|
|
市場調査レポート
商品コード
1327712
RNAiドラッグデリバリー:各種技術と世界の市場RNAi Drug Delivery: Technologies and Global Markets |
||||||
|
|||||||
| RNAiドラッグデリバリー:各種技術と世界の市場 |
|
出版日: 2023年08月08日
発行: BCC Research
ページ情報: 英文 141 Pages
納期: 即納可能
|
- 全表示
- 概要
- 図表
- 目次
世界の核酸ドラッグデリバリーの市場規模は、2023年の11億米ドルから、予測期間中は20.5%のCAGRで推移し、2028年には27億米ドルの規模に成長すると予測されています。
タイプ別で見ると、カプセル化の部門は、2023年の6億2,940万米ドルから、22.1%のCAGRで推移し、2028年には17億米ドルの規模に成長すると予測されています。また、共役の部門は、2023年の4億3,260万米ドルから、17.9%のCAGRで推移し、2028年には9億8,460万米ドルの規模に成長すると予測されています。
当レポートでは、世界のRNAiドラッグデリバリーの市場を調査し、市場概要、市場影響因子および市場機会の分析、市場規模の推移・予測、各種区分・地域別の詳細分析、ESGの展開、新興技術と新たな開発、競合情勢、主要企業のプロファイルなどをまとめています。
目次
第1章 イントロダクション
第2章 サマリー・ハイライト
第3章 市場概要
- 核酸医薬品の歴史
- アンチセンスオリゴヌクレオチド
- RNA
- ドラッグデリバリーシステム
- 核酸医薬品
- 阻害タイプ
- アンチセンスオリゴヌクレオチド
- 法規制
- 前臨床INDA
- RNAiドラッグデリバリー市場に対するCOVID-19の影響分析
- COVID-19のプラスの影響
- COVID-19のマイナスの影響
第4章 市場力学
- 市場促進要因
- 市場抑制要因
- 市場機会
第5章 核酸ドラッグデリバリー技術市場:タイプ別
- カプセル化
- ウイルスベクターベース
- 非ウイルスベクターベースの送達システム
- 共役
第6章 市場内訳:用途別
- 医薬品開発・発見
- RNAベース創薬プロセス
- RNAi薬の設計
- シーケンスの最適化
- 化学修飾
- 標的化デリバリー
- 治療
第7章 市場内訳:投与経路別
第8章 市場内訳:分子別
- 低分子干渉リボ核酸
- アンチセンスオリゴヌクレオチド
- フォミビルセン (Vitravene)
- ミポメルセン
- ヌシネルセン (Spinraza)
- イノテルセン (Tegsedi)
- エテプリルセン (Exondys 51)
- ゴロディルセン (Vyondys 53)
- Milasen:ユニークな個別化医療
- メッセンジャーRNA
- その他
- アプタマー
- マイクロRNA
第9章 市場内訳:治療領域別
- 腫瘍
- 希少疾患・遺伝性疾患
- 中枢神経系
- 呼吸器系
- その他の病気
第10章 市場内訳:地域別
- 北米
- 欧州
- アジア太平洋
- 日本
- 韓国
- 中国
- インド
- オーストラリア
- その他の地域
第11章 バイオテクノロジー分野におけるESG (環境・社会・ガバナンス)
- バイオテクノロジー産業における主なESG問題
- バイオテクノロジー産業のESGのパフォーマンス分析
- 環境面のパフォーマンス
- 社会面のパフォーマンス
- ガバナンス面のパフォーマンス
- バイオテクノロジーにおけるESG:消費者の視点
- ケーススタディ
- BCC Researchによる総論
第12章 新たな技術と開発
- 肺へのドラッグデリバリー
- ナノテクノロジー
- AIと機械学習の利用
第13章 臨床試験・特許の分析
- 臨床試験
- 特許
第14章 M&A・資金調達の見通し
- 核酸送達技術のスタートアップによる資金調達
第15章 競合情報
第16章 企業プロファイル
- ALNYLAM PHARMACEUTICALS INC.
- ARCTURUS THERAPEUTICS INC.
- ARBUTUS BIOPHARMA CORP.
- ARROWHEAD PHARMACEUTICALS INC.
- BENITEC BIOPHARMA
- CELLECTA INC.
- ELEVEN THERAPEUTICS
- GENEVANT SCIENCES CORP.
- IONIS PHARMACEUTICALS INC.
- MIRIMUS, INC.
- NOVO NORDISK
- NANODE THERAPEUTICS INC.
- OLIX PHARMACEUTICALS
- PHIO PHARMACEUTICALS CORP.
- SILENCE THERAPEUTICS PLC
- SIRNAOMICS INC.
- SOMAGENICS, INC.
List of Tables
- Summary Table : Global Market for Nucleic Acid Drug Delivery, by Type, Through 2028
- Table 1 : Challenges for the Delivery of Nucleic Acid Drugs
- Table 2 : Differences Between Small Molecule Drugs and Biological Drugs
- Table 3 : Nucleic Acid Drug Classification
- Table 4 : Nucleic Acid Drug Types
- Table 5 : siRNA Therapeutics for SARS-CoV-2, SARS-CoV-1 and MERS-CoV, 2021
- Table 6 : Orphan Drug Incentives in Various Countries
- Table 7 : Direct Methods of Insertion into the Cell
- Table 8 : Advantages and Disadvantages of Various Kinds of Drug Delivery Systems
- Table 9 : Advantages and Disadvantages of Viral Vectors
- Table 10 : Global Market for Nucleic Acid Drug Delivery, by Encapsulation Material Type, Through 2028
- Table 11 : Lipid Nanoparticles and Their Functions
- Table 12 : Lipid Nanoparticle Types and Their Advantages and Disadvantages
- Table 13 : Comparison of Polymeric Vectors and Lipid-Based Vectors
- Table 14 : Polymer Types for Nucleic Acid Drug Delivery and Their Properties
- Table 15 : Global Market for Nucleic Acid Drug Delivery, by Application, Through 2028
- Table 16 : Approved Nucleic Acid Drugs, upto June 2023
- Table 17 : Routes of Administration for Nucleic Acid Drugs
- Table 18 : Global Market for Nucleic Acid Drug Delivery, by Route of Aministration, Through 2028
- Table 19 : Characteristics of Different Molecule Types for Nucleic Acid Drug
- Table 20 : Global Market for Nucleic Acid Drug Delivery, by Molecule Type, Through 2028
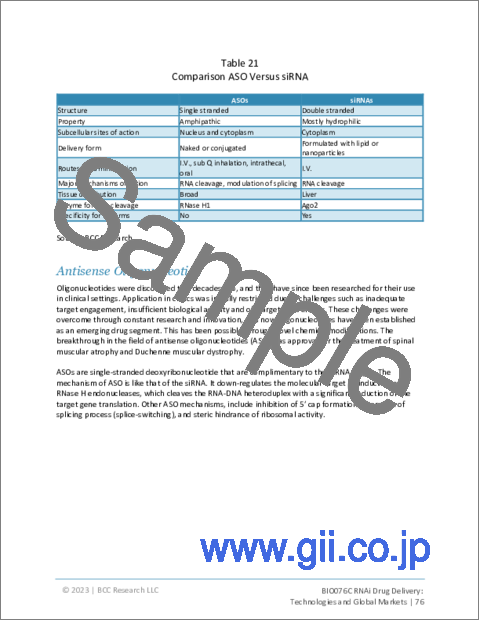
- Table 21 : Comparison ASO Versus siRNA
- Table 22 : Various mRNA Drugs Under Clinical Trials, June 2023
- Table 23 : Comaprision of miRNA and siRNA
- Table 24 : Global Market for Nucleic Acid Drug Delivery, by Therapeutic Area, Through 2028
- Table 25 : Global Market for Nucleic Acid Drug Delivery, by Region, Through 2028
- Table 26 : North American Market for Nucleic Acid Drug Delivery, by Type, Through 2028
- Table 27 : North American Market for Non-Viral Vector Nucleic Acid Drug Delivery, by Type, Through 2028
- Table 28 : U.S. Biotech Private Rounds for Nucleic Acid-Based, June 2022
- Table 29 : European Market for Nucleic Acid Drug Delivery, by Type, Through 2028
- Table 30 : European Market for Non-Viral Vector Nucleic Acid Drug Delivery, by Type, Through 2028
- Table 31 : Asia-Pacific Market for Nucleic Acid Drug Delivery, by Type, Through 2028
- Table 32 : Asia-Pacific Market for Non-Viral Vector Nucleic Acid Drug Delivery, by Type, Through 2028
- Table 33 : Rest of the World Market for Nucleic Acid Drug Delivery, by Type, Through 2028
- Table 34 : Rest of the World Market for Non-Viral Vector Nucleic Acid Drug Delivery, by Type, Through 2028
- Table 35 : Alnylam ESG initiatives
- Table 36 : Patents Issued on Nucleic Acid Drug Deivery, 2000-2020
- Table 37 : Collaboration in Nucleic Acid Drug Delivery Market, 2021-2023
- Table 38 : Start-Up Funding in the Nucleic Acid Drug Delivery Industry, January 2022 and March 2023
- Table 39 : Collaborations in the Nucleic Acid Drug Delivery Market, 2021-2023
- Table 40 : Clinical Trials and Approvals in the Nucliec Acid Drug Delivery Market, 2021-2023
- Table 41 : Other Strategic Developments in Nucleic Acid Drug Delivery Market, 2021-2023
- Table 42 : Alnylam Pharmaceuticals Inc.: Annual Revenue, 2022
- Table 43 : Alnylam Pharmaceuticals: Pipeline Products
- Table 44 : Alnylam Pharmaceuticals: Product Portfolio
- Table 45 : Alnylam Pharmaceuticals: Recent Developments, 2021-2023
- Table 46 : Arcturus Therapeutics Inc.: Annual Revenue, 2022
- Table 47 : Arcturus Therapeutics: Pipeline Products
- Table 48 : Arcturus Therapeutics: Recent Developments, 2021-2023
- Table 49 : Arbutus Biopharma Corp.: Collaborations, 2021-2023
- Table 50 : Arbutus Biopharma Corp.: Annual Revenue, 2022
- Table 51 : Arbutus Biopharma Corp.: Recent Developments, 2021-2023
- Table 52 : Pipeline of Arrowhead Pharmaceuticals
- Table 53 : Benitec Biopharma: Annual Revenue, 2022
- Table 54 : Eleven Therapeutics: Recent Developments, 2022
- Table 55 : Genevant: Recent Developments, 2021 and 2022
- Table 56 : Ionis Phamaceutical Inc.: Annual Revenue, 2022
- Table 57 : Pipeline Products of Ionis Pharmaceuticals
- Table 58 : Ionis Pharmaceuticals Inc.: Recent Developments, 2023
- Table 59 : Mirimus Inc.: Recent Developments, 2021 and 2022
- Table 60 : Novo Nordisk: Annual Revenue, 2022
- Table 61 : Pipeline Products of Novo Nordisk
- Table 62 : Pipeline Products of Nanode Therapeutics
- Table 63 : Olix Pharmaceuticals: Recent Developments, 2021-2023
- Table 64 : Slience Therapeutics: Annual Revenue, 2022
- Table 65 : Pipeline of Silence Therapeutics
- Table 66 : Silence Therapeutics: Recent Developments, 2023
- Table 67 : Pipeline of Sirnaomics Inc.
- Table 68 : Sirnaomics Inc.: Recent Developments, 2021-2023
List of Figures
- Summary Figure : Global Market for Nucleic Acid Drug Delivery, by Type, 2020-2028
- Figure 1 : Timeline of Nucleic Acid Therapeutics, Since 1950s
- Figure 2 : Enzymes Needed for RNA-Based Drugs
- Figure 3 : Basic Function of a Drug Delivery System
- Figure 4 : Challenges Faced by RNA-Based Drug Delivery
- Figure 5 : Nucleic Acid Drug Types
- Figure 6 : Clinical Trial Flow
- Figure 7 : Clinical Trial Specifications
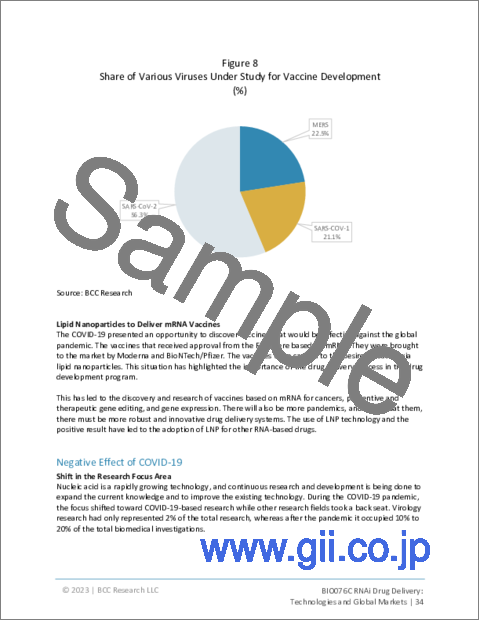
- Figure 8 : Share of Various Viruses Under Study for Vaccine Development
- Figure 9 : Research on Virologoly During the COVID-19 Pandemic
- Figure 10 : RNA-Based Drugs Under Development, 2017-2022
- Figure 11 : Total RNA-Based Funding, by Venture Capitalists, 2017-2021
- Figure 12 : Corporate-Backed RNA Financing, 2017-2021
- Figure 13 : Financial Benefits for Orphan Drugs
- Figure 14 : Nano-Delivery Types Used for Nucleic Acid Drug Delivery
- Figure 15 : Global Market Shares of Nucleic Acid Drug Delivery Sytems, by Type, 2022
- Figure 16 : Drug Delivery of Nucleic Acid Drugs, by Types
- Figure 17 : Viral Vector Types
- Figure 18 : Global Market for Conjugate Drug Delivery Systems of Nucleic Acids, 2020-2028
- Figure 19 : Gene Therapy Types
- Figure 20 : RNA-Based Drugs in Research and Development, 2022
- Figure 21 : Initial Issues with RNA-Based Drugs
- Figure 22 : RNA Drug Designing
- Figure 23 : Market Breakdown by Route of Administration Types and Subtypes
- Figure 24 : siRNA Delivery System
- Figure 25 : Antisense Oligonucleotide Mechanism of Action
- Figure 26 : Global Market Shares of Nulceic Acid Drug Delivery Systems, by Therapeutic Area, 2022
- Figure 27 : Global Market for Nucleic Acid Drug Delivery, by Region, 2020-2028
- Figure 28 : Share of Biotechnology Firms Reporting and Not Reporting ESG Data, 2022
- Figure 29 : ESG Disclosure in Various Company Specific Documents in Biotechnology Industry, 2022
- Figure 30 : Share of ESG Disclosure During the Forecasted Period in the Biotechnology Industry
- Figure 31 : Share of ESG Significnace in the Biotechnology Industry
- Figure 32 : Major Environmental Concerns in the Biotechnology Industry, 2023
- Figure 33 : Major Social Concerns in the Biotechnology Industry
- Figure 34 : Major Governance Concerns in the Biotechnology Industry
- Figure 35 : Share of Clinical Trials of RNA Therapeutics, by Clinical Trial Phase, 2020-2022
- Figure 36 : Share of Clinical Trials of RNA Therapeutics, by Age Group, 2022
- Figure 37 : Share of Clinical Trials of RNA Therapeutics, by Gender, 2022
- Figure 38 : Share of Clinical Trials of RNA Therapeutics, by Investors, 2022
- Figure 39 : Share of Clinical Trials of RNA Therapeutics, by Recruitment Status, 2022
- Figure 40 : Share of Clinical Trials of RNA Therapeutics Based on Study Results
- Figure 41 : Start-Up Funding in the Nucleic Acid Drug Delivery Industry, by Various Rounds, 2022 and 2023
- Figure 42 : Distribution Share of Start-Up Funding in the Nucleic Acid Drug Delivery Industry, by Various Rounds, 2022 and 2023
- Figure 43 : Global Market Shares of Nucleic Acid Drug Deivery, by Various Growth Strategies, 2022
- Figure 44 : Alnylam Pharmaceuticals: Annual Revenue, 2021 and 2022
- Figure 45 : Alnylam Pharmaceuticals: Revenue Share, by Products, 2022
- Figure 46 : Alnylam Pharmaceuticals: Revenue Share, by Region, 2022
- Figure 47 : Arcturus Therapeutics: Annual Revenue, 2021 and 2022
- Figure 48 : Arbutus Biopharma Corp.: Annual Revenue, 2021 and 2022
- Figure 49 : Arbutus Biopharma Corp.: Revenue Share, by Type, 2022
- Figure 50 : Benitec Biopharma: Annual Revenue, 2021 and 2022
- Figure 51 : Ionis Phamaceutical: Annual Revenue, 2021 and 2022
- Figure 52 : Novo Nordisk: Annual Revenue, 2021 and 2022
- Figure 53 : Novo Nordisk: Revenue Share, by Segments, 2022
- Figure 54 : Novo Nordisk: Revenue Share, by Region, 2022
Highlights:
The global nucleic acid drug delivery market should reach $2.7 billion by 2028 from $1.1 billion in 2023 at a compound annual growth rate (CAGR) of 20.5% for the forecast period of 2023 to 2028.
Encapsulated segment of the global nucleic acid drug delivery market is expected to grow from $629.4 million in 2023 to $1.7 billion in 2028 at a CAGR of 22.1% for the forecast period of 2023 to 2028.
Conjugated segment of the global nucleic acid drug delivery market is expected to grow from $432.6 million in 2023 to $984.6 million in 2028 at a CAGR of 17.9% for the forecast period of 2023 to 2028.
Report Scope:
This research report categorizes the market for nucleic acid drugs by type. The major product segments are conjugated and encapsulated. The non-viral vectors used for encapsulation are divided into polymers, lipids and others (e.g., inorganic material, hybrid systems). The market is also segmented based on the molecule types, delivery modes and therapeutic areas. It is divided by application types into therapeutic applications and research-based applications. The markets in North America, Europe, the Asia-Pacific region and Rest of the World (RoW) are covered.
Report Includes:
- 24 data tables and 45 additional tables
- An up-to-date overview and industry analysis of the global markets for RNA interference (RNAi) drug delivery technologies
- Analyses of the global market trends, with historic market revenue (sales figures) from 2020 to 2022, estimates for 2023, and projections of compound annual growth rates (CAGRs) through 2028
- Estimation of the actual market size and revenue forecast for the RNAi drug delivery technologies market, and corresponding market share analysis based on type of RNAi, therapeutic area, and region
- Discussion of major growth drivers, industry-specific challenges, regulatory aspects, and technology advancement that will shape the market for RNAi drug delivery technologies as a basis for projecting demand in the next few years (2023-2028)
- Review of key patent grants and patent applications on RNAi drug delivery markets, and emerging technologies and new developments within the marketplace
- Latest information on the mergers and acquisition deals, partnerships, agreements, collaborations, and other strategic alliances within the marketplace
- Insight into the recent industry structure, competitive aspects of each product segments, increasing investment on R&D activities, market growth strategies, and company revenue share analysis
- A look at commercial opportunities in the RNAi research tools and reagents, recent progress and future opportunities for RNAi therapeutics in various disease classifications, clinical trial applications, and potential markets for future developments
- Identification of the major stakeholders and analysis of the competitive landscape based on recent developments, segmental revenues, and operational integration
- Descriptive company profiles of the leading global players of the industry, including Alnylam Pharmaceuticals, Benitec Biopharma, Ionis Phamaceutical, Novo Nordisk and Sirnaomics Inc.
Table of Contents
Chapter 1 Introduction
- Study Goals and Objectives
- Reasons for Doing the Study
- Scope of Report
- What's New in This Update?
- Research Methodology
- Geographic Breakdown
Chapter 2 Summary and Highlights
- Market Outlook
Chapter 3 Market Overview
- Introduction
- History of Nucleic Acid Drugs
- Antisense Oligonucleotides
- RNA
- Drug Delivery System
- Nucleic Acid Drugs
- Inhibition Type
- Antisense Oligonucleotide
- Regulations
- Pre-Clinical INDA
- COVID-19 Impact Analysis on the RNAi Drug Delivery Markets
- Positive Impact of COVID-19
- Negative Effect of COVID-19
Chapter 4 Market Dynamics
- Market Drivers
- Approved RNAi Drugs
- Venture Funding Increased in RNAi Drugs
- RNAi Drugs Designated as Orphan Drugs
- New Nanoparticle Technologies for Drug Delivery Systems
- Market Restraints
- High Drug Costs
- Lack of Awareness Regarding Rare Diseases
- Clustered Regularly Interspaced Short Palindromic Repeats Technology Application Growing
- Market Opportunities
- Application in Cancer Treatment
- Infectious Disease and RNAi Drugs
Chapter 5 Nucleic Acid Drug Delivery Technology Market by Type
- Encapsulation
- Viral Vector-Based
- Non-Viral Vector-Based Delivery System
- Conjugates
Chapter 6 Market Breakdown by Application
- Drug Development and Discovery
- Process of RNA-Based Drug Discovery
- Design of RNAi Drugs
- Sequence Optimization
- Chemical Modification
- Targeted Delivery
- Therapeutic Application
Chapter 7 Market Breakdown by Route of Administration
Chapter 8 Market Breakdown by Molecules
- Small Interfering Ribonucleic Acid
- Antisense Oligonucleotide
- Fomivirsen (Vitravene)
- Mipomersen
- Nusinersen (Spinraza)
- Inotersen (Tegsedi)
- Eteplirsen (Exondys 51)
- Golodirsen (Vyondys 53)
- Milasen: A Unique Personalized Medicine
- Messenger RNA
- Others
- Aptamers
- Micro RNA
Chapter 9 Market Breakdown by Therapeutic Area
- Oncology
- Rare and Genetic Diseases
- Central Nervous System
- Respiratory
- Other Disease
Chapter 10 Market Breakdown by Region
- North America
- Europe
- Asia-Pacific
- Japan
- South Korea
- China
- India
- Australia
- Rest of the World
Chapter 11 Environmental, Social and Governance in the Biotechnology Sector
- Key Environmental, Social and Governance Issues in the Biotechnology Industry
- Biotechnology Industry Environmental, Social and Governance Performance Analysis
- Environmental Performance
- Social Performance
- Governance Performance
- Consumer Perspective on ESG in Biotechnology
- Case Study
- Concluding Remarks from BCC Research
Chapter 12 Emerging Technologies and Developments
- Drug Delivery to Lungs
- Nanotechnology
- Use of Artificial Intelligence and Machine Learning
Chapter 13 Clinical Trial and Patent Analysis
- Clinical Trials Analysis
- Patent Analysis
Chapter 14 Mergers and Acquisitions and Funding Outlook
- Start-Up Funding in Nucleic Acid Delivery Technology
Chapter 15 Competitive Intelligence
Chapter 16 Company Profiles
- ALNYLAM PHARMACEUTICALS INC.
- ARCTURUS THERAPEUTICS INC.
- ARBUTUS BIOPHARMA CORP.
- ARROWHEAD PHARMACEUTICALS INC.
- BENITEC BIOPHARMA
- CELLECTA INC.
- ELEVEN THERAPEUTICS
- GENEVANT SCIENCES CORP.
- IONIS PHARMACEUTICALS INC.
- MIRIMUS, INC.
- NOVO NORDISK
- NANODE THERAPEUTICS INC.
- OLIX PHARMACEUTICALS
- PHIO PHARMACEUTICALS CORP.
- SILENCE THERAPEUTICS PLC
- SIRNAOMICS INC.
- SOMAGENICS, INC.