|
|
市場調査レポート
商品コード
1385520
重症筋無力症治療の世界市場:予測・動向Global Myasthenia Gravis Treatment Market: Forecast and Trends |
||||||
|
|||||||
| 重症筋無力症治療の世界市場:予測・動向 |
|
出版日: 2023年11月23日
発行: BCC Research
ページ情報: 英文 120 Pages
納期: 即納可能
|
全表示
- 概要
- 図表
- 目次
世界の重症筋無力症治療の市場規模は、2023年の13億米ドルから、予測期間中は10.5%のCAGRで推移し、2028年には22億米ドルの規模に成長すると予測されています。
タイプ別で見ると、全身型重症筋無力症治療の部門は、2023年の11億米ドルから、同期間中10.6%のCAGRで推移し、2028年には19億米ドルの規模に成長すると予測されています。また、眼筋型重症筋無力症治療の部門は、2023年の1億240万米ドルから、9.3%のCAGRで推移し、2028年には1億5,960万米ドルの規模に成長すると予測されています。
当レポートでは、世界の重症筋無力症治療の市場を調査し、市場および疾患・診断・治療の概要、市場影響因子および市場機会の分析、市場規模の推移・予測、各種区分・地域別の詳細分析、技術および特許の動向、パイプラインの分析、ESGの展開、競合情勢、主要企業のプロファイルなどをまとめています。
目次
第1章 イントロダクション
第2章 サマリー・ハイライト
- 市場の見通し
- レポートハイライト
第3章 市場概要
- 重症筋無力症
- 重症筋無力症眼科
- 全身型重症筋無力症
- 一過性新生児重症筋無力症
- 重症筋無力症に対する世界の規制構造
- 医薬品の承認と規制
- 臨床試験
- 患者アクセスと健康保険
- 薬価設定と償還
- 診療ガイドライン
- 希少疾患指定
- 患者の権利擁護と支援
- 重症筋無力症治療の価格と償還
- 北米
- 欧州
- アジア太平洋
- 重症筋無力症治療に使用される治療法・治療薬
- 薬剤
- 手術
- 静脈内 (IV) 療法
- 薬剤
第4章 市場力学
- 市場促進要因
- 高齢者人口の増加
- 発生率と有病率の増加
- 重症筋無力症の診断の進歩
- R&D
- 規制環境
- 個別化医療
- 市場機会
- アンメットニーズ
- R&Dの進歩
- 生物学的製剤と免疫療法
- 世界的展開
- 意識の向上
- カスタマイズされた治療アプローチ
- 医療費償還
- 患者中心のケア
- 競合情勢
- 重症筋無力症の慢性的性質
- 研究協力
- 市場抑制要因
- 限られた患者数
- 診断上の課題
- 疾患修飾療法の欠如
- 償還の問題
- 新しい治療法のリスク
- その他の抑制要因
第5章 市場内訳:地域別
- 市場概要
- 北米
- 欧州
- アジア太平洋
第6章 市場内訳:タイプ別
- 市場概要
- 全身型重症筋無力症
- 一過性新生児重症筋無力症
- 眼筋型重症筋無力症
第7章 市場内訳:治療法別
- 薬剤
- 手術
- 点滴療法
第8章 市場内訳:製品タイプ別
- モノクローナル抗体
- コリンエステラーゼ阻害剤
- 静脈内免疫グロブリン
- 免疫抑制剤
- コルチコステロイド
第9章 ESGの展開
- 環境
- 社会
- ガバナンス
- ケーススタディ
- AstraZeneca
- BCCによる総論
第10章 新たな技術と開発
第11章 競合情報
- 概要
- 業界シナリオ
- 企業シェア
第12章 特許分析
第13章 パイプライン分析
- 臨床試験
第14章 M&Aとベンチャー資金調達の見通し
第15章 企業プロファイル
- ABBVIE
- AMYASTHENIA GRAVISEN INC.
- ASTELLAS PHARMA INC.
- ASTRAZENECA
- BAUSCH HEALTH COMPANIES INC.
- BAYER AG
- BIOGEN
- BRISTOL-MYERS SQUIBB
- CSL
- DAIICHI SANKYO CO. LTD.
- F. HOFFMANN-LA ROCHE AG
- GILEAD SCIENCES INC.
- GLAXOSMITHKLINE PLC.
- MERCK & CO. INC.
- NOVARTIS
- PFIZER INC.
- SANOFI
- SERVIER LABORATORIES
- TEVA PHARMACEUTICAL INDUSTRIES LTD.
第16章 付録:頭字語
List of Tables
- Summary Table : Global Market for the Treatment of Myasthenia Gravis, by Type of Myasthenia Gravis, Through 2028
- Table 1 : Global Population, Aged 60+, 2010-2050
- Table 2 : Factors Leading to the Increasing Incidence of Myasthenia Gravis Worldwide
- Table 3 : Global Serological Testing for Myasthenia Gravis, by Type
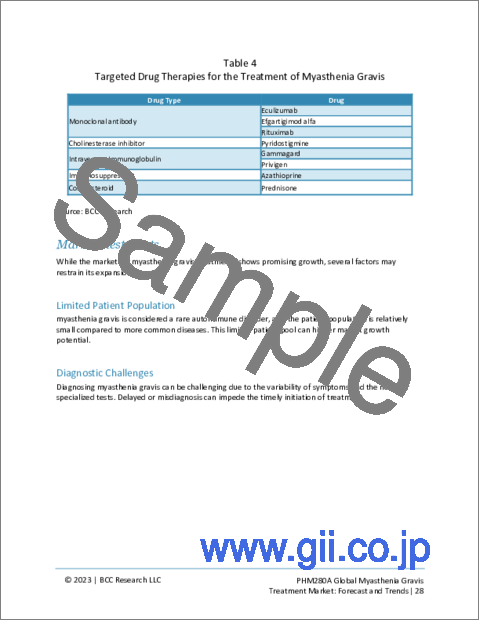
- Table 4 : Targeted Drug Therapies for the Treatment of Myasthenia Gravis
- Table 5 : Diagnostic Challenges in the Treatment of Myasthenia Gravis
- Table 6 : Risks of Emerging Therapies for Myasthenia Gravis, by Type
- Table 7 : Global Market for the Treatment of Myasthenia Gravis, by Region, Through 2028
- Table 8 : North American Market for the Treatment of Myasthenia Gravis, by Country, Through 2028
- Table 9 : European Market for the Treatment of Myasthenia Gravis, by Country, Through 2028
- Table 10 : Asia-Pacific Market for the Treatment of Myasthenia Gravis, by Country, Through 2028
- Table 11 : Global Prevalence of Myasthenia Gravis, by Type
- Table 12 : Global Market for the Treatment of Generalized Myasthenia Gravis, by Region, Through 2028
- Table 13 : Global Market for the Treatment of Ocular Myasthenia Gravis, by Region, Through 2028
- Table 14 : Global Market for the Treatment of Transient Neonatal Myasthenia Gravis, by Region, Through 2028
- Table 15 : Global Market for Medications for the Treatment of Myasthenia Gravis, by Region, Through 2028
- Table 16 : Global Market for Surgical Treatment of Myasthenia Gravis, by Region, Through 2028
- Table 17 : Global Market for Intravenous Therapy for the Treatment of Myasthenia Gravis, by Region, Through 2028
- Table 18 : Global Market for the Treatment of Myasthenia Gravis, by Type of Product, Through 2028
- Table 19 : Global Market for Monoclonal Antibodies for the Treatment of Myasthenia Gravis, by Type of Product, Through 2028
- Table 20 : Global Market for Cholinesterase Inhibitors for the Treatment of Myasthenia Gravis, by Type of Product, Through 2028
- Table 21 : Global Market for Intravenous Immunoglobulins for the Treatment of Myasthenia Gravis, by Type of Product, Through 2028
- Table 22 : Global Market for Immunosuppressants for the Treatment of Myasthenia Gravis, by Type of Product, Through 2028
- Table 23 : Global Market for Corticosteroids for the Treatment of Myasthenia Gravis, by Type of Product, Through 2028
- Table 24 : ESG Framework for the Global Pharmaceutical Industry
- Table 25 : Social Framework of the Global Pharmaceutical Industry
- Table 26 : Top Pharma Companies: ESG Ratings
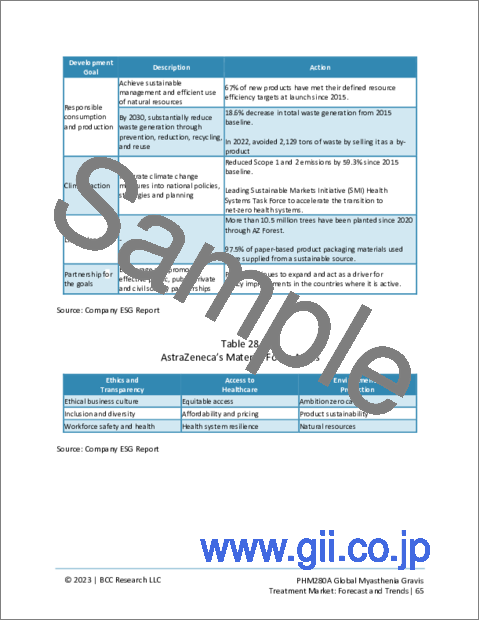
- Table 27 : AstraZeneca's Contribution to Sustainable Development Goals
- Table 28 : AstraZeneca's Material Focus Areas
- Table 29 : New Technologies for Myasthenia Gravis Treatments
- Table 30 : New Pharmaceuticals for the Treatment of Myasthenia Gravis
- Table 31 : Global Company Market Shares of Myasthenia Gravis Treatments, 2022
- Table 32 : Global Product Shares of Myasthenia Gravis Treatments, by Brand, 2022
- Table 33 : Global Sales of Myasthenia Gravis Treatments, by Brand, 2022
- Table 34 : Key Financials of Pharma Companies Offering Myasthenia Gravis Treatment Products, 2022
- Table 35 : Patents on Myasthenia Gravis Treatments, 2018-Present
- Table 36 : Selected Manufacturer Patents on Myasthenia Gravis Drugs, 2018-Present
- Table 37 : Patents on Eculizumab, 2018-Present
- Table 38 : Selected Manufacturer Patents on Eculizumab, 2018-Present
- Table 39 : Patents on Efgartigimod alfa, 2018-Present
- Table 40 : Selected Manufacturer Patents on Efgartigimod alfa, 2018-Present
- Table 41 : Patents on Rituximab, 2018-Present
- Table 42 : Selected Manufacturer Patents on Rituximab, 2018-Present
- Table 43 : Patents on Pyridostigmine, 2018-Present
- Table 44 : Selected Manufacturer Patents on Pyridostigmine, 2018-Present
- Table 45 : Patents on Privigen, 2018-Present
- Table 46 : Selected Manufacturer Patents on Privigen, 2018-Present
- Table 47 : Clinical Trials on Myasthenia Gravis, as of 2023
- Table 48 : Mergers and Acquisitions, 2019-2021
- Table 49 : AbbVie: Product Information
- Table 50 : AbbVie: Financial Overview, 2020-2022
- Table 51 : AbbVie: Key Developments, 2021-2023
- Table 52 : Amgen: Revenue, 2020-2022
- Table 53 : Amgen: Key Developments, 2021 and 2022
- Table 54 : Astellas Pharma: Revenue, 2020-2022
- Table 55 : Astellas Pharma: Key Developments, 2018-2022
- Table 56 : AstraZeneca: Product Information
- Table 57 : AstraZeneca: Revenue, 2020-2022
- Table 58 : AstraZeneca: Key Developments, 2022 and 2023
- Table 59 : Bausch Health: Product Information
- Table 60 : Bausch Health: Revenue, 2020-2022
- Table 61 : Bayer AG: Product Information
- Table 62 : Bayer AG: Financial Overview, 2020-2022
- Table 63 : Bayer AG: Key Developments, 2022 and 2023
- Table 64 : Biogen: Financial Overview, 2020-2022
- Table 65 : Biogen: Key Developments, 2018-2023
- Table 66 : Bristol-Myers Squibb: Product Information
- Table 67 : Bristol-Myers Squibb: Financial Overview, 2020-2022
- Table 68 : Bristol-Myers Squibb: Key Developments, 2021-2023
- Table 69 : CSL: Product Information
- Table 70 : CSL: Revenue, 2020-2022
- Table 71 : CSL: Recent Developments, 2022
- Table 72 : Daiichi Sankyo: Product Information
- Table 73 : Daiichi Sankyo: Financial Overview, 2020-2022
- Table 74 : Daiichi Sankyo: Key Developments, 2022 and 2023
- Table 75 : Roche: Product Information
- Table 76 : Roche: Revenue, 2020-2022
- Table 77 : Roche: Key Developments, 2021-2023
- Table 78 : Gilead Sciences: Financial Overview, 2020-2022
- Table 79 : Gilead Sciences: Key Developments, 2022
- Table 80 : GlaxoSmithKline plc: Product Segments
- Table 81 : GlaxoSmithKline plc: Financial Overview, 2020-2022
- Table 82 : GlaxoSmithKline plc: Recent Developments, 2022
- Table 83 : Merck & Co. Inc.: Business Segments
- Table 84 : Merck & Co. Inc.: Financial Overview, 2020-2022
- Table 85 : Merck & Co. Inc.: Key Developments, 2022
- Table 86 : Novartis: Product Information
- Table 87 : Novartis: Financial Overview, 2020-2022
- Table 88 : Novartis: Key Developments, 2023
- Table 89 : Pfizer Inc: Product Segments
- Table 90 : Pfizer Inc: Financial Overview, 2020-2022
- Table 91 : Pfizer Inc: Top Brands, 2022
- Table 92 : Pfizer Inc: Key Developments, 2019-2023
- Table 93 : Sanofi: Business Segments
- Table 94 : Sanofi: Annual Revenue, 2020-2022
- Table 95 : Sanofi: Key Developments, 2021-2023
- Table 96 : Servier: Annual Revenue, 2020-2022
- Table 97 : Servier: Key Developments, 2022 and 2023
- Table 98 : Teva: Financial Overview, 2020-2022
- Table 99 : Teva: Key Developments, 2021-2023
- Table 100 : Acronyms
List of Figures
- Summary Figure A : Global Market for the Treatment of Myasthenia Gravis, by Type of Myasthenia Gravis, 2020-2028
- Summary Figure B : Global Market Shares of the Treatment of Myasthenia Gravis, by Type of Myasthenia Gravis, 2022
- Figure 1 : Global Factors Leading to the Prevalence of Myasthenia Gravis, by Region, 2022
- Figure 2 : Global Market Shares of Myasthenia Gravis Treatments, by Region, 2022
- Figure 3 : North American Market Shares of Myasthenia Gravis Treatments, by Country, 2022
- Figure 4 : European Market Shares of Myasthenia Gravis Treatments, by Country, 2022
- Figure 5 : Asia-Pacific Market Shares of Myasthenia Gravis Treatments, by Country, 2022
- Figure 6 : Global Distribution of Types of Myasthenia Gravis, 2022
- Figure 7 : Global Market Shares of Myasthenia Gravis Treatments, by Treatment Modality, 2022
- Figure 8 : Global Market Shares of Myasthenia Gravis Treatments, by Type of Product, Through 2022
- Figure 9 : Global Electricity Production from Renewable Sources, 2017-2021
- Figure 10 : Global Recycled Waste, 2017-2021
- Figure 11 : Global CO2 Emissions, 2017-2021
- Figure 12 : Global Company Market Shares of Myasthenia Gravis Treatments, 2022
- Figure 13 : Global Sales of Myasthenia Gravis Treatments, by Brand, 2022
- Figure 14 : AbbVie: Revenue Shares, by Country, 2022
- Figure 15 : Bristol-Myers Squibb: Revenue Share, by Country/Region, 2022
- Figure 16 : Daiichi Sankyo: Market Share, by Country/Region, 2022
- Figure 17 : Gilead Sciences: Revenue, by Type of Product, 2022
- Figure 18 : GlaxoSmithKline plc: Annual Revenue, by Segment, 2022
- Figure 19 : Merck & Co. Inc.: Annual Revenue, Pharmaceutical Segment, 2022
- Figure 20 : Novartis Innovative Medicines: Market Share, by Country/Region, 2022
- Figure 21 : Novartis Sandoz: Market Share, by Country/Region 2022
- Figure 22 : Sanofi: Revenue, by Region, 2022
- Figure 23 : Sanofi: Revenue, by Segment, 2022
- Figure 24 : Teva: Market Share, by Region, 2022
Highlights:
The global market for myasthenia gravis treatment is estimated to increase from $1.3 billion in 2023 to reach $2.2 billion by 2028, at a compound annual growth rate (CAGR) of 10.5% from 2023 through 2028.
The global market for generalized myasthenia gravis treatment is estimated to increase from $1.1 billion in 2023 to reach $1.9 billion by 2028, at a compound annual growth rate (CAGR) of 10.6% from 2023 through 2028.
The global market for ocular myasthenia gravis treatment is estimated to increase from $102.4 million in 2023 to reach $159.6 million by 2028, at a compound annual growth rate (CAGR) of 9.3% from 2023 through 2028.
Report Scope:
This report aims to study the myasthenia gravis treatment global market size comprehensively. Current and historical market revenues can be estimated based on the product type, treatment by drug and region.
Report Includes:
- 54 data tables and 47 additional tables
- An overview and analysis of the global market for myasthenia gravis (MG) treatments
- Analyses of market trends, with historical market revenue data (sales figures) for 2020-2022, estimates for 2023, and projections of compound annual growth rates (CAGRs) through 2028
- Estimates of the market size, revenue forecasts and potential growth share analysis for MG treatments based on product, type, treatment modality, and region
- In-depth information (facts and figures) pertaining to the major factors influencing the market (drivers, restraints, opportunities and challenges)
- Analysis of the market growth opportunities through Porter's Five Forces and PESTLE analyses, taking into consideration the prevailing micro- and macro environmental factors
- An examination of the importance of ESG in the myasthenia gravis market, taking into account consumer attitudes, risks and opportunities, and the ESG practices of pharmaceutical and biotech companies
- Discussion of the factors driving the market, industry trends and new developments
- Analysis of relevant patents
- Identification of the leaders in the field of MG
- Profiles of the leading market players, including Profiles of the leading market players, including AbbVie, Bausch Health, Gilead Sciences, Merck & Co. Inc., Servier, and Teva
Table of Contents
Chapter 1 Introduction
- Study Goals and Objectives
- Reasons for Doing This Study
- Scope of Report
- Methodology
- Information Sources
- Primary Research
- Secondary Research
- Geographic Breakdown
- Segmentation Breakdown
Chapter 2 Summary and Highlights
- Market Outlook
- Report Highlights
Chapter 3 Market Overview
- Myasthenia Gravis
- Ocular Myasthenia Gravis
- Generalized Myasthenia Gravis
- Transient Neonatal Myasthenia Gravis
- Global Regulatory Structure for Myasthenia Gravis
- Drug Approvals and Regulation
- Clinical Trials
- Patient Access and Health Insurance
- Pharmaceutical Pricing and Reimbursement
- Clinical Practice Guidelines
- Rare Disease Designation
- Patient Advocacy and Support
- Pricing and Reimbursement for the Treatment of Myasthenia Gravis
- North America
- Europe
- Asia-Pacific
- Therapies/Drugs Used to Treat Myasthenia Gravis
- Medications
- Surgery
- Intravenous (IV) Therapy
- Medications
Chapter 4 Market Dynamics
- Market Drivers
- Growing Elderly Population
- Increasing Incidence and Prevalence
- Advancements in Myasthenia Gravis Diagnostics
- Research and Development
- Regulatory Environment
- Personalized Medicine
- Market Opportunities
- Unmet Medical Needs
- Advancements in Research and Development
- Biologics and Immunotherapies
- Global Expansion
- Increased Awareness
- Tailored Treatment Approaches
- Healthcare Reimbursement
- Patient-Centric Care
- Competitive Landscape
- Chronic Nature of Myasthenia Gravis
- Research Collaboration
- Market Restraints
- Limited Patient Population
- Diagnostic Challenges
- Lack of Disease-Modifying Therapies
- Reimbursement Issues
- Risks of Emerging Therapies
- Other Restraints
Chapter 5 Market Breakdown by Region
- Market Overview
- North America
- Europe
- Asia-Pacific
Chapter 6 Market Breakdown by Type
- Market Overview
- Generalized Myasthenia Gravis
- Transient Neonatal Myasthenia Gravis
- Ocular Myasthenia Gravis
Chapter 7 Market Breakdown by Treatment Modality
- Introduction
- Medications
- Surgery
- Intravenous Therapy
Chapter 8 Market Breakdown by Type of Product
- Monoclonal Antibodies
- Brands
- Cholinesterase Inhibitors
- Brand
- Intravenous Immunoglobulins
- Brands
- Immunosuppressants
- Brand
- Corticosteroids
- Brand
Chapter 9 ESG Development
- Introduction
- Environment
- Social
- Governance
- Case Study
- AstraZeneca
- Concluding Remarks from BCC Research
Chapter 10 Emerging Technologies and Developments
- Introduction
Chapter 11 Competitive Intelligence
- Overview
- Industry Scenario
- Company Shares
Chapter 12 Patent Analysis
- Patent Analysis by Manufacturer
Chapter 13 Pipeline Analysis
- Clinical Trials
Chapter 14 M&A and Venture Funding Outlook
- Introduction
Chapter 15 Company Profiles
- ABBVIE
- AMYASTHENIA GRAVISEN INC.
- ASTELLAS PHARMA INC.
- ASTRAZENECA
- BAUSCH HEALTH COMPANIES INC.
- BAYER AG
- BIOGEN
- BRISTOL-MYERS SQUIBB
- CSL
- DAIICHI SANKYO CO. LTD.
- F. HOFFMANN-LA ROCHE AG
- GILEAD SCIENCES INC.
- GLAXOSMITHKLINE PLC.
- MERCK & CO. INC.
- NOVARTIS
- PFIZER INC.
- SANOFI
- SERVIER LABORATORIES
- TEVA PHARMACEUTICAL INDUSTRIES LTD.