic Testing Market, $Million, 2020-2031
- Figure 92: Germany: Lung Cancer Genomic Testing Market, $Million, 2020-2031
- Figure 93: Italy Lung Cancer Genomic Testing Market, $Million, 2020-2031
- Figure 94: Spain Lung Cancer Genomic Testing Market, $Million, 2020-2031
- Figure 95: France Lung Cancer Genomic Testing Market, $Million, 2020-2031
- Figure 96: Rest-of-Europe Lung Cancer Genomic Testing Market, $Million, 2020-2031
- Figure 97: Asia-Pacific Lung Cancer Genomic Testing Market (by Country), $Million, 2020 and 2031
- Figure 98: Japan Lung Cancer Genomic Testing Market, $Million, 2020-2031
- Figure 99: China Lung Cancer Genomic Testing Market, $Million, 2020-2031
- Figure 100: South-Korea Lung Cancer Genomic Testing Market, $Million, 2020-2031
- Figure 101: Singapore Lung Cancer Genomic Testing Market, $Million, 2020-2031
- Figure 102: Australia Lung Cancer Genomic Testing Market, $Million, 2020-2031
- Figure 103: India Lung Cancer Genomic Testing Market, $Million, 2020-2031
- Figure 104: Rest-of-Asia-Pacific Lung Cancer Genomic Testing Market, $Million, 2020-2031
- Figure 105: Rest-of-the-World Lung Cancer Genomic Testing Market, $Million, 2020-2031
- Figure 106: Total Number of Companies Profiled
- Figure 107: QIAGEN N.V.: Product Portfolio
- Figure 108: QIAGEN N.V.: Overall Financials, 2018-2020
- Figure 109: QIAGEN N.V.: Revenue (by Business Segment), 2018-2020
- Figure 110: QIAGEN N.V.: Revenue (by Region), 2018-2020
- Figure 111: QIAGEN N.V.: R&D Expenditure, 2018-2020
- Figure 112: QIAGEN N.V.: SWOT Analysis
- Figure 113: Agilent Technologies, Inc.: Product Portfolio
- Figure 114: Agilent Technologies, Inc.: Overall Financials, 2018-2021
- Figure 115: Agilent Technologies, Inc.: Revenue (by Business Segment), 2018-2021
- Figure 116: Agilent Technologies, Inc.: Revenue (by Product Type), 2018-2021
- Figure 117: Agilent Technologies, Inc.: Revenue (by Region), 2019-2021
- Figure 118: Agilent Technologies, Inc.: R&D Expenditure, 2018-2021
- Figure 119: Agilent Technologies, Inc.: SWOT Analysis
- Figure 120: Thermo Fisher Scientific, Inc.: Product Portfolio
- Figure 121: Thermo Fisher Scientific, Inc.: Overall Financials, 2018-2021
- Figure 122: Thermo Fisher Scientific, Inc.: Revenue (by Business Segment), 2018-2020
- Figure 123: Thermo Fisher Scientific, Inc.: Revenue (by Region), 2018-2020
- Figure 124: Thermo Fisher Scientific, Inc.: R&D Expenditure, 2018-2020
- Figure 125: Thermo Fisher Scientific, Inc.: SWOT Analysis
- Figure 126: Quest Diagnostics Incorporated.: Product Portfolio
- Figure 127: Quest Diagnostics: Overall Financials, 2018-2021
- Figure 128: Quest Diagnostics Incorporated: Revenue (by Business Segment), 2018-2021
- Figure 129: Quest Diagnostics Incorporated: SWOT Analysis
- Figure 130: Laboratory Corporation of America Holdings: Product Portfolio
- Figure 131: Laboratory Corporation of America Holdings: Overall Financials, 2018-2020
- Figure 132: Laboratory Corporation of America Holdings: Revenue (by Segment), 2018-2020
- Figure 133: Laboratory Corporation of America Holdings: Revenue (by Region), 2018-2020
- Figure 134: Laboratory Corporation of America Holdings: SWOT Analysis
- Figure 135: CENTOGENE N.V.: Product Portfolio
- Figure 136: CENTOGENE N.V.: Overall Financials, 2018-2020
- Figure 137: CENTOGENE N.V.: Revenue (by Business Segment), 2018-2020
- Figure 138: CENTOGENE N.V.: Revenue (by Region), 2018-2020
- Figure 139: CENTOGENE N.V.: R&D Expenditure, 2018-2020
- Figure 140: CENTOGENE N.V.: SWOT Analysis
- Figure 141: BGI: Product Portfolio
- Figure 142: BGI: SWOT Analysis
- Figure 143: CeGaT GmbH: Product Portfolio
- Figure 144: CeGaT GmbH: SWOT Analysis
- Figure 145: Illumina, Inc.: Product Portfolio
- Figure 146: Illumina, Inc.: Overall Financials, 2018-2021
- Figure 147: Illumina, Inc.: Revenue (by Business Segment), 2018-2020
- Figure 148: Illumina, Inc.: Revenue (by Business Segment, by Technology), 2018-2020
- Figure 149: Illumina, Inc.: Revenue (by Region), 2018-2020
- Figure 150: Illumina, Inc.: R&D Expenditure, 2018-2020
- Figure 151: Illumina, Inc.: SWOT Analysis
- Figure 152: F. Hoffmann-La Roche Ltd.: Product Portfolio
- Figure 153: F. Hoffmann-La Roche Ltd.: Overall Financials, 2018-2021
- Figure 154: F. Hoffmann-La Roche Ltd.: Revenue (by Business Segment), 2018-2021
- Figure 155: Hoffmann-La Roche Ltd.: Revenue (by Region), 2018-2020
- Figure 156: F. Hoffmann-La Roche Ltd.: R&D Expenditure, 2018-2020
- Figure 157: F. Hoffmann-La Roche Ltd.: SWOT Analysis
- Figure 158: Abbott Laboratories: Product Portfolio
- Figure 159: Abbott Laboratories: Overall Financials, 2018-2020
- Figure 160: Abbott Laboratories: Revenue (by Segment), 2018-2021
- Figure 161: Abbott Laboratories: Revenue (by Region), 2018-2020
- Figure 162: Abbott Laboratories: R&D Expenditure, 2018-2020
- Figure 163: Abbott Laboratories: SWOT Analysis
- Figure 164: CD Genomics: Product Portfolio
- Figure 165: CD Genomics: SWOT Analysis
- Figure 166: NeoGenomics Laboratories: Product Portfolio
- Figure 167: NeoGenomics Laboratories: Overall Financials, 2018-2021
- Figure 168: NeoGenomics Laboratories: Revenue (by Business Segment), 2018-2021
- Figure 169: NeoGenomics Laboratories: R&D Expenditure, 2018-2021
- Figure 170: NeoGenomics Laboratories: SWOT Analysis
- Figure 171: Admera Health: Product Portfolio
- Figure 172: Admera Health: SWOT Analysis
- Figure 173: OncoDNA: Product Portfolio
- Figure 174: OncoDNA: SWOT Analysis
- Figure 175: OPKO Health, Inc.: Product Portfolio
- Figure 176: OPKO Health, Inc.: Overall Financials, 2018-2021
- Figure 177: OPKO Health, Inc.: Revenue (by Region), 2018-2020
- Figure 178: OPKO Health, Inc.: Revenue (by Business Segment), 2018-2021
- Figure 179: OPKO Health, Inc.: R&D Expenditure, 2018-2021
- Figure 180: OPKO Health, Inc.: SWOT Analysis
- Figure 181: Invitae Corporation: Product Portfolio
- Figure 182: Invitae Corporation: Overall Financials, 2018-2021
- Figure 183: Invitae Corporation: Revenue (by Business Segment), 2018-2021
- Figure 184: Invitae Corporation: R&D Expenditure, 2018-2021
- Figure 185: Invitae Corporation: SWOT Analysis
- Figure 186: Veracyte, Inc.: Product Portfolio
- Figure 187: Veracyte, Inc.: Overall Financials, 2018-2021, $Million
- Figure 188: Veracyte, Inc.: Revenue (by Business Segment), 2018-2021
- Figure 189: Veracyte, Inc.: Revenue (by Region), 2018-2021
- Figure 190: Veracyte, Inc.: R&D Expenditure, 2018-2021
- Figure 191: Veracyte, Inc.: SWOT Analysis
List of Tables
- Table 1: Priority Genetic Testing at Diagnosis for Advanced Non-Squamous Non-Small Cell Lung Cancer
- Table 2: Classification of Medical Device
- Table 3: Lung Cancer Genetic Mutation and Targeted Therapies
- Table 4: Companies Providing Kits and Assays
- Table 5: Companies Offering Services
- Table 6: FDA Approved Lung Cancer NGS Assays
- Table 7: Lung Cancer Insights in 2018
- Table 8: Hoffmann-La Roche Ltd.: Revenue for Tissue Diagnostics Segment
“Global Lung Cancer Genomic Testing Market to Reach $3,279.8 Million by 2031.”
Global Lung Cancer Genomic Testing Market Industry Overview
The lung cancer genomic testing medicine market was valued at $1,262.0 million in 2020 and is expected to reach $3,279.8 million by 2031, growing at a CAGR of 8.97% during the forecast period 2021-2031. The growth in the global lung cancer genomic testing market is expected to be driven by increasing awareness and adoption of the lung cancer genomic testing market, recent launches of novel lung cancer genomic testing, and the expanding research in the field of lung cancer with an emphasis on pharmacogenomics and development of companion diagnostics.
Market Lifecycle Stage
The global lung cancer genomic testing market is still in the nascent phase. Various companies are increasing investments in research and development to facilitate the development of lung cancer genomic testing, which is expected to further increase the adoption of lung cancer genomic tests.
The shift of healthcare systems toward precision diagnostic and precision medicine will drive the adoption of lung cancer genomic testing facilitating informed treatment decision making and improving healthcare outcomes. Increasing recommendations by the international oncology societies for the use of genomic testing for lung cancer diagnosis provides major opportunities in the global lung cancer genomic testing market.
Impact
- The presence of major in-vitro diagnostics (IVD) product providers of lung cancer genomic tests in regions such as North America and Europe has a major impact on the market. For instance, Qiagen provides therascreen Solid Tumor assays, a real-time polymerase chain reaction (PCR) test for EGFR detection in non-small cell lung cancer (NSCLC) patients. Additionally, Roche provides Cobas EGFR Mutation Test, a PCR-based test, and FoundationOne CDx by Foundation Medicine Inc., which is a next-generation sequencing (NGS) based assay for NSCLC.
- The presence of major laboratory developed tests (LDTs) service providers companies offer lung cancer genomic testing in regions such as North America and Europe, which has a major impact on the market. The LDTs are offered by Quest Diagnostics and Laboratory Corporation of America (Labcorp), targeting various lung cancer genes, for example, EGFR, MET, ALK, and ROS, based on Fluorescence in situ Hybridization (FISH) and NGS. The presence of these companies has a positive impact on market growth.
Impact of COVID-19
The global lung cancer genomic testing market is dominated by the utility in diagnostic laboratories, hospitals, and clinics. During the beginning of the COVID-19 pandemic, multiple countries witnessed a complete or a partial lockdown, and all elective surgeries and procedures were halted in the healthcare settings. Since the lung cancer genomic testing is categorized under the elective procedure, the impact of the COVID-19 pandemic on the lung cancer genomic testing was negative. BIS Research analysis has concluded that the market witnessed a drop of 3.24% in the annual growth rate of global lung cancer genomic testing market.
Market Segmentation
Segmentation 1: by Product Type
- Products
- Services
The global lung cancer genomic testing market services segment is expected to be dominated. This is owing to the easy availability, accessibility, and adaptation of LDTs, due to lower cost when compared with the product's market consisting of IVD.
Segmentation 2: by Technology
- Polymerase Chain Reaction (PCR)
- Next-Generation Sequencing (NGS)
- Fluorescence In Situ Hybridization
- Others
The global lung cancer genomic testing market by polymerase chain reaction (PCR) is expected to be dominated. This is owing to the overall cost efficiency and high sensitivity of the PCR-based genomic test for the detection of disease-causing mutations in lung cancer.
Segmentation 3: by Sample Type
- Tissue Biopsy
- Liquid Biopsy
The global lung cancer genomic testing market by tissue biopsy sample type is expected to be dominated. This is owing to the standard healthcare practice of extracting a lung tissue biopsy for lung cancer diagnosis, which is further utilized for lung cancer genomic testing.
Segmentation 4: by Panel Type
- Multi-Gene Panel
- Single-Gene Panel
The global lung cancer genomic testing market by multi-gene panel type is expected to be dominated. This is owing to the standard healthcare practice and familiarity of healthcare professionals with the extraction of lung tissue biopsy for lung cancer diagnosis, which is further utilized for lung cancer genomic testing.
Segmentation 5: by End User
- Research Organization
- Hospitals/Clinics
- Diagnostic Laboratories
- Other End Users
The global lung cancer genomic testing market by research organization end user is expected to be dominated. This is owing to a large number of clinical research and academic organizations where lung cancer genomic testing is utilized for drug development, development of companion diagnostics, and enrolment of patients in clinical trials.
Segmentation 6: by Region
- North America - U.S. and Canada
- Latin America - Brazil, Mexico, and Rest-of-Latin America
- Europe - U.K., Germany, Italy, Spain, France, and Rest-of-Europe
- Asia-Pacific - Japan, China, South Korea, Singapore, Australia, India, and Rest-of-Asia-Pacific
- Rest-of-the-World
The global lung cancer genomic testing market is expected to be dominated by North America, generating the highest revenue of $713.5 million. This is owing to the presence of a large number of research organizations and products and services companies in the U.S.
Recent Developments in Global Lung Cancer Genomic Testing Market
- In December 2021, FDA approved Thermo Fisher Scientific's NGS-based companion diagnostic for EGFR Exon20 insertion mutant non-small cell lung cancer tumor tissue Oncomine Dx Target Test, now approved for 12 NSCLC targeted therapies globally.
- In September 2021, FDA approved Thermo Fisher Scientific's tissue-based NGS companion diagnostic for Takeda's targeted therapy for NSCLC patients with EGFR Exon20 insertion mutations- Oncomine Dx Target Test now approved as CDx for five targeted NSCLC therapies in the U.S.
- In September 2020, Laboratory Corporation of America Holdings entered into a commercial partnership with Resolution Bioscience; the company rolled out Resolution ctDx lung liquid biopsy test.
- In May 2021, Qiagen launched the first FDA-approved tissue companion diagnostic to identify the KRAS G12C mutation in NSCLC tumors and expanded precision medicine options in lung cancer.
- In January 2020, Qiagen built a global collaboration with Amgen for companion diagnostic development in non-small cell lung cancer.
Demand - Drivers and Limitations
Following are the demand drivers for the global lung cancer genomics market:
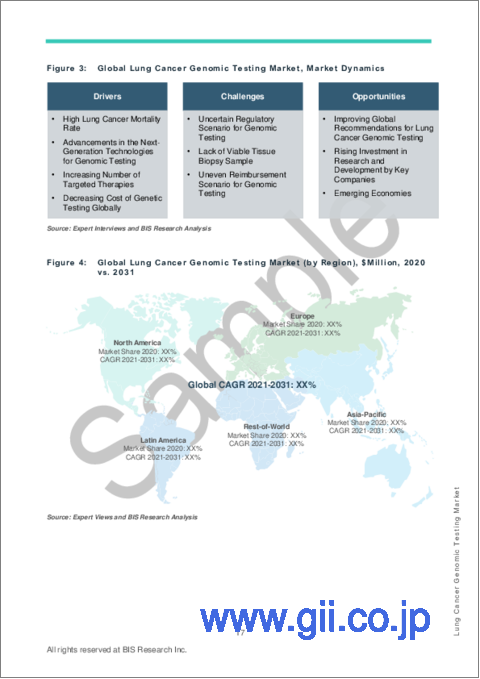
- High Lung Cancer Mortality Rate
- Advancements in the Next-Generation Technologies for Genomic Testing
- Increasing Number of Targeted Therapies
- Decreasing Cost of Genetic Testing Globally
The market is expected to face some limitations too due to the following challenges:
- Uncertain Regulatory Scenario for Genomic Testing
- Lack of Viable Tissue Biopsy Sample
- Uneven Reimbursement Scenario for Genomic Testing
How Can This Report Add Value to an Organization?
- Product/Innovation Strategy: The product segment helps the reader understand the different types of products, i.e., products (in-vitro diagnostic test) and services (laboratory developed tests) with respect to lung cancer genomic testing. Additionally, the study provides the reader a detailed understanding of the segmentation of the product and market analysis by technology, sample type, panel type, and understand the growth potential of the market by utilization based on the different end users.
- Growth/Marketing Strategy: The global lung cancer genomic testing market has seen major development by key players operating in the market, such as product launches, synergistic activities, mergers and acquisitions, business expansion, partnership, collaboration, joint venture, and funding. The favored strategy for the companies has been synergistic activities and mergers and acquisitions, strengthening the product portfolio and their position in the lung cancer genomic testing market. For instance, in February 2022, Foundation Medicine Inc. announced its collaboration with Eli Lilly and Company to develop FoundationOne CDx and FoundationOne Liquid CDx as companion diagnostics for RETEVMO, which was FDA approved in May for non-small cell lung cancer patients.
- Competitive Strategy: Key players in the global lung cancer genomic testing market analyzed and profiled in the study involve the product manufacturer and test service providers. Moreover, a detailed competitive benchmarking of the players operating in the global lung cancer genomic testing market has been done to help the readers understand how players stack against each other, presenting a clear market landscape. Additionally, comprehensive competitive strategies such as partnerships, agreements, and collaborations will aid the reader in understanding the untapped revenue pockets in the market.
Key Market Players and Competition Synopsis
The companies that are profiled have been selected based on inputs gathered from primary experts and analyzing company coverage, product portfolio, key developments, and market penetration.
Some of the prominent names established in this market are:
- QIAGEN N.V.
- Agilent Technologies, Inc.
- Thermo Fisher Scientific, Inc.
- Quest Diagnostics Incorporated
- Laboratory Corporation of America Holdings
- CENTOGENE N.V.
- BGI
- CeGaT GmbH
- Illumina, Inc.
- F. Hoffmann-La Roche Ltd.
- Abbott Laboratories
- CD Genomics
- NeoGenomics Laboratories
- Admera Health
- OncoDNA
- OPKO Health, Inc.
- Invitae Corporation
- Veracyte, Inc.
Companies that are not a part of the above-mentioned pool have been well represented across different sections of the report (wherever applicable).
Table of Contents
1 Markets
- 1.1 Product Definition
- 1.1.1 Lung Cancer Genomic Testing
- 1.1.2 Inclusion and Exclusion
- 1.1.3 Market Scope
- 1.1.3.1 Key Questions Answered by this Report:
- 1.2 Research Methodology
- 1.2.1 Primary Data Sources
- 1.2.2 Secondary Data Sources
- 1.3 Market Estimation Model
- 1.4 Criteria for Company Profiling
2 Market Overview
- 2.1 Introduction
- 2.2 Global Prevalence of Lung Cancer
- 2.3 Significance of Genomic Testing in Lung Cancer
- 2.4 COVID-19 Impact on Lung Cancer Genomic Testing Market
- 2.4.1 Disruption in Global Lung Cancer Genomic Testing Market: Pre- and Post-COVID-19 Market Analysis
- 2.4.2 COVID-19 Affecting Supply Chain of Global Lung Cancer Genomic Testing Market
- 2.4.3 Interruption in Research and Clinical Development and Commercial Operation
- 2.4.4 Navigating Crisis Recovery and Looking to the Future
3 Industry Analysis
- 3.1 Global Legal Requirements and Regulations
- 3.1.1 Legal Requirements and Frameworks in the U.S.
- 3.1.1.1 Centers for Medicare & Medicaid Services Regulation
- 3.1.2 Legal Requirements and Frameworks in Europe
- 3.1.3 Legal Requirements and Frameworks in Asia-Pacific
- 3.1.3.1 China
- 3.1.3.2 Japan
- 3.1.4 Latin America (Brazil and Mexico)
- 3.1.1 Legal Requirements and Frameworks in the U.S.
- 3.2 Patent Landscape
- 3.2.1 Patent Filing Trend
- 3.2.2 Patent Analysis by Country
- 3.2.3 Patent Analysis by Technology
4 Market Dynamics
- 4.1 Overview
- 4.2 Impact Analysis
- 4.3 Market Driving Factors
- 4.3.1 High Lung Cancer Mortality Rate
- 4.3.2 Advancements in the Next-Generation Technologies for Genomic Testing
- 4.3.3 Increasing Number of Targeted Therapies
- 4.3.4 Decreasing Cost of Genetic Testing Globally
- 4.4 Market Restraining Factors
- 4.4.1 Uncertain Regulatory Scenario for Genomic Testing
- 4.4.2 Lack of Viable Tissue Biopsy Sample
- 4.4.3 Uneven Reimbursement Scenario for Genomic Testing
- 4.5 Market Opportunities
- 4.5.1 Improving Global Recommendations for Lung Cancer Genomic Testing
- 4.5.1.1 American Society of Clinical Oncology (ASCO) Recommendations
- 4.5.1.2 European Society of Clinical Oncology (ESMO) Recommendations
- 4.5.1.3 Pan-Asia Clinical Practical Guidelines
- 4.5.2 Rising Investment in Research and Development
- 4.5.3 Emerging Economies
- 4.5.1 Improving Global Recommendations for Lung Cancer Genomic Testing
5 Competitive Landscape
- 5.1 Key Strategies and Developments
- 5.1.1 Synergistic Activities
- 5.1.2 Regulatory Approvals
- 5.1.3 Product Launches and Upgradations
- 5.1.4 Mergers and Acquisitions
- 5.1.5 Business Expansion
- 5.1.6 Investment, Funding, and Joint Venture
- 5.1.7 Market Share Analysis by Company
- 5.1.8 Growth-Share Analysis by Company
6 Global Lung Cancer Genomic Testing Market, (by Product Type), $Million, 2020-2031
- 6.1 Overview
- 6.1.1 Products
- 6.1.2 Services
7 Global Lung Cancer Genomic Testing Market, (by Technology), $Million, 2020-2031
- 7.1 Overview
- 7.2 Next-Generation Sequencing
- 7.3 Polymerase Chain Reaction (PCR)
- 7.4 Fluorescence In Situ Hybridization (FISH)/In Situ Hybridization (ISH)
- 7.5 Others
8 Global Lung Cancer Genomic Testing Market, (by Panel Type), $Million, 2020-2031
- 8.1 Overview
- 8.2 Single-Gene
- 8.3 Multi-Gene Panel
9 Global Lung Cancer Genomic Testing Market, (by Sample Type), $Million, 2020-2031
- 9.1 Overview
- 9.2 Tissue Biopsy
- 9.3 Liquid Biopsy
- 9.3.1 cfDNA
- 9.3.2 ctDNA
10 Global Lung Cancer Genomic Testing Market, (by End User), $Million, 2020-2031
- 10.1 Overview
- 10.2 Research Organization
- 10.3 Hospitals/Clinics
- 10.4 Diagnostic Laboratories
- 10.5 Other End Users
11 Global Lung Cancer Genomic Testing Market (by Region), $Million, 2020-2031
- 11.1 Overview
- 11.2 North America
- 11.2.1 North America: Market Dynamics
- 11.2.2 North America: Key Trends
- 11.2.3 U.S.
- 11.2.4 Canada
- 11.3 Latin America
- 11.3.1 Brazil
- 11.3.2 Mexico
- 11.3.3 Rest-of-Latin America (RoLA)
- 11.4 Europe
- 11.4.1 Europe: Market Dynamics
- 11.4.2 Europe: Key Trends
- 11.4.3 U.K.
- 11.4.4 Germany
- 11.4.5 Italy
- 11.4.6 Spain
- 11.4.7 France
- 11.4.8 Rest-of-Europe
- 11.5 Asia-Pacific
- 11.5.1 Asia-Pacific Lung Cancer Genomic Testing: Market Dynamics
- 11.5.2 Asia-Pacific: Key Trends
- 11.5.3 Japan
- 11.5.4 China
- 11.5.5 South-Korea
- 11.5.6 Singapore
- 11.5.7 Australia
- 11.5.8 India
- 11.5.9 Rest-of-Asia-Pacific
- 11.6 Rest-of-the-World
- 11.6.1 Key Trends in Rest-of-the-World Lung Cancer Genomic Testing Market
12 Company Profiles
- 12.1 Overview
- 12.2 QIAGEN N.V.
- 12.2.1 Company Overview
- 12.2.2 Role of QIAGEN N.V. in the Global Lung Cancer Genomic Testing Market
- 12.2.3 Key Competitors
- 12.2.4 Key Customers
- 12.2.5 Financials
- 12.2.5.1 Key Insights About Financial Health of the Company
- 12.2.6 Corporate Strategies
- 12.2.6.1 Mergers and Acquisitions
- 12.2.6.2 Synergistic Activities
- 12.2.6.3 Business Expansion and Funding
- 12.2.6.4 Product Launches and Upgradation
- 12.2.7 SWOT Analysis
- 12.3 Agilent Technologies, Inc.
- 12.3.1 Company Overview
- 12.3.2 Role of Agilent Technologies, Inc. in the Global Lung Cancer Genomic Testing Market
- 12.3.3 Key Competitors
- 12.3.4 Key Customers
- 12.3.5 Financials
- 12.3.5.1 Key Insights About Financial Health of the Company
- 12.3.6 Corporate Strategies
- 12.3.6.1 Mergers and Acquisitions
- 12.3.6.2 Synergistic Activities
- 12.3.6.3 Business Expansion and Funding
- 12.3.6.4 Product Launches and Upgradation
- 12.3.7 SWOT Analysis
- 12.4 Thermo Fisher Scientific, Inc.
- 12.4.1 Company Overview
- 12.4.2 Role of Thermo Fisher Scientific, Inc. in the Global Lung Cancer Genomic Testing Market
- 12.4.3 Key Competitors
- 12.4.4 Key Customers
- 12.4.5 Financials
- 12.4.5.1 Key Insights About Financial Health of the Company
- 12.4.6 Corporate Strategies
- 12.4.6.1 Mergers and Acquisitions
- 12.4.6.2 Synergistic Activities
- 12.4.6.3 Business Expansion and Funding
- 12.4.6.4 Product Launches and Upgradation
- 12.4.7 SWOT Analysis
- 12.5 Quest Diagnostics Incorporated
- 12.5.1 Company Overview
- 12.5.2 Role of Quest Diagnostics Incorporated in the Global Lung Cancer Genomic Testing Market
- 12.5.3 Key Competitors
- 12.5.4 Key Customers
- 12.5.5 Financials
- 12.5.6 Corporate Strategies
- 12.5.6.1 Mergers and Acquisitions
- 12.5.6.2 Synergistic Activities
- 12.5.6.3 Business Expansion and Funding
- 12.5.6.4 Product Launches and Upgradation
- 12.5.7 SWOT Analysis
- 12.6 Laboratory Corporation of America Holdings
- 12.6.1 Company Overview
- 12.6.2 Role of Laboratory Corporation of America Holdings in the Global Lung Cancer Genomic Testing Market
- 12.6.3 Key Competitors
- 12.6.4 Key Customers
- 12.6.5 Financials
- 12.6.6 Corporate Strategies
- 12.6.6.1 Mergers and Acquisitions
- 12.6.6.2 Synergistic Activities
- 12.6.6.3 Business Expansion and Funding
- 12.6.6.4 Product Launches and Upgradation
- 12.6.7 SWOT Analysis
- 12.7 CENTOGENE N.V.
- 12.7.1 Company Overview
- 12.7.2 Role of CENTOGENE N.V. in the Global Lung Cancer Genomic Testing Market
- 12.7.3 Key Competitors
- 12.7.4 Key Customers
- 12.7.5 Financials
- 12.7.6 Key Insights About Financial Health of the Company
- 12.7.7 Corporate Strategies
- 12.7.7.1 Mergers and Acquisitions
- 12.7.7.2 Synergistic Activities
- 12.7.7.3 Business Expansion and Funding
- 12.7.7.4 Product Launches and Upgradation
- 12.7.8 SWOT Analysis
- 12.8 BGI
- 12.8.1 Company Overview
- 12.8.2 Role of BGI in the Global Lung Cancer Genomic Testing Market
- 12.8.3 Key Competitors
- 12.8.4 Key Customers
- 12.8.5 Corporate Strategies
- 12.8.5.1 Mergers and Acquisitions
- 12.8.5.2 Synergistic Activities
- 12.8.5.3 Business Expansion and Funding
- 12.8.5.4 Product Launches and Upgradation
- 12.8.6 SWOT Analysis
- 12.9 CeGaT GmbH
- 12.9.1 Company Overview
- 12.9.2 Role of CeGaT GmbH in the Global Lung Cancer Genomic Testing Market
- 12.9.3 Key Competitors
- 12.9.4 Key Customers
- 12.9.5 Corporate Strategies
- 12.9.5.1 Mergers and Acquisitions
- 12.9.5.2 Synergistic Activities
- 12.9.5.3 Business Expansion and Funding
- 12.9.5.4 Product Launches and Upgradation
- 12.9.6 SWOT Analysis
- 12.1 Illumina, Inc.
- 12.10.1 Company Overview
- 12.10.2 Role of Illumina, Inc. in the Global Lung Cancer Genomic Market
- 12.10.3 Key Competitors
- 12.10.4 Key Customers
- 12.10.5 Financials
- 12.10.5.1 Key Insights About Financial Health of the Company
- 12.10.6 Corporate Strategies
- 12.10.6.1 Mergers and Acquisitions
- 12.10.6.2 Synergistic Activities
- 12.10.6.3 Business Expansion and Funding
- 12.10.6.4 Product Launches and Upgradation
- 12.10.7 SWOT Analysis
- 12.11 F. Hoffmann-La Roche Ltd.
- 12.11.1 Company Overview
- 12.11.2 Role of F. Hoffmann-La Roche Ltd. in the Global Lung Cancer Genomic Testing Market
- 12.11.3 Key Competitors
- 12.11.4 Key Customers
- 12.11.5 Financials
- 12.11.6 Key Insights About Financial Health of the Company
- 12.11.7 Corporate Strategies
- 12.11.7.1 Mergers and Acquisitions
- 12.11.7.2 Synergistic Activities
- 12.11.7.3 Business Expansion and Funding
- 12.11.7.4 Product Launches and Upgradation
- 12.11.8 SWOT Analysis
- 12.12 Abbott Laboratories
- 12.12.1 Company Overview
- 12.12.2 Role of Abbott Laboratories in the Global Lung Genomic Testing Market
- 12.12.3 Key Competitors
- 12.12.4 Key Customers
- 12.12.5 Financials
- 12.12.6 Key Insights About Financial Health of the Company
- 12.12.7 Corporate Strategies
- 12.12.7.1 Mergers and Acquisitions
- 12.12.7.2 Synergistic Activities
- 12.12.7.3 Business Expansion and Funding
- 12.12.7.4 Product Launches and Upgradation
- 12.12.8 SWOT Analysis
- 12.13 CD Genomics
- 12.13.1 Company Overview
- 12.13.2 Role of CD Genomics in the Global Lung Cancer Genomic Testing Market
- 12.13.3 Key Competitors
- 12.13.4 Key Customers
- 12.13.5 Corporate Strategies
- 12.13.5.1 Mergers and Acquisitions
- 12.13.5.2 Synergistic Activities
- 12.13.5.3 Business Expansion and Funding
- 12.13.5.4 Product Launches and Upgradation
- 12.13.6 SWOT Analysis
- 12.14 NeoGenomics Laboratories
- 12.14.1 Company Overview
- 12.14.2 Role of NeoGenomics Laboratories in the Global Lung Cancer Genomic Testing Market
- 12.14.3 Key Competitors
- 12.14.4 Key Customers
- 12.14.5 Financials
- 12.14.6 Key Developments
- 12.14.7 Key Insights About Financial Health of the Company
- 12.14.8 Corporate Strategies
- 12.14.8.1 Mergers and Acquisitions
- 12.14.8.2 Synergistic Activities
- 12.14.8.3 Business Expansion and Funding
- 12.14.8.4 Product Launches and Upgradation
- 12.14.9 SWOT Analysis
- 12.15 Admera Health
- 12.15.1 Company Overview
- 12.15.2 Role of Admera Health in the Global Lung Cancer Genomic Testing Market
- 12.15.3 Key Competitors
- 12.15.4 Key Customers
- 12.15.5 Corporate Strategies
- 12.15.5.1 Mergers and Acquisitions
- 12.15.5.2 Synergistic Activities
- 12.15.5.3 Business Expansion and Funding
- 12.15.5.4 Product Launches and Upgradation
- 12.15.6 SWOT Analysis
- 12.16 OncoDNA
- 12.16.1 Company Overview
- 12.16.2 Role of OncoDNA in the Global Lung Cancer Genomic Testing Market
- 12.16.3 Key Competitors
- 12.16.4 Key Customers
- 12.16.5 Corporate Strategies
- 12.16.5.1 Mergers and Acquisitions
- 12.16.5.2 Synergistic Activities
- 12.16.5.3 Business Expansion and Funding
- 12.16.5.4 Product Launches and Upgradation
- 12.16.6 SWOT Analysis
- 12.17 OPKO Health, Inc.
- 12.17.1 Company Overview
- 12.17.2 Role of OPKO Health, Inc. in the Global Lung Cancer Genomic Testing Market
- 12.17.3 Key Competitors
- 12.17.4 Key Customers
- 12.17.5 Financials
- 12.17.6 Key Insights About Financial Health of the Company
- 12.17.7 Corporate Strategies
- 12.17.7.1 Mergers and Acquisitions
- 12.17.7.2 Synergistic Activities
- 12.17.7.3 Business Expansion and Funding
- 12.17.7.4 Product Launches and Upgradation
- 12.17.8 SWOT Analysis
- 12.18 Emerging Companies
- 12.18.1 Invitae Corporation
- 12.18.1.1 Company Overview
- 12.18.1.2 Role of Invitae Corporation in the Global Lung Cancer Genomic Testing Market
- 12.18.1.3 Key Competitors
- 12.18.1.4 Key Customers
- 12.18.1.5 Financials
- 12.18.1.6 Key Insights About Financial Health of the Company
- 12.18.1.7 Corporate Strategies
- 12.18.1.7.1 Mergers and Acquisitions
- 12.18.1.7.2 Product Launches and Upgradation
- 12.18.1.8 SWOT Analysis
- 12.18.2 Veracyte, Inc.
- 12.18.2.1 Company Overview
- 12.18.2.2 Role of Veracyte, Inc. in the Global Lung Cancer Genomic Testing Market
- 12.18.2.3 Key Competitors
- 12.18.2.4 Key Customers
- 12.18.2.5 Financials
- 12.18.2.6 Key Insights About Financial Health of the Company
- 12.18.2.7 Corporate Strategies
- 12.18.2.7.1 Mergers and Acquisitions
- 12.18.2.7.2 Product Launches and Upgradation
- 12.18.2.8 SWOT Analysis
- 12.18.1 Invitae Corporation