|
|
市場調査レポート
商品コード
1735440
ヒトマイクロバイオーム由来の医薬品および診断薬:世界市場Human Microbiome-based Drugs and Diagnostics: Global Markets |
||||||
|
|||||||
| ヒトマイクロバイオーム由来の医薬品および診断薬:世界市場 |
|
出版日: 2025年05月23日
発行: BCC Research
ページ情報: 英文 116 Pages
納期: 即納可能
|
全表示
- 概要
- 図表
- 目次
世界のヒトマイクロバイオーム由来の医薬品および診断薬の市場規模は、2025年の3億9,340万ドルから、2030年末には12億ドルに拡大すると見込まれており、2025年から2030年にかけてのCAGRは25.6%と予測されています。
北米の市場規模は、2025年の2億2,570万ドルから、2030年末には5億7,890万ドルに拡大すると見込まれており、同期間のCAGRは20.7%とされています。
欧州の市場規模は、2025年の6,930万ドルから、2030年末には3億590万ドルに拡大すると見込まれており、同期間のCAGRは34.6%と予測されています。
当レポートでは、世界のヒトマイクロバイオーム由来の医薬品および診断薬の市場を調査し、市場概要、市場影響因子および市場機会の分析、法規制環境、新興技術および技術開発の動向、市場規模の推移・予測、各種区分・地域別の詳細分析、競合情勢、主要企業のプロファイルなどをまとめています。
目次
第1章 エグゼクティブサマリー
- 市場見通し
- 調査範囲
- 市場サマリー
- 市場力学
- 新興技術
- セグメント別分析
- 地域分析
- 結論
第2章 市場概要
- 概要と市場定義
- ヒトにおけるさまざまなマイクロバイオーム
- マイクロバイオームと人の健康・疾患の関係
- マイクロバイオーム研究を支える技術
- 培養および栽培技術
- マイクロバイオーム治療薬の開発戦略
- 添加型マイクロバイオーム療法
- 調節型マイクロバイオーム療法
- マクロ経済要因の分析
- 地政学的要因
- インフレおよび為替変動
- ポーターのファイブフォース分析
第3章 市場力学
- サマリー
- 市場促進要因
- マイクロバイオームと疾患の相関に関する証拠の増加
- 疾病予防とモニタリングのためのマイクロバイオームに基づく診断
- 市場抑制要因
- 臨床試験設計における課題
- 確立された規制枠組みの欠如
- マイクロバイオーム治療の高コスト
- 市場機会
- 消費者向けマイクロバイオーム検査の普及
- 肺と皮膚のマイクロバイオームに対する薬剤と診断
第4章 規制状況
- 規制面
- 北米
- 欧州
- アジア太平洋
第5章 新興技術
- サマリー
- 新興技術
- 微生物生態系療法
- メタトランスクリプトームシーケンシング
- 遺伝子改変マイクロバイオーム治療薬
- マイクロバイオーム由来医薬品との併用療法および補助療法
- 新規前臨床モデル
第6章 市場セグメンテーション分析
- セグメンテーションの内訳
- 市場分析:タイプ別
- マイクロバイオームベース医薬品
- 市場分析:用途別
- 感染症
- 消化器疾患
- 代謝障害
- がん
- その他
- 市場分析:エンドユーザー別
- 病院・診療所
- 研究機関
- 製薬会社
- 地理的内訳
- 市場分析:地域別
- 北米
- 欧州
- アジア太平洋
- 南米
- 中東・アフリカ
第7章 競合情報
- サマリー
- 企業シェア分析
- 競合分析
- ベンチャー資金調達と投資情勢
- 最近の動向
第8章 ヒトマイクロバイオームに基づく医薬品・診断産業における持続可能性:ESGの観点
- ESG:イントロダクション
- ESGリスク評価
- 結論
第9章 付録
- 調査手法
- 出典
- 略語
- 企業プロファイル
- BIOMEBANK
- ENTEROBIOTIX LTD.
- ENTEROME
- FERRING
- GENETIC ANALYSIS
- ILLUMINA INC.
- MICROBIOME INSIGHTS
- MICROBIOTICA
- NESTLE HEALTH SCIENCE
- OXFORD NANOPORE TECHNOLOGIES PLC
- PACBIO
- SFA THERAPEUTICS INC.
- THERMO FISHER SCIENTIFIC INC.
- VEDANTA BIOSCIENCES INC.
List of Tables
- Summary Table : Global Market for Human Microbiome-based Drugs and Diagnostics, by Region, Through 2030
- Table 1 : Technology for Microbiome Research
- Table 2 : Diseases Associated with Microbiome Dysbiosis
- Table 3 : Microbiome-based Drug Nomenclature
- Table 4 : Global Market for Human Microbiome-based Drugs and Diagnostics, by Type, Through 2030
- Table 5 : Global Market for Human Microbiome-based Drugs, by Route of Administration, Through 2030
- Table 6 : Global Market for Human Microbiome-based Drugs, by Region, Through 2030
- Table 7 : Global Market for Human Microbiome-based Drugs, by Rectal Route of Administration, by Region, Through 2030
- Table 8 : Global Market for Human Microbiome-based Drugs, by Oral Route of Administration, by Region, Through 2030
- Table 9 : Global Market for Human Microbiome-based Diagnostics, by Region, Through 2030
- Table 10 : Global Market for Human Microbiome-based Diagnostics, by Product Type, Through 2030
- Table 11 : Global Market for Human Microbiome-based Diagnostics Reagents and Kits, by Region, Through 2030
- Table 12 : Global Market for Human Microbiome-based Diagnostic Instruments, by Region, Through 2030
- Table 13 : Global Market for Human Microbiome-based Drugs and Diagnostics, by Application, Through 2030
- Table 14 : Global Market for Human Microbiome-based Drugs and Diagnostics for Infectious Diseases, by Region, Through 2030
- Table 15 : Global Market for Human Microbiome-based Drugs and Diagnostics for GI Disorders, by Region, Through 2030
- Table 16 : Global Market for Human Microbiome-based Drugs and Diagnostics for Metabolic Disorders, by Region, Through 2030
- Table 17 : Global Market for Human Microbiome-based Drugs and Diagnostics for Cancer, by Region, Through 2030
- Table 18 : Microbiome-based Drugs in the Pipeline for Cancer Therapy
- Table 19 : Global Market for Human Microbiome-based Drugs and Diagnostics for Other Diseases, by Region, Through 2030
- Table 20 : Global Market for Human Microbiome-based Drugs and Diagnostics, by End User, Through 2030
- Table 21 : Global Market for Microbiome-based Drugs and Diagnostics for Hospitals and Clinics, by Region, Through 2030
- Table 22 : Global Market for Microbiome-based Drugs and Diagnostics for Research Institutions, by Region, Through 2030
- Table 23 : Global Market for Microbiome-based Drugs and Diagnostics for Pharmaceutical Firms, by Region, Through 2030
- Table 24 : Global Market for Human Microbiome-based Drugs and Diagnostics, by Region, Through 2030
- Table 25 : North American Market for Human Microbiome-based Drugs and Diagnostics, by Country, Through 2030
- Table 26 : North American Market for Human Microbiome-based Drugs and Diagnostics, by Type, Through 2030
- Table 27 : North American Market for Microbiome-based Drugs, by Route of Administration, Through 2030
- Table 28 : North American Market for Microbiome-based Diagnostics, by Product Type, Through 2030
- Table 29 : North American Market for Microbiome-based Drugs and Diagnostics, by Application, Through 2030
- Table 30 : North American Market for Microbiome-based Drugs and Diagnostics, by End User, Through 2030
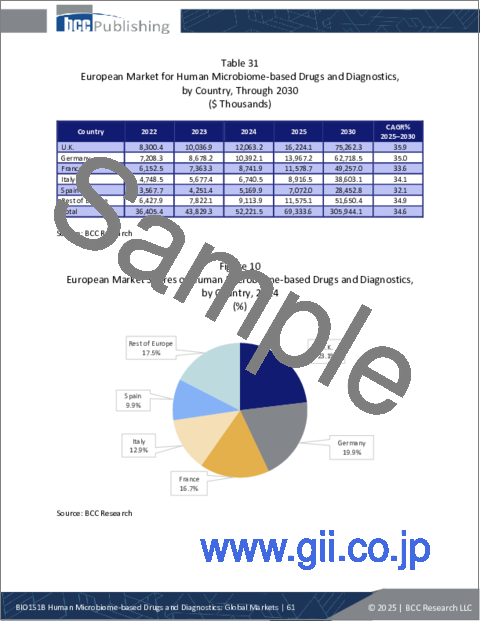
- Table 31 : European Market for Human Microbiome-based Drugs and Diagnostics, by Country, Through 2030
- Table 32 : European Market for Human Microbiome-based Drugs and Diagnostics, by Type, Through 2030
- Table 33 : European Market for Microbiome-based Drugs, by Route of Administration, Through 2030
- Table 34 : European Market for Microbiome-based Diagnostics, by Product Type, Through 2030
- Table 35 : European Market for Microbiome-based Drugs and Diagnostics, by Application, Through 2030
- Table 36 : European Market for Microbiome-based Drugs and Diagnostics, by End User, Through 2030
- Table 37 : Asia-Pacific Market for Human Microbiome-based Drugs and Diagnostics, by Country, Through 2030
- Table 38 : Asia-Pacific Market for Human Microbiome-based Drugs and Diagnostics, by Type, Through 2030
- Table 39 : Asia-Pacific Market for Microbiome-based Drugs, by Route of Administration, Through 2030
- Table 40 : Asia-Pacific Market for Microbiome-based Diagnostics, by Product Type, Through 2030
- Table 41 : Asia-Pacific Market for Microbiome-based Drugs and Diagnostics, by Application, Through 2030
- Table 42 : Asia-Pacific Market for Microbiome-based Drugs and Diagnostics, by End User, Through 2030
- Table 43 : South American Market for Human Microbiome-based Drugs and Diagnostics, by Type, Through 2030
- Table 44 : South American Market for Microbiome-based Drugs, by Route of Administration, Through 2030
- Table 45 : South American Market for Microbiome-based Diagnostics, by Product Type, Through 2030
- Table 46 : South American Market for Microbiome-based Drugs and Diagnostics, by Application, Through 2030
- Table 47 : South American Market for Microbiome-based Drugs and Diagnostics, by End User, Through 2030
- Table 48 : MEA Market for Human Microbiome-based Drugs and Diagnostics, by Type, Through 2030
- Table 49 : MEA Market for Microbiome-based Drugs, by Administration Route, Through 2030
- Table 50 : MEA Market for Microbiome-based Diagnostics, by Product Type, Through 2030
- Table 51 : MEA Market for Microbiome-based Drugs and Diagnostics, by Application, Through 2030
- Table 52 : MEA Market for Microbiome-based Drugs and Diagnostics, by End User, Through 2030
- Table 53 : Competitive Analysis of Human Microbiome-based Diagnostics Market
- Table 54 : Venture Funding in the Human Microbiome-based Drugs and Diagnostics Market, 2024
- Table 55 : Recent Developments in the Human Microbiome-based Drug and Diagnostics Market, 2022-2025
- Table 56 : ESG Goals of Human Microbiome-based Drugs and Diagnostics Companies
- Table 57 : ESG Risk Ratings for Human Microbiome-based Drugs and Diagnostics Firms, 2025
- Table 58 : Report Information Sources
- Table 59 : Abbreviations Used in this Report
- Table 60 : BiomeBank: Company Snapshot
- Table 61 : BiomeBank: Product Portfolio
- Table 62 : BiomeBank: News/Key Developments, 2024
- Table 63 : EnteroBiotix Ltd.: Company Snapshot
- Table 64 : EnteroBiotix Ltd.: Product Portfolio
- Table 65 : EnteroBiotix Ltd.: News/Key Developments, 2024
- Table 66 : Enterome: Company Snapshot
- Table 67 : Enterome: Product Portfolio
- Table 68 : Enterome: News/Key Developments, 2022
- Table 69 : Ferring: Company Snapshot
- Table 70 : Ferring: Financial Performance, FY 2022 and 2023
- Table 71 : Ferring: Product Portfolio
- Table 72 : Ferring: News/Key Developments, 2022 and 2023
- Table 73 : Genetic Analysis: Company Snapshot
- Table 74 : Genetic Analysis: Financial Performance, FY 2023 and 2024
- Table 75 : Genetic Analysis: Product Portfolio
- Table 76 : Genetic Analysis: News/Key Developments, 2024
- Table 77 : Illumina Inc.: Company Snapshot
- Table 78 : Illumina Inc.: Financial Performance, FY 2023 and 2024
- Table 79 : Illumina Inc.: Product Portfolio
- Table 80 : Microbiome Insights: Company Snapshot
- Table 81 : Microbiome Insights: Product Portfolio
- Table 82 : Microbiome Insights: News/Key Developments, 2024
- Table 83 : Microbiotica: Company Snapshot
- Table 84 : Microbiotica: Product Portfolio
- Table 85 : Microbiotica: News/Key Developments, 2023
- Table 86 : Nestle Health Science: Company Snapshot
- Table 87 : Nestle Health Science: Product Portfolio
- Table 88 : Nestle Health Science: News/Key Developments, 2024
- Table 89 : Oxford Nanopore Technologies plc: Company Snapshot
- Table 90 : Oxford Nanopore Technologies plc: Financial Performance, FY 2023 and 2024
- Table 91 : Oxford Nanopore Technologies plc.: Product Portfolio
- Table 92 : PacBio: Company Snapshot
- Table 93 : PacBio: Financial Performance, FY 2023 and 2024
- Table 94 : PacBio: Product Portfolio
- Table 95 : PacBio: News/Key Developments, 2025
- Table 96 : SFA Therapeutics Inc.: Company Snapshot
- Table 97 : SFA Therapeutics Inc.: Product Portfolio
- Table 98 : SFA Therapeutics Inc.: News/Key Developments, 2024
- Table 99 : Thermo Fisher Scientific Inc.: Company Snapshot
- Table 100 : Thermo Fisher Scientific Inc.: Financial Performance, FY 2023 and 2024
- Table 101 : Thermo Fisher Scientific Inc.: Product Portfolio
- Table 102 : Vedanta Biosciences Inc.: Company Snapshot
- Table 103 : Vedanta Biosciences Inc.: Product Portfolio
- Table 104 : Vedanta Biosciences Inc.: News/Key Developments, 2023
- Table 105 : Emerging Startups
List of Figures
- Summary Figure : Global Market Shares of Human Microbiome-based Drugs and Diagnostics, by Region, 2024
- Figure 1 : Essential Functions of the Human Microbiome
- Figure 2 : Porter's Five Forces Analysis of the Human Microbiome-based Drugs and Diagnostics Market
- Figure 3 : Human Microbiome-based Drugs and Diagnostics Market Dynamics
- Figure 4 : Diseases and Conditions Associated with Dysbiosis
- Figure 5 : Global Market Shares of Human Microbiome-based Drugs and Diagnostics, by Type, 2024
- Figure 6 : Global Market Shares of Human Microbiome-based Drugs and Diagnostics, by Application, 2024
- Figure 7 : Global Market Shares of Human Microbiome-based Drugs and Diagnostics, by End User, 2024
- Figure 8 : Global Market Shares of Human Microbiome-based Drugs and Diagnostics, by Region, 2024
- Figure 9 : North American Market Shares of Human Microbiome-based Drugs and Diagnostics, by Country, 2024
- Figure 10 : European Market Shares of Human Microbiome-based Drugs and Diagnostics, by Country, 2024
- Figure 11 : Asia-Pacific Market Shares of Human Microbiome-based Drugs and Diagnostics, by Country, 2024
- Figure 12 : Company Shares in the Human Microbiome-based Drug Market, 2024
- Figure 13 : Ferring: Revenue Share, by Business Unit, FY 2023
- Figure 14 : Ferring: Revenue Share, by Country/Region, FY 2023
- Figure 15 : Genetic Analysis: Revenue Share, by Business Unit, FY 2024
- Figure 16 : Genetic Analysis: Revenue Share, by Country/Region, FY 2024
- Figure 17 : Illumina Inc.: Revenue Share, by Business Unit, FY 2024
- Figure 18 : Illumina Inc.: Revenue Share, by Country/Region, FY 2024
- Figure 19 : Oxford Nanopore Technologies plc: Revenue Share, by Business Unit, FY 2024
- Figure 20 : Oxford Nanopore Technologies plc: Revenue Share, by Country/Region, FY 2024
- Figure 21 : PacBio: Revenue Share, by Business Unit, FY 2024
- Figure 22 : PacBio: Revenue Share, by Country/Region, FY 2024
- Figure 23 : Thermo Fisher Scientific Inc.: Revenue Share, by Business Unit, FY 2024
- Figure 24 : Thermo Fisher Scientific Inc.: Revenue Share, by Country/Region, FY 2024
The global market for human microbiome-based drugs and diagnostics is expected to grow from $393.4 million in 2025 to reach $1.2 billion by the end of 2030, at a compound annual growth rate (CAGR) of 25.6% from 2025 through 2030.
The North American market for human microbiome-based drugs and diagnostics is expected to grow from $225.7 million in 2025 to reach $578.9 million by the end of 2030, at a CAGR of 20.7% from 2025 through 2030.
The European market for human microbiome-based drugs and diagnostics is expected to grow from $69.3 million in 2025 to reach $305.9 million by the end of 2030, at a CAGR of 34.6% from 2025 through 2030.
Report Scope
The report analyzes the global market for human microbiome-based drugs and diagnostics and market trends. It includes global revenues for the base year 2024, estimated data for 2025 and the compound annual growth rates (CAGRs) for forecast period of 2025 to 2030. The market is segmented by type, application, end user and region. The microbiome-based drug segment is further segmented by route of administration: oral or rectal. Microbiome-based diagnostics are segmented based on product type into instruments, and reagents and kits. End-user segments include hospitals and clinics, pharmaceutical companies and research institutes. Applications include metabolic, gastrointestinal (GI) and infectious diseases, cancer and others. The regions covered include North America, Europe, Asia-Pacific, South America and the Middle East and Africa (MEA). The report discusses emerging technologies and analyzes the competitive landscape, providing the ranking/market shares of leading companies in the market. It also includes a chapter on environmental, social and corporate governance (ESG) developments.
Human microbiome-based products that are intended for wellness or as dietary supplements are outside the scope of the report, as are nonstandardized fecal microbiota transplantation (FMT) procedures. Microbiome-based diagnostics include only products that claim to detect or screen for any disease or condition.
Report Includes
- 55 data tables and 51 additional tables
- Analyses of the trends in global markets for human microbiome-based drugs and diagnostics, with revenue data from 2022 to 2024, estimates for 2025, and projected CAGRs through 2030
- Estimates of the size and revenue prospects for the global market, along with a market share analysis by type, drug route of administration, diagnostics product type, application, end user, and region
- Facts and figures pertaining to market dynamics, opportunities and deterrents, technological advances, regulations, and the impacts of macroeconomic variables
- Review of the prevalence of infectious diseases, metabolic disorders and chronic ailments
- An assessment of current products, clinical trials and identification of new potential markets for novel products and assay development
- Overview of the sustainability trends and ESG developments in the industry, with emphasis on the ESG practices followed by leading companies, their ESG ratings, and consumer attitudes
- An analysis of the key patent grants and recently published patents
- Analysis of the industry structure and value chain, and the competitive landscape, including companies' market shares, strategic alliances, M&A activity, venture fundings and investment outlook
- Profiles of the leading companies, including Ferring Pharmaceuticals, Nestle Health Science, BiomeBank, Genetic Analysis AS, and Vedanta Biosciences Inc.
Table of Contents
Chapter 1 Executive Summary
- Market Outlook
- Scope of Report
- Market Summary
- Market Dynamics
- Emerging Technologies
- Analysis by Segment
- Regional Analysis
- Conclusion
Chapter 2 Market Overview
- Overview and Market Definition
- Different Microbiomes in Humans
- Microbiome, Human Health and Disease
- Technologies Aiding Microbiome Research
- Culturing and Cultivation
- Strategies for the Development of Microbiome Therapeutics
- Additive Microbiome Therapy
- Modulatory Microbiome Therapy
- Analysis of Macroeconomic Factors
- Geopolitical Factors
- Inflation and Currency Exchange Fluctuations
- Porter's Five Forces Analysis
Chapter 3 Market Dynamics
- Takeaways
- Market Drivers
- Growing Evidence of Microbiome-disease Correlation
- Microbiome-based Diagnostics for Disease Prevention and Monitoring
- Market Restraints
- Challenges in Clinical Trial Design
- Lack of Established Regulatory Frameworks
- High Costs of Microbiome Therapeutics
- Market Opportunities
- Direct-to-Consumer Microbiome Testing
- Drugs and Diagnostics for Lung and Skin Microbiomes
Chapter 4 Regulatory Landscape
- Regulatory Aspects
- North America
- Europe
- Asia-Pacific
Chapter 5 Emerging Technologies
- Takeaways
- Emerging Technologies
- Microbial Ecosystem Therapeutics
- Metatranscriptome Sequencing
- Genetically Modified Microbiome Therapeutics
- Combination and Adjuvant Therapies with Microbiome-based Drugs
- Novel Preclinical Models
Chapter 6 Market Segmentation Analysis
- Segmentation Breakdown
- Market Analysis by Type
- Microbiome-based Drugs
- Market Analysis by Application
- Infectious Diseases
- GI Disorders
- Metabolic Disorders
- Cancer
- Other Diseases
- Market Analysis by End User
- Hospitals and Clinics
- Research Institutions
- Pharmaceutical Companies
- Geographic Breakdown
- Market Analysis by Region
- North America
- Europe
- Asia-Pacific
- South America
- Middle East and Africa
Chapter 7 Competitive Intelligence
- Takeaways
- Company Share Analysis
- Competitive Analysis
- Venture Funding and Investment Landscape
- Recent Developments
Chapter 8 Sustainability in the Human Microbiome-based Drugs and Diagnostics Industry: An ESG Perspective
- Introduction to ESG
- ESG Risk Ratings
- Concluding Remarks
Chapter 9 Appendix
- Methodology
- Sources
- Abbreviations
- Company Profiles
- BIOMEBANK
- ENTEROBIOTIX LTD.
- ENTEROME
- FERRING
- GENETIC ANALYSIS
- ILLUMINA INC.
- MICROBIOME INSIGHTS
- MICROBIOTICA
- NESTLE HEALTH SCIENCE
- OXFORD NANOPORE TECHNOLOGIES PLC
- PACBIO
- SFA THERAPEUTICS INC.
- THERMO FISHER SCIENTIFIC INC.
- VEDANTA BIOSCIENCES INC.
- Emerging Start-ups/Market Disruptors