|
|
市場調査レポート
商品コード
1071901
3D細胞培養市場:スキャフォールド形態別(スキャフォールドベース、スキャフォールドフリーシステム)、製品別(ハイドロゲル/細胞外マトリックス(ECM)、3Dバイオリアクター、3Dシャーレ、ハンギングドロッププレート、マイクロ流体システム)3D Cell Culture Market by Scaffold Format (Scaffold Based and Scaffold Free System), Products (Hydrogel / Extracellular Matrix (ECM), 3D Bioreactor, 3D Petri Dish, Hanging Drop Plate, Microfluidic System, |
||||||
|
● お客様のご希望に応じて、既存データの加工や未掲載情報(例:国別セグメント)の追加などの対応が可能です。 詳細はお問い合わせください。 |
|||||||
| 3D細胞培養市場:スキャフォールド形態別(スキャフォールドベース、スキャフォールドフリーシステム)、製品別(ハイドロゲル/細胞外マトリックス(ECM)、3Dバイオリアクター、3Dシャーレ、ハンギングドロッププレート、マイクロ流体システム) |
|
出版日: 2022年04月29日
発行: Roots Analysis
ページ情報: 英文 481 Pages
納期: 即日から翌営業日
|
- 全表示
- 概要
- 図表
- 目次
ハイライト例
目次
第1章 序文
第2章 エグゼクティブサマリー
第3章 イントロダクション
- 章の概要
- 細胞培養の種類
- 培養中の細胞の形態
- 2D細胞培養と3D細胞培養
- 3D細胞培養の概要
- 細胞培養の確立と維持
- 健康な細胞培養を維持するための要件
- 3D細胞培養システムの応用
- モデルシステム
- 創薬と前臨床研究
- 癌研究
- ウイルス学調研究
- 遺伝子工学と遺伝子治療の研究
- 3D細胞培養システムの利点と制限
- 今後の展望
第4章 3D細胞培養システムの分類
- 3D細胞培養分類
- スキャフォールドベースの3D細胞培養
- スキャフォールドフリーの3D細胞培養
- オルガノイド
第5章 3Dマトリックスとスキャフォールドの作成
- 章の概要
- 多孔質スキャフォールドの製造方法
- 繊維状スキャフォールドの製造方法
- ヒドロゲルの製造方法
- カスタムスキャフォールドの製造方法
- マイクロスフェアの製造方法
- ネイティブスキャフォールドの製造方法
第6章 3D細胞培養システム:開発者の情勢
- 章の概要
- 3D細胞培養システム開発者:全体的な市場情勢
- 3D細胞培養:サービスプロバイダーのリスト
- 3D細胞培養:提携アッセイ、キット、試薬のリスト
第7章 市場情勢:スキャフォールドベースの製品
- 章の概要
- スキャフォールドベースの製品:全体的な市場情勢
- スキャフォールドベースの製品:開発者の情勢
第8章 市場情勢:スキャフォールドフリー製品
- 章の概要
- スキャフォールドフリー製品:全体的な市場情勢
- スキャフォールドフリー製品:開発者の情勢
第9章 市場情勢:3Dバイオ市場情勢
- 章の概要
- 3Dバイオリアクター:全体的な市場情勢
- 3Dバイオリアクター:開発者の情勢
第10章 主要な応用分野
- 章の概要
- 癌研究における3D細胞培養システム
- 創薬および毒性スクリーニングにおける3D細胞培養システム
- 幹細胞調査における3D細胞培養システム
- 再生医療および組織工学における3D細胞培養
- 3D細胞培養システム:主要な応用分野別分析
第11章 企業プロファイル概要:スキャフォールドベースの製品(ヒドロゲル/ECM開発者)
- 章の概要
- 3D Biotek
- Advanced BioMatrix
- Alphabioregen
- Corning Life Sciences
- RREPROCELL
第12章 企業プロファイル概要:スキャフォールドフリー製品(オーガンオンチップ開発者)
- 章の概要
- CN Bio Innovations
- Emulate
- InSphero
- MIMETAS
- TissUse
第13章 企業プロファイル概要:3Dバイオリアクター
- 章の概要
- BISS TGT
- Celartia
- Cell Culture
- EBERS
- Flexcell International
- PBS Biotech
- Synthecon
第14章 資金調達と投資の分析
第15章 パートナーシップとコラボレーション
第16章 特許分析
- 章の概要
- 範囲と調査手法
- 3D細胞培養システム:特許分析
- 3D細胞培養システム:特許評価分析
- 主要な特許:引用数別分析
第17章 出版物の分析
第18章 製品競争力分析
第19章 ケーススタディ
第20章 市場予測
- 章の概要
- 重要な仮定と予測調査手法
- 世界の3D細胞培養市場、2022年~2035年
- 世界の3D細胞培養市場:ビジネスセグメント別分布
- 世界の3D細胞培養システム市場:3D細胞培養形態別分布
- 世界の3D細胞培養システム市場:製品タイプ別分布
- 世界の3D細胞培養システム市場:適用分野別分布
- 世界の3D細胞培養システム市場:目的別分布
- 世界の3D細胞培養システム市場:地域別分布
第21章 調査分析
第22章 結論
第23章 エグゼクティブインサイト
第24章 付録I:集計データ
第25章 付録II:企業と組織のリスト
List Of Tables
- Table 3.1 Morphology of Cells in a Culture
- Table 3.2 Differences between 2D and 3D Cell Cultures
- Table 3.3 Features of 3D Spheroids generated via 3D Cell Culture Systems
- Table 4.1 Advantages and Disadvantages of Scaffold Based and Scaffold Free Systems
- Table 4.2 Advantages and Disadvantages of Natural and Synthetic Scaffolds
- Table 4.3 Advantages and Disadvantages of Natural and Synthetic Hydrogels
- Table 4.4 Cell Cultures Used in Magnetic Levitation
- Table 4.5 Origin and Culture Techniques Used for Organoids
- Table 5.1 Advantages and Disadvantages of Methods Used for Fabrication for Porous Scaffolds
- Table 5.2 3D Cell Culture Studies Using Porous Scaffolds
- Table 5.3 Methods for Fabrication Used of Fibrous Scaffolds
- Table 5.4 Advantages and Disadvantages of Methods Used for Fabrication of Fibrous Scaffolds
- Table 5.5 3D Cell Culture Studies Using Fibrous Scaffolds
- Table 5.6 Advantages and Disadvantages of Methods Used for Fabrication of Hydrogels
- Table 5.7 3D Cell Culture Studies Using Hydrogels
- Table 5.8 Advantages and Disadvantages of Methods Used for Fabrication of Custom Scaffolds
- Table 5.9 3D Cell Culture Studies Using Custom Scaffolds
- Table 5.10 Advantages and Disadvantages of Methods Used for Fabrication of Microspheres
- Table 5.11 3D Cell Culture Studies Using Microspheres
- Table 5.12 3D Cell Culture Studies Using Native Scaffolds
- Table 6.1 3D Cell Culture Systems: List of Developers
- Table 6.2 3D Cell Culture Systems: List of Service Providers
- Table 6.3 3D Cell Culture Systems: List of Assays, Kits and Reagents
- Table 7.1 Scaffold Based Products: List of Products
- Table 7.2 Scaffold Based Products: List of Developers
- Table 8.1 Scaffold Free Products: List of Products
- Table 8.2 Scaffold Free Products: List of Developers
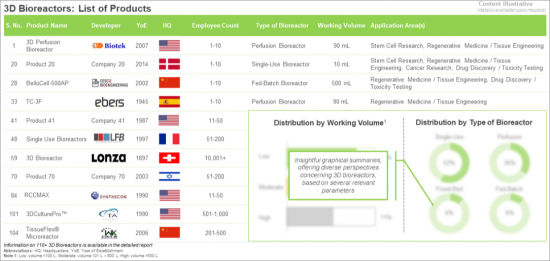
- Table 9.1 3D Bioreactors: List of Products
- Table 9.2 3D Bioreactors: List of Developers
- Table 10.1 Scaffold Based Products: Information on Key Application Areas
- Table 10.2 Scaffold Free Products: Information on Key Application Areas
- Table 10.3 3D Bioreactors: Information on Key Application Areas
- Table 11.1 Scaffold Based Products (Hydrogel / ECM Developers): List of Companies Profiled
- Table 11.2 3D Biotek: Company Snapshot
- Table 11.3 3D Biotek: Key Characteristics of Hydrogels / ECMs
- Table 11.4 3D Biotek: Recent Developmnets and Future Outlook
- Table 11.5 Advanced BioMatrix: Company Snapshot
- Table 11.6 Advanced BioMatrix: Key Characteristics of Hydrogels / ECMs
- Table 11.7 Advanced BioMatrix: Recent Developments and Future Outlook
- Table 11.8 Alphabioregen: Company Snapshot
- Table 11.9 Alphabioregen: Key Characteristics of Hydrogels / ECMs
- Table 11.10 Alphabioregen: Recent Developments and Future Outlook
- Table 11.11 Corning Life Sciences: Company Snapshot
- Table 11.12 Corning Life Sciences: Key Characteristics of Hydrogels / ECMs
- Table 11.13 Corning Life Sciences: Recent Developments and Future Outlook
- Table 11.14 REPROCELL: Company Snapshot
- Table 11.15 REPROCELL: Key Characteristics of Hydrogels / ECMs
- Table 11.16 REPROCELL: Recent Developments and Future Outlook
- Table 12.1 Scaffold Free Products (Organ-on-Chips): List of Companies Profiled
- Table 12.2 CN Bio Innovations: Company Snapshot
- Table 12.3 CN Bio Innovations: Information on Financial Instances
- Table 12.4 CN Bio Innovations: Key Characteristics of Organ-on-Chips Products
- Table 12.5 CN Bio Innovations: Recent Developments and Future Outlook
- Table 12.6 Emulate: Company Snapshot
- Table 12.7 Emulate: Information on Funding Instances
- Table 12.8 Emulate: Key Characteristics of Organ-on-Chips Products
- Table 12.9 Emulate: Recent Developments and Future Outlook
- Table 12.10 InSphero: Company Snapshot
- Table 12.11 InSphero: Information on Funding Instances
- Table 12.12 InSphero: Key Characteristics of Organ-on-Chips Products
- Table 12.13 InSphero: Recent Developments and Future Outlook
- Table 12.14 MIMETAS: Company Snapshot
- Table 12.15 MIMETAS: Information on Funding Instances
- Table 12.16 MIMETAS: Key Characteristics of Organ-on-Chips Products
- Table 12.17 MIMETAS: Recent Developments and Future Outlook
- Table 12.18 TissUse: Company Snapshot
- Table 12.19 TissUse: Key Characteristics of Organ-on-Chips Products
- Table 12.20 TissUse: Recent Developments and Future Outlook
- Table 13.1 3D Bioreactors: List of Companies Profiled
- Table 13.2 BISS TGT: Company Snapshot
- Table 13.3 BISS TGT: Key Characteristics of 3D Bioreactors
- Table 13.4 BISS TGT: Recent Developments and Future Outlook
- Table 13.5 Celartia: Company Snapshot
- Table 13.6 Celartia: Key Characteristics of 3D Bioreactors
- Table 13.7 Celartia: Recent Developments and Future Outlook
- Table 13.8 Cell Culture: Company Snapshot
- Table 13.9 Cell Culture: Key Characteristics of 3D Bioreactors
- Table 13.10 EBERS: Company Snapshot
- Table 13.11 EBERS: Key Characteristics of 3D Bioreactors
- Table 13.12 EBERS: Recent Developments and Future Outlook
- Table 13.13 Flexcell International: Company Snapshot
- Table 13.14 Flexcell International: Key Characteristics of 3D Bioreactors
- Table 13.15 Flexcell International: Recent Developments and Future Outlook
- Table 13.16 PBS Biotech: Company Snapshot
- Table 13.17 PBS Biotech: Key Characteristics of 3D Bioreactors
- Table 13.18 PBS Biotech: Recent Developments and Future Outlook
- Table 13.19 Synthecon: Company Snapshot
- Table 13.20 Synthecon: Key Characteristics of 3D Bioreactors
- Table 13.21 Synthecon: Recent Developments and Future Outlook
- Table 14.1 Funding and Investments: Information on Year of Investment, Type of Funding, Amount Raised and Investor, 2016 - 2021
- Table 14.2 Funding and Investments: Information on Year of Establishment, Location of Headquarters of Recipients, Focus Area, and Type of Product, 2016 -2021
- Table 15.1 Partnerships and Collaborations: Information on Year of Partnership, Type of Partnership, and Partner, 2016 - Q1 2022
- Table 15.2 Partnerships and Collaborations: Information on Type of Agreement, Focus Area, and Type of Product, 2016 - Q1 2022
- Table 16.1 Patent Analysis: CPC Symbols
- Table 16.2 Patent Analysis: Most Popular CPC Symbols
- Table 16.3 Patent Analysis: List of Top 10 CPC Symbols
- Table 16.4 Patent Analysis: List of Relatively High Value Patents
- Table 18.1 Survey Insights: Overview of Respondents
- Table 18.2 Survey Insights: Designation and Seniority Level
- Table 18.3 Survey Insights: Focus Area of the Company
- Table 18.4 Survey Insights: Type of 3D Cell Culture Products Offered
- Table 18.5 Survey Insights: Status of Development of Product(s)
- Table 18.6 Survey Insights: Method of Fabrication Used
- Table 18.7 Survey Insights: Source of 3D Cultured Cells
- Table 18.8 Survey Insights: Key Areas of Application
- Table 18.9 Survey Insights: 3D Cell Culture Services Offered
- Table 18.10 Survey Insights: Current Market Opportunity (2022)
- Table 18.11 Survey Insights: Future Market Opportunity (2035)
- Table 20.1 Cellendes: Company Snapshot
- Table 20.2 Synthecon: Company Snapshot
- Table 20.3 BRTI Life Sciences: Company Snapshot
- Table 20.4 Kirkstall: Company Snapshot
- Table 20.5 QGel: Company Snapshot
- Table 20.6 Xylyx Bio: Company Snapshot
- Table 20.7 InSphero: Company Snapshot
- Table 20.8 GSI: Company Snapshot
- Table 20.9 Nanofiber Solutions: Company Snapshot
- Table 20.10 FlexCell International: Company Snapshot
- Table 20.11 MBL International: Company Snapshot
- Table 21.1 3D Cell Culture System Developers: Distribution by Year of Establishment
- Table 21.2 3D Cell Culture System Developers: Distribution by Company Size
- Table 21.3 3D Cell Culture System Developers: Distribution by Location of Headquarters
- Table 21.4 3D Cell Culture System Developers: Distribution by 3D Cell Culture Format
- Table 21.5 3D Cell Culture System Developers: Distribution by Type of Product
- Table 21.6 3D Cell Culture System Developers: Distribution by Number of Products
- Table 21.7 Heat Map Representation: Distribution by 3D Cell Culture Format and Location of Headquarters
- Table 21.8 Tree Map Representation: Distribution by Company Size and Type of Product
- Table 21.9 World Map Representation: Distribution by Location of Regional Headquarters
- Table 21.10 Scaffold Based Products: Distribution by Status of Development
- Table 21.11 Scaffold Based Products: Distribution by Type of Product
- Table 21.12 Scaffold Based Products: Distribution by Source of 3D Cultured Cells
- Table 21.13 Scaffold Based Products: Distribution by Method Used for Fabrication
- Table 21.14 Scaffold Based Products: Distribution by Material Used for Fabrication
- Table 21.15 Scaffold Based Products: Distribution by Type of Product and Source of 3D Cultured Cells
- Table 21.16 Scaffold Based Products: Distribution by Type of Product and Method Used for Fabrication
- Table 21.17 Scaffold Based Product Developers: Distribution by Year of Establishment
- Table 21.18 Scaffold Based Product Developers: Distribution by Company Size
- Table 21.19 Scaffold Based Product Developers: Distribution by Location of Headquarters
- Table 21.20 Leading Developers: Distribution by Number of Scaffold Based Products
- Table 21.21 Tree Map Representation: Distribution by Type of Product and Company Size
- Table 21.22 Scaffold Free Products: Distribution by Status of Development
- Table 21.23 Scaffold Free Products: Distribution by Type of Product
- Table 21.24 Scaffold Free Products: Distribution by Source of 3D Cultured Cells
- Table 21.25 Scaffold Free Products: Distribution by Method Used for Fabrication
- Table 21.26 Scaffold Free Products: Distribution by Material Used for Fabrication
- Table 21.27 Scaffold Free Products: Distribution by Type of Product and Source of 3D Cultured Cells
- Table 21.28 Scaffold Free Products: Distribution by Type of Product and Method Used for Fabrication
- Table 21.29 Scaffold Free Product Developers: Distribution by Year of Establishment
- Table 21.30 Scaffold Free Product Developers: Distribution by Company Size
- Table 21.31 Scaffold Free Product Developers: Distribution by Location of Headquarters
- Table 21.32 Leading Developers: Distribution by Number of Scaffold Free Products
- Table 21.33 Tree Map Representation: Distribution by Type of Product and Company Size
- Table 21.34 3D Bioreactors: Distribution by Type of 3D Bioreactor
- Table 21.35 3D Bioreactors: Distribution by Working Volume
- Table 21.36 3D Bioreactor Developers: Distribution by Year of Establishment
- Table 21.37 3D Bioreactor Developers: Distribution by Company Size
- Table 21.38 3D Bioreactor Developers: Distribution by Location of Headquarters
- Table 21.39 Leading Developers: Distribution by Number of 3D Bioreactors
- Table 21.40 3D Cell Culture Systems: Distribution by Key Application Areas
- Table 21.41 3D Cell Culture Systems: Distribution by Key Application Areas and 3D Cell Culture Format
- Table 21.42 Scaffold Based Products: Distribution by Key Application Areas
- Table 21.43 Scaffold Free Products: Distribution by Key Application Areas
- Table 21.44 3D Bioreactors: Distribution by Key Application Areas
- Table 21.45 Funding and Investments: Distribution of Recipient Companies by Year of Establishment and Type of Funding, 2015 - 2021
- Table 21.46 Funding and Investments: Cumulative Number of Instances by Year, 2015 - 2021
- Table 21.47 Funding and Investments: Cumulative Amount Invested, 2015 - 2021 (USD Million)
- Table 21.48 Funding and Investments: Distribution of Instances by Type of Funding, 2015 -2021
- Table 21.49 Funding and Investments: Year-Wise Distribution by Number of Instances and Type of Funding, 2015 - 2021
- Table 21.50 Funding and Investments: Distribution of Amount Invested by Type of Funding, 2015 - 2021 (USD Million)
- Table 21.51 Funding and Investments: Year-Wise Distribution of Amount Invested and Type of Funding, 2015 -2021
- Table 21.52 Funding and Investments: Distribution of Instances and Amount Invested by 3D Cell Culture Format, 2015 - 2021
- Table 21.53 Funding and Investments: Distribution of Instances and Amount Invested by Type of Product, 2015 - 2021
- Table 21.54 Funding and Investments: Distribution by Geography
- Table 21.55 Funding and Investments: Regional Distribution by Total Amount Invested, 2015 - 2021
- Table 21.56 Most Active Players: Distribution by Number of Funding Instances, 2015 - 2021
- Table 21.57 Most Active Players: Distribution by Amount Raised, 2015 - 2021 (USD Million)
- Table 21.58 Most Active Investors: Distribution by Funding Instances, 2015 - 2021
- Table 21.59 Partnerships and Collaborations: Cumulative Year-Wise Trend, 2015 - 2021
- Table 21.60 Partnerships and Collaborations: Distribution by Type of Partnership
- Table 21.61 Partnerships and Collaborations: Distribution by Year of Partnership and Type of Partnership
- Table 21.62 Partnerships and Collaborations: Distribution by Company Size and Type of Partnership
- Table 21.63 Partnerships and Collaborations: Distribution by Type of Partner
- Table 21.64 Partnerships and Collaborations: Distribution by Year of Partnership and Type of Partner
- Table 21.65 Partnerships and Collaborations: Distribution by Type of Partnership and Type of Partner
- Table 21.66 Partnerships and Collaborations: Distribution by 3D Cell Culture Format
- Table 21.67 Partnerships and Collaborations: Distribution by Year of Partnership and 3D Cell Culture Format
- Table 21.68 Partnerships and Collaborations: Distribution by Type of Partnership and 3D Cell Culture Format
- Table 21.69 Partnerships and Collaborations: Distribution by Type of Product
- Table 21.70 Partnerships and Collaborations: Distribution by Year of Partnership and Type of Product
- Table 21.71 Partnerships and Collaborations: Distribution by Type of Partnership and Type of Product
- Table 21.72 Most Active Players: Distribution by Number of Partnerships
- Table 21.73 Partnerships and Collaborations: Regional Distribution
- Table 21.74 Partnerships and Collaborations: Intercontinental and Intracontinental Agreements
- Table 21.75 Patent Analysis: Distribution by Type of Patent
- Table 21.76 Patent Analysis: Cumulative Distribution by Publication Year, 2016 - Q1 2022
- Table 21.77 Patent Analysis: Distribution of Granted Patents by Publication Year, 2016-Q1 2022
- Table 21.78 Patent Analysis: Distribution of Filed Patents Publication Year, 2016-Q1 2022
- Table 21.79 Patent Analysis: Distribution by Number of Patent Type and Publication Year, 2016-Q1 2022
- Table 21.80 Patent Analysis: Distribution by Issuing Authorities Involved
- Table 21.81 Patent Analysis: Cumulative Year-wise Distribution by Type of Applicant, 2016-Q1 2022
- Table 21.82 Leading Industry Players: Distribution by Number of Patents
- Table 21.83 Leading Non-Industry Players: Distribution by Number of Patents
- Table 21.84 Patent Analysis: Distribution by Patent Age, 2002-2022
- Table 21.85 Patent Analysis: Valuation Analysis
- Table 21.86 Publication Analysis: Distribution by Publication Year, 2019-Q1 2022
- Table 21.87 Top Authors: Analysis by Number of Publications
- Table 21.88 Patent Analysis: Key Journals based on Number of Publications
- Table 21.89 Patent Analysis: Key Publisher based on Number of Publications
- Table 21.90 Leading Funding Institute: Distribution by Number of Publications
- Table 21.91 Global 3D Cell Culture Market, Conservative, Base and Optimistic Scenarios, 2022-2035 (USD Million)
- Table 21.92 Global 3D Cell Culture Market: Distribution by Business Segment, 2022 and 2035
- Table 21.93 3D Cell Culture Systems Market, Conservative, Base and Optimistic Scenarios, 2022-2035 (USD Million)
- Table 21.94 3D Cell Culture Consumables Market, Conservative, Base and Optimistic Scenarios, 2022-2035 (USD Million)
- Table 21.95 3D Cell Culture Services Market, Conservative, Base and Optimistic Scenarios, 2022-2035 (USD Million)
- Table 21.96 Global 3D Cell Culture Systems Market: Distribution by 3D Cell Culture Format, 2022 and 2035
- Table 21.97 3D Cell Culture Systems Market for Scaffold based Products, Conservative, Base and Optimistic Scenarios, 2022-2035 (USD Million)
- Table 21.98 3D Cell Culture Systems Market for Scaffold Free Products, Conservative, Base and Optimistic Scenarios, 2022-2035 (USD Million)
- Table 21.99 3D Cell Culture Systems Market for Market 3D Bioreactors, Conservative, Base and Optimistic Scenarios, 2022-2035 (USD Million)
- Table 21.100 Global 3D Cell Culture Systems Market: Distribution by Type of Product, 2022 and 2035
- Table 21.101 3D Cell Culture Systems Market for Attachment Resistant Surfaces, Conservative, Base and Optimistic Scenarios, 2022-2035 (USD Million)
- Table 21.102 3D Cell Culture Systems Market for Hydrogels / ECMs, Conservative, Base and Optimistic Scenarios, 2022-2035 (USD Million)
- Table 21.103 3D Cell Culture Systems Market for Micropatterned Surface, Conservative, Base and Optimistic Scenarios, 2022-2035 (USD Million)
- Table 21.104 3D Cell Culture Systems Market for Microcarriers, Conservative, Base and Optimistic Scenarios, 2022-2035 (USD Million)
- Table 21.105 3D Cell Culture Systems Market for Microfluidic Systems, Conservative, Base and Optimistic Scenarios, 2022-2035 (USD Million)
- Table 21.106 3D Cell Culture Systems Market for Solid Scaffolds, Conservative, Base and Optimistic Scenarios, 2022-2035 (USD Million)
- Table 21.107 3D Cell Culture Systems Market for Suspension Culture Systems, Conservative, Base and Optimistic Scenarios, 2022-2035 (USD Million)
- Table 21.108 Global 3D Cell Culture Systems Market: Distribution by Area of Application, 2022 and 2035
- Table 21.109 3D Cell Culture Systems Market for Cancer Research, Conservative, Base and Optimistic Scenarios, 2022-2035 (USD Million)
- Table 21.110 3D Cell Culture Systems Market for Drug Discovery and Toxicity Testing, Conservative, Base and Optimistic Scenarios, 2022-2035 (USD Million)
- Table 21.111 3D Cell Culture Systems Market for Stem Cell Research, Conservative, Base and Optimistic Scenarios, 2022-2035 (USD Million)
- Table 21.112 3D Cell Culture Systems Market for Regenerative Medicine and Tissue Engineering, Conservative, Base and Optimistic Scenarios, 2022-2035 (USD Million)
- Table 21.113 Global 3D Cell Culture Systems Market: Distribution by Purpose, 2022 and 2035
- Table 21.114 3D Cell Culture Systems Market for Research Use, Conservative, Base and Optimistic Scenarios, 2022-2035 (USD Million)
- Table 21.115 3D Cell Culture Systems Market for Therapeutic Use, Conservative, Base and Optimistic Scenarios, 2022-2035 (USD Million)
- Table 21.116 Global 3D Cell Culture Systems Market: Distribution by Geography, 2022 and 2035
- Table 21.117 3D Cell Culture Systems Market in North America, Conservative, Base and Optimistic Scenarios, 2022-2035 (USD Million)
- Table 21.118 3D Cell Culture Systems Market in Europe, Conservative, Base and Optimistic Scenarios, 2022-2035 (USD Million)
- Table 21.119 3D Cell Culture Systems Market in Asia-Pacific, Conservative, Base and Optimistic Scenarios, 2022-2035 (USD Million)
- Table 21.120 3D Cell Culture Systems Market in Latin America, Conservative, Base and Optimistic Scenarios, 2025-2035 (USD Million)
- Table 21.121 3D Cell Culture Systems Market in Middle East and North Africa (MENA), Conservative, Base and Optimistic Scenarios, 2022-2035 (USD Million)
- Table 21.122 3D Cell Culture Systems Market in Rest of the World, Conservative, Base and Optimistic Scenarios, 2022-2035 (USD Million)
- Table 21.123 Global 3D Cell Culture Systems Market: Distribution by Leading Players, 2022
- Table 21.124 Global 3D Cell Culture Systems Market: Conservative, Base and Optimistic Scenarios, 2022, 2028 and 2035 (USD Million)
- Table 21.125 Survey Insights: Distribution of Respondents by Year of Establishment of Company
- Table 21.126 Survey Insights: Distribution of Respondents by Company Size
- Table 21.127 Survey Insights: Distribution of Respondents by Location of Company Headquarters (Region-Wise)
- Table 21.128 Survey Insights: Distribution of Respondents by Location of Company Headquarters (Country-Wise)
- Table 21.129 Survey Insights: Distribution of Respondents by Designation and Seniority Level
- Table 21.130 Survey Insights: Distribution by Focus Area
- Table 21.131 Survey Insights: Distribution by Type of 3D Cell Culture Products Offered
- Table 21.132 Survey Insights: Distribution by Development Status of Product(s)
- Table 21.133 Survey Insights: Distribution by Method of Fabrication Used
- Table 21.134 Survey Insights: Distribution by Source of Cultured Cells
- Table 21.135 Survey Insights: Distribution by Key Application Areas
- Table 21.136 Survey Insights: Distribution by 3D Cell Culture Services Offered
- Table 21.137 Survey Insights: Distribution by Current and Future Market Opportunity, 2022 and 2035
List Of Companies
The following companies and organizations have been mentioned in the report
- 1. 101Bio
- 2. 3D Biomatrix
- 3. 3D Biotechnology Solutions
- 4. 3D Biotek
- 5. 3Dnamics
- 6. 4Dcell
- 7. 4titude
- 8. AbbVie Ventures
- 9. abc biopply
- 10. Abcam
- 11. ABL Europe
- 12. Abo Akademi University
- 13. Abstraction Ventures
- 14. Abzena
- 15. Accellta
- 16. Accurate International Biotechnology
- 17. Advanced BioMatrix
- 18. Advanced Regenerative Manufacturing Institute (ARMI)/BiofabUSA
- 19. Advanced Scientifics
- 20. Aetos Biologics
- 21. Afirmus Biosource
- 22. AGC
- 23. Agency for Science, Technology and Research (A*STAR)
- 24. AIM Biotech
- 25. Akero Therapeutics
- 26. Akron Biotech
- 27. Alector
- 28. Allevi
- 29. Alnylam Pharmaceuticals
- 30. Alphabioregen
- 31. ALS Investment Fund
- 32. AlveoliX
- 33. American Laboratory Products
- 34. AMS Biotechnology
- 35. AnaPath Services
- 36. Angel Investors
- 37. AngelMD
- 38. Angels 5K
- 39. Angels in MedCity
- 40. Angels Santé
- 41. Anthrogenesis
- 42. Aquitaine Science Transfert
- 43. Aquiti Gestion
- 44. AR Brown
- 45. Arizona State University
- 46. ARL Design
- 47. ARTeSYN Biosolutions
- 48. AS ONE INTERNATIONAL
- 49. AstraZeneca
- 50. Arizona State University
- 51. ATEL Ventures
- 52. Atera
- 53. Avantor
- 54. Axol Bioscience
- 55. AxoSim
- 56. AXT
- 57. Axxicon
- 58. BASF
- 59. Bayer
- 60. B-CULTURE
- 61. BEOnChip
- 62. Bi/ond
- 63. Bio-Byblos Biomedical
- 64. BioCat
- 65. BioConcept
- 66. BIOFABICS
- 67. Biogelx
- 68. Bioinspired Solutions
- 69. BioInvent International
- 70. BIOKÉ
- 71. BioLamina
- 72. Biomaterials USA
- 73. Biomerix
- 74. BiomimX
- 75. Biopredic International
- 76. Bio-Techne
- 77. BioTek Instruments
- 78. BISS TGT
- 79. Barcelona Liver Bioservices (BLB)
- 80. Bonus BioGroup
- 81. Bpifrance
- 82. BRAIN
- 83. BrainXell
- 84. Brammer Bio
- 85. Braveheart Investment Group
- 86. Bristol-Myers Squibb
- 87. Broad Institute
- 88. BRTI Life Sciences
- 89. Cambridge Bioscience
- 90. CarThera
- 91. Cedars-Sinai Medical Center
- 92. Celartia
- 93. Cell Applications
- 94. Cell Culture Company (C3)
- 95. CellFiber
- 96. Cell Guidance Systems
- 97. CELLEC Biotek
- 98. Cellendes
- 99. Cellevate
- 100. CELLnTEC
- 101. CELLphenomics
- 102. CellSpring
- 103. CellSystems
- 104. Celprogen
- 105. CelVivo
- 106. Center for Drug Evaluation and Research (CDER)
- 107. Center for the Advancement of Science in Space (CASIS)
- 108. CESCO Bioengineering
- 109. Charles River Laboratories
- 110. Cherry Biotech
- 111. China Regenerative Medicine International (CRMI)
- 112. Cincinnati Children's Hospital Medical Center
- 113. CITIC Securities
- 114. CN Bio Innovations
- 115. CN Innovations
- 116. Collagen Solutions
- 117. Commission for Technology and Innovation
- 118. Commonwealth Serum Laboratories
- 119. Comune di Milano
- 120. Corning Life Sciences
- 121. Cosmo Bio
- 122. Creative Bioarray
- 123. CSL
- 124. Curi Bio
- 125. Cyprio
- 126. Cyprotex
- 127. Cytiva
- 128. Danaher
- 129. Deepbridge Capital
- 130. Demcon
- 131. Development Bank of Wales
- 132. DiPole Materials
- 133. Downing Ventures
- 134. Dynamic42
- 135. EBERS
- 136. Ectica Technologies
- 137. EDITHGEN
- 138. EDmicBio
- 139. Electrospinning
- 140. Emulate
- 141. Enso Discoveries
- 142. eNUVIO
- 143. Eppendorf
- 144. Esco Aster
- 145. Esperante
- 146. Ethicon
- 147. Etica Technologies
- 148. European Commission
- 149. European Life Sciences Growth Fund (ELSGF)
- 150. European Research Council (ERC)
- 151. European Union (EU)
- 152. Eurostars
- 153. EU-ToxRisk
- 154. Eva Scientific
- 155. Evotec
- 156. Executive Agency for Small and Medium-sized Enterprises (EASME)
- 157. faCellitate
- 158. Food and Drug Administration
- 159. Fennik Life Sciences
- 160. Ferentis
- 161. FHNW University
- 162. FiberCell Systems
- 163. Fibralign
- 164. Finep
- 165. Finesse Solutions
- 166. Finovam Gestion
- 167. Flexcell International
- 168. Fundação para a Ciência e a Tecnologia (FCT)
- 169. Foundation for Technological Innovation
- 170. Founder
- 171. Founders Fund
- 172. Freeline
- 173. French Government
- 174. Frequency Therapeutics
- 175. FroggaBio
- 176. Fujifilm
- 177. FUJIFILM Wako Pure Chemical
- 178. Funakoshi
- 179. Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP)
- 180. Gabriel Investments
- 181. Galapagos
- 182. Galia Gestion
- 183. Gamma 3
- 184. Gelmetix
- 185. Gelomics
- 186. Gemini Bio
- 187. Gemstone Biotherapeutics
- 188. Genome Institute of Singapore
- 189. Georgia Research Alliance
- 190. German Research Foundation
- 191. GlassWall Syndicate
- 192. GlaxoSmithKline
- 193. Global Cell Solutions
- 194. Government of China
- 195. Government of the Netherlands
- 196. Great Stuff Ventures
- 197. GSI
- 198. HµREL
- 199. Hamilton
- 200. Harvard Apparatus
- 201. Harvard College
- 202. HCS Pharma
- 203. Helvoet
- 204. Heraeus Medical
- 205. Hesperos
- 206. Histogenics
- 207. Hokkaido Soda
- 208. HP Wild Holding
- 209. Hubrecht Organoid Technology
- 210. Human Models for Analysis of Pathways (HMAPs) Center
- 211. Humanetics
- 212. Hyamedix
- 213. ibidi
- 214. IMSS-Gulf Bio Analytical
- 215. INITIO CELL
- 216. Innovate UK
- 217. Innovation Fund Denmark
- 218. InoCure
- 219. Inova Health System
- 220. inRegen
- 221. InSphero
- 222. Institute for Molecular Medicine Finland
- 223. Invest Northern Ireland
- 224. Invitrocue
- 225. InvivoSciences
- 226. Ionis Pharmaceuticals
- 227. Irdi Soridec Gestion
- 228. Janssen Biotech
- 229. Japan Vilene Company
- 230. Jellagen Marine Biotechnologies
- 231. Johns Hopkins University
- 232. JRI Orthopaedics
- 233. JVCKENWOOD
- 234. Kero
- 235. Kim & Friends
- 236. Kirkstall
- 237. KIYATEC
- 238. KOKEN
- 239. Koninklijke Nederlandse Akademie Van Wetenschappen
- 240. Kuraray
- 241. LabCorp
- 242. Laboratory for Integrated Micro Mechatronic Systems
- 243. Laconia
- 244. LAMBDA Laboratory Instruments
- 245. Lantern Pharma
- 246. Lawrence J. Ellison Institute for Transformative Medicine
- 247. LBA Healthcare Management
- 248. Lena Biosciences
- 249. LFB Biomanufacturing
- 250. Life Technologies
- 251. Lifecore Biomedical
- 252. LifeNet Health
- 253. Laboratory for Integrated Micro-Mechatronic Systems (LIMMS)
- 254. Lineage Cell Therapeutics
- 255. Locate Bio
- 256. London School of Hygiene & Tropical Medicine
- 257. Lonza
- 258. Lund University
- 259. LuoLabs
- 260. Manchester BIOGEL
- 261. Mario Negri Institute for Pharmacological Research
- 262. Maryland Momentum Fund
- 263. Maryland Stem Cell Research Fund (MSCRF)
- 264. Massachusetts Institute of Technology
- 265. MassChallenge
- 266. MatTek Life Sciences
- 267. MBL International
- 268. Menicon Life Science
- 269. Merck Accelerator
- 270. Merck KGaA
- 271. Michael J. Fox Foundation
- 272. Michigan Technological University
- 273. MicorFIT
- 274. MicroDigital
- 275. Micronit
- 276. MicroTissues
- 277. Midven
- 278. MIMETAS
- 279. Minerva Business Angel Network
- 280. Ministry of Higher Education, Research and Innovation (France)
- 281. Mirage Biomedicals
- 282. Molecular Devices
- 283. MTTlab
- 284. Nano Dimension
- 285. Nanobiose
- 286. Nanofiber Solutions
- 287. Nanogaia
- 288. NanoSurface Biomedical
- 289. National Aeronautics and Space Administration (NASA)
- 290. National Cancer Institute (NCI)
- 291. National Center for Advancing Translational Sciences (NCATS)
- 292. National Centre for the Replacement, Refinement and Reduction of Animals in Research (NC3Rs)
- 293. National Institutes of Health (NIH)
- 294. National Institute on Aging (NIA)
- 295. National Institutes for Food and Drug Control (NIFDC)
- 296. National Natural Science Foundation of China
- 297. National Science Foundation (NSF)
- 298. National University Hospital
- 299. National University of Singapore
- 300. NETRI
- 301. Neuromics
- 302. New Orleans BioFund
- 303. Newable Private Investing
- 304. Nexcelom Bioscience
- 305. Nichirei Biosciences
- 306. Nord France Amorquage
- 307. Northwick Park Institute for Medical Research
- 308. Nortis
- 309. Nova Biomedical
- 310. Novartis Venture Fund
- 311. Noviocell
- 312. Nucleus Biologics
- 313. NYU Winthrop Hospital
- 314. Olaregen Therapeutix
- 315. Omni Life Science
- 316. Oregon Health & Science University
- 317. Organovo
- 318. Orthomimetics
- 319. OS Fund
- 320. Oxford MEStar
- 321. Pairnomix
- 322. Pall Corporation
- 323. Particle3D
- 324. Path BioAnalytics
- 325. PBS Biotech
- 326. Peak Capital Advisors
- 327. Pelo Biotech
- 328. Pensees
- 329. PepGel
- 330. Percell Biolytica
- 331. PerkinElmer
- 332. Pfizer
- 333. Phase Holographic Imaging (PHI)
- 334. Pitch@Palace
- 335. PL BioScience
- 336. Plasticell
- 337. Pluristem Therapeutics
- 338. Portugal Ventures
- 339. Precision Biologics
- 340. Premedical Laboratories
- 341. Primorigen Biosciences
- 342. Principia SGR
- 343. ProBio
- 344. ProBioGen
- 345. Prodizen
- 346. PromoCell
- 347. Protista International
- 348. PT Rajawali Medika Mandiri
- 349. QGel Bio
- 350. QIAGEN
- 351. Quintech Life Sciences
- 352. RASA
- 353. React4life
- 354. Real Research
- 355. RealBio Technology
- 356. Regemat3D
- 357. Repligen
- 358. ReproCell
- 359. Research Without Animal Experiment
- 360. Revivocell
- 361. Rigenerand
- 362. Roche
- 363. RoosterBio
- 364. Roswell Park Comprehensive Cancer Center
- 365. Royal Netherlands Academy of Arts and Sciences
- 366. Saguaro Technologies
- 367. SAICO Biosystems
- 368. Sanofi Ventures
- 369. SARSTEDT
- 370. Sartorius
- 371. S-BIO
- 372. Science and Technology Facilities Council (STFC)
- 373. ScienCell
- 374. ScienCell Research Laboratories
- 375. SciFi VC
- 376. SciKon Innovation
- 377. Scinus Cell Expansion
- 378. Scottish Investment Bank
- 379. ScreenIn3D
- 380. Seres Therapeutics
- 381. Shanghai Cienle Medical Technology
- 382. Shanghai Institute of Biochemistry and Cell Biology (SIBCB)
- 383. Shanghai Institute of Materia Medica
- 384. Siemens Technology
- 385. Sigma-Aldrich
- 386. SKE Research Equipment
- 387. SmiLe Incubator
- 388. SoloHill Engineering
- 389. Sphere Fluidics
- 390. Spheritech
- 391. Spiber Technologies
- 392. Stanford University
- 393. Start-Up Chile
- 394. State Key Laboratory of Experimental Hematology
- 395. StemCell Systems
- 396. STEMCELL Technologies
- 397. Stemmatters
- 398. StemoniX
- 399. StemTek Therapeutics
- 400. SUN bioscience
- 401. Swiss Federal Laboratories for Materials Science and Technology
- 402. SyndicateRoom
- 403. Synthecon
- 404. SynVivo
- 405. TA Instruments
- 406. Takeda
- 407. Tampere University
- 408. Tantti Laboratory
- 409. tebu-bio
- 410. Technical University of Berlin
- 411. TEDCO
- 412. Terumo
- 413. Texas Tech University Health Sciences Center (TTUHSC)
- 414. The Idea Village
- 415. Mario Negri Institute for Pharmacological Research
- 416. Thermo Fisher Scientific
- 417. TheWell Bioscience
- 418. Tianjin Weikai Biological Engineering
- 419. Tissue Click
- 420. TissueLabs
- 421. TissUse
- 422. Tokyo Future Style
- 423. TPG
- 424. TreeFrog Therapeutics
- 425. Trevigen
- 426. Triumvirate Environmental
- 427. Twinhelix
- 428. U.S. Small Business Administration (SBA)
- 429. UK Innovation & Science Seed Fund
- 430. UK Science and Technology Facilities Council
- 431. United States Department of Defense
- 432. University College London
- 433. University Hospital Zurich (USZ)
- 434. University of Alberta
- 435. University of Arkansas for Medical Sciences
- 436. University of Bath
- 437. University of Brescia
- 438. University of Bristol
- 439. University of California
- 440. University of Cambridge
- 441. University of Central Florida
- 442. University of Genoa
- 443. University of Manchester
- 444. University of Mannheim
- 445. University of Milan
- 446. University of Nottingham
- 447. University of Sheffield
- 448. University of Strathclyde
- 449. University of Washington School of Pharmacy
- 450. University of Zurich
- 451. UPM Biomedicals
- 452. UW Medicine
- 453. VA Portland Health Care System
- 454. Vanderbilt University
- 455. Venture Kick
- 456. Venturecraft
- 457. VentureSouth
- 458. Viscofan BioEngineering
- 459. Visikol
- 460. Vivo Biosciences
- 461. VWR
- 462. Wake Forest Institute for Regenerative Medicine
- 463. Women Who Tech
- 464. XAnge
- 465. Xenos
- 466. XP Biomed
- 467. Xylyx Bio
- 468. Zhejiang University
- 469. zPREDICTA
Title:
3D Cell Culture
Market by Scaffold Format (Scaffold Based and Scaffold Free System), Products (Hydrogel / Extracellular Matrix (ECM), 3D Bioreactor, 3D Petri Dish, Hanging Drop Plate, Microfluidic System, Micropatterned Surface, Microcarrier, Solid Scaffold, and Suspension System), Application Areas (Cancer Research, Drug Discovery and Toxicology, Stem Cell Research, Tissue Engineering and Regenerative Medicine), Purpose (Research Use and Therapeutic Use), and Key Geographical Regions (North America, Europe, Asia-Pacific and Rest of the World): Industry Trends and Global Forecasts (4th Edition), 2022-2035.
Example Highlights:

Overview:
Animal testing has been the most common method in various experimental studies in biomedical research, given their resemblance to humans in terms of genetics, anatomy, and physiology. Specifically, mice genome has 80% similarity with humans, which makes them excellent models for various research purposes. However, the use of animals in scientific research is associated with several ethical concerns, which led to the establishment of the principle of 3Rs- Replacement, Reduction and Refinement, to address the ethical concerns related to animal welfare and limit the use of animals in scientific research. As of 2018, this initiative led to 50% reduction in the use of animals as compared to the statistics noted in 1985. Further, the process of animal breeding / housing for scientific purposes is also associated with high costs and requires skilled labor. Moreover, it has been demonstrated that animal cell cultures are unable to accurately mimic the natural (in vivo) microenvironment as the cells cultured in monolayers are both morphologically and physiochemically different from their in vivo counterparts. These concerns have necessitated a transition from animal-based testing to the use of 3-dimensional (3D) cell culture models. Over time, advances in biotechnology and materials science have enabled the development of a variety of 3D cell culture systems in order to drive research across different application areas, including cancer research, drug discovery, tissue engineering and others.
At present, more than 140 companies offer 3D cell culture systems in a variety of formats, including scaffold-based products, scaffold-free products and 3D bioreactors. These systems have demonstrated to be capable of more accurately simulating the natural tissue microenvironment, offer increased cell-to-cell and cell-to-ECM interactions, more accurate evaluation of drug toxicity and cellular responses, and co-cultuirng of multiple cell types together. Moreover, there are certain complex 3D cell culture models that can even replace animal models exhibiting reproducible results and thereby, serving as better in vivo models across multiple application areas. Given the various benefits of such systems, the field has garnered the attention of various venture capital firms and strategic investors that have been providing financial support to drive research efforts focused on exploring different formats of 3D cell culture systems, including organoids and organ-on-chips across multiple application areas. Moreover, there has been an increase in scientific literature on 3D cell culture systems and collaborations for 3D bioreactors and cell culture products. Given the ongoing innovation in this field, and the paradigm shift from 2D cell culture systems and animal testing to 3D cell culture models, the market is likely to witness a significant growth in the foreseen future.
Scope of the Report:
The "3D Cell Culture Market by Scaffold Format (Scaffold Based and Scaffold Free System), Products (Hydrogel / Extracellular Matrix (ECM), 3D Bioreactor, 3D Petri Dish, Hanging Drop Plate, Microfluidic System, Micropatterned Surface, Microcarrier, Solid Scaffold, and Suspension System), Application Areas (Cancer Research, Drug Discovery and Toxicology, Stem Cell Research, Tissue Engineering and Regenerative Medicine), Purpose (Research Use and Therapeutic Use), and Key Geographical Regions (North America, Europe, Asia-Pacific, Latin America, MENA and Rest of the World): Industry Trends and Global Forecasts (4th Edition), 2022-2035" report features an extensive study of the current landscape, offering an informed opinion on the likely evolution of the market in the mid to long term. The study also features an in-depth analysis, highlighting the capabilities of various industry stakeholders engaged in this domain. Amongst other elements, the report includes:
- A detailed discussion on the classification of 3D cell culture systems, categorized as scaffold based systems (hydrogels / ECMs, solid scaffolds, micropatterned surfaces and microcarriers), scaffold free systems (attachment resistant surfaces, suspension systems and microfluidic systems) and 3D bioreactors.
- An elaborate discussion on the methods used for fabrication of 3D matrices and scaffolds, highlighting the materials used, the process of fabrication, merits and demerits, and the applications of different fabrication methods.
- An overview of the current market landscape of companies offering various 3D cell culture systems, including information on a number of relevant parameters, such as year of establishment, size of employee base, geographical presence, 3D cell culture format (scaffold based products, scaffold free products and 3D bioreactors), and type of product (hydrogels / ECMs, micropatterned surfaces, solid scaffolds, microcarriers, attachment resistant surfaces, suspension systems and microfluidic systems). In addition, the chapter provides information related to the companies providing 3D culture related services, and associated reagents / consumables.
- A detailed assessment of the overall landscape of scaffold based products, along with analyses based on a number of relevant parameters, such as status of development (under development, developed not commercialized, and commercialized), type of product (hydrogels / ECMs, micropatterned surfaces, solid scaffolds, and microcarriers), source of scaffold (human based, chemical based, animal based, plant based, and polymer based), and fabrication material used. In addition, it presents details of the companies involved in the development of scaffold based products, providing information on their year of establishment, company size, and location of headquarters.
- A detailed assessment of the overall landscape of scaffold free products, along with analyses based on a number of relevant parameters, such as status of development (under development, developed and not commercialized, and commercialized), type of product (attachment resistant surfaces, suspension systems and microfluidic systems), type of material (human based, animal based, plant based and polymer based), and material used for fabrication. In addition, it presents details of the companies involved in the development of scaffold free products, providing information on their year of establishment, company size, and location of headquarters.
- A detailed assessment of the overall landscape of 3D bioreactors, along with analyses based on a number of relevant parameters, such as type of 3D bioreactor (single-use, perfusion, fed-batch, and fixed-bed), status of development (under development, developed and not commercialized, and commercialized), typical working volume, scale of operation (lab scale, pre-clinical / clinical scale and commercial scale), type of manufacturing process (batch-continuous, fed-batch and continuous), type of cell culture system (mammalian cell, insect cell, microbial cell, and plant cell), type of molecule processed (vaccine, monoclonal antibody, recombinant protein, stem cell, cell therapy and gene therapy), and application area (drug discovery / toxicity testing, stem cell research, regenerative medicine / tissue engineering and cancer research). In addition, it presents details of the companies involved in the development of 3D bioreactors, providing information on their year of establishment, company size, and location of headquarters.
- A detailed review of the key application areas (cancer research, drug discovery and toxicology, stem cell research, tissue engineering and regenerative medicine) for which various 3D cell culture products are being developed / used.
- Elaborate profiles of prominent players offering Scaffold-based, Scaffold-free cell culture systems and 3D bioreactors (shortlisted based on the number of products being offered) that are engaged in the development of 3D cell culture products. Each company profile includes a brief overview of the company, financial / funding information (if available), details on its product portfolio, recent developments, and an informed future outlook.
- An analysis of the investments made in the period between 2016-2022, including instances of seed financing, venture capital financing, debt financing, grants / awards, capital raised from IPOs and subsequent offerings, at various stages of development in small and mid-sized companies (established after 2005; with less than 200 employees) that are engaged in the development of 3D cell culture products.
- An analysis of the various partnerships related to 3D cell culture products, which have been established since 2015, based on several parameters, such as year of agreement, type of partnership (product development and commercialization agreements, product integration and utilization agreements, product licensing agreement, research and development agreements, distribution agreements, acquisitions, joint venture and other agreements), 3D cell culture format (scaffold based products, scaffold free products and 3D bioreactor), type of product (hydrogels / ECMs, micropatterned surfaces, solid scaffolds, microcarriers, attachment resistant surfaces, suspension systems and microfluidic systems), and most active players. It also provides the regional distribution of players involved in the collaborations.
- An in-depth analysis of over 6,400 patents that have been filed / granted for 3D cell culture products, between 2016-2021, based on parameters, such as type of patent, publication year, issuing authority involved, CPC symbols, type of applicant, emerging focus areas, leading patent assignees (in terms of number of patents filed / granted), patent characteristics and geography. It also includes a detailed patent valuation analysis.
- An analysis of more than 3,800 peer-reviewed scientific articles related to 3D cell culture and its technologies, published since 2019, based on several parameters, such as year of publication, emerging focus areas, most popular authors, and most popular journals (in terms of number of articles published in the given time period and journal impact factor), top publisher and type of funding institute.
- An in-depth competitiveness analysis of 3D bioreactors, taking into consideration the supplier power (based on the year of establishment of the 3D bioreactors developer) and key features of bioreactors, such as scale of operation (lab scale, pre-clinical / clinical scale and commercial scale), type of molecule supported (vaccine, monoclonal antibody, recombinant protein, stem cell, cell therapy and gene therapy), type of cell culture supported (mammalian cell, insect cell, microbial cell, and plant cell) and application area (drug discovery / toxicity testing, stem cell research, regenerative medicine/tissue engineering and cancer research).
- A case study on the 3D cell culture products for organoids and organ-on-chips, along with analysis based on parameters, such as status of development, and area of applications. In addition, it presents details of the developer companies, along with information on their year of establishment, company size, and location of headquarters.
- Insights from an industry-wide survey, featuring inputs solicited from various experts who are directly / indirectly involved in the development of 3D cell culture products, emphasized on the focus area of their company, type of 3D cell culture products offered, development status of the product(s), method of fabrication used, source of 3D cultured cells, application area of product(s), type of service(s) offered, and present and future market opportunity.
One of the key objectives of the report was to identify the primary growth drivers and estimate the potential future size of the 3D cell culture market. Based on various parameters, such as business segment, price of 3D cell culture products, and likely adoption of the 3D cell culture products, we have developed informed estimates on the likely evolution of the 3D cell culture systems market, for the period 2022-2035. Our year-wise projections of the current and forecasted opportunity have further been segmented across 3D cell culture format (scaffold based systems, scaffold free systems, and 3D bioreactors), type of product (hydrogels / ECMs, micropatterned surfaces, solid scaffolds, microcarriers, attachment resistant surfaces, suspension systems, and microfluidic systems), area of application (cancer research, drug discovery / toxicity testing, stem cell research, and regenerative medicine / tissue engineering), purpose (research use and therapeutic use), key geographical regions (North America, Europe, Asia-Pacific, Latin America, MENA and rest of the world), and leading product developers. In order to account for future uncertainties and to add robustness to our model, we have provided three forecast scenarios, namely conservative, base and optimistic scenarios, representing different tracks of the industry's growth.
The opinions and insights presented in the report were influenced by discussions held with senior stakeholders in the industry. The report features detailed transcripts of interviews held with the following industry and non-industry players:
- Brigitte Angres (Co-founder, Cellendes)
- Bill Anderson (President and CEO, Synthecon)
- Anonymous (President and CEO, Anonymous)
- Anonymous (Co-founder and Vice President, Anonymous)
- Scott Brush (Vice President, BRTI Life Sciences)
- Malcolm Wilkinson (Managing Director, Kirkstall)
- Ryder Clifford (Director, QGel) and Simone Carlo Rizzi (Chief Scientific Officer, QGel)
- Tanya Yankelevich (Director, Xylyx Bio)
- Jens Kelm (Chief Scientific Officer, InSphero)
- Walter Tinganelli (Group Leader, GSI)
- Darlene Thieken (Project Manager, Nanofiber Solutions)
- Andrea Picon (Director, Business Development, FlexCell International)
- Frank Junker (Chief Business Officer, InSphero)
- Mohammed Mamunur Rahman (Manager, Business Development, MBL International)
Key Questions Answered:
- Who are the leading industry players engaged in the development of 3D cell culture products?
- Which are the most popular 3D cell culture products?
- Which are the different application areas for which 3D cell culture products are being developed?
- What are the key factors that are likely to influence the evolution of 3D cell culture systems market?
- What is the trend of capital investments in the 3D cell culture systems market?
- Which partnership models are commonly adopted by stakeholders in 3D cell culture market?
- How is the current and future opportunity likely to be distributed across key market segments?
- What are the anticipated future trends related to 3D cell culture systems market?
Chapter Outlines:
Chapter 2 is an executive summary of the insights captured in our research. It offers a high-level view on the current state and the likely evolution of the 3D cell culture systems market in the mid to long term.
Chapter 3 provides a general introduction to 3D cell culture systems. The chapter presents information on the different types of cell cultures, methods of cell culturing and their application areas. The chapter also features a comparative analysis of 2D and 3D cultures, as well as highlights the current need and advantages of 3D culture systems.
Chapter 4 provides an overview of the classification of 3D culture systems, namely scaffold based systems (hydrogels / ECMs, solid scaffolds, micropatterned surfaces and microcarriers), scaffold free systems (attachment resistant surfaces, suspension systems and microfluidic systems) and 3D bioreactors. It also provides insights on the underlying concepts, advantages and disadvantages of the aforementioned products.
Chapter 5 presents summary of different techniques that are commonly used for fabrication of 3D matrices and scaffolds. In addition, the chapter provides information on the working principle, benefits and limitations associated with each method used for fabricating scaffolds. In addition, the chapter features key takeaways from various research studies focused on matrices fabricated using the aforementioned methods.
Chapter 6 includes information on around 140 industry players offering various 3D cell culture products. It features detailed analyses of developers, based on year of establishment, company size, location of headquarters, 3D cell culture format (scaffold based products, scaffold free products and 3D bioreactors), and type of product (hydrogels / ECMs, micropatterned surfaces, solid scaffolds, microcarriers, attachment resistant surfaces, suspension systems and microfluidic systems). In addition, the chapter provides different insightful representations, which include [A] a heat map representation, illustrating the distribution of developers, based on 3D cell culture format and location of headquarters, [B] tree map representation, presenting the distribution of developers, based on type of product and company size, and [C] world map representation, highlighting the regional distribution of headquarters of the developer companies.
Chapter 7 presents information on around 200 scaffold based products that are either commercialized or under development. It features detailed analysis of these products based on a number of relevant parameters, such as status of development (under development, developed and not commercialized, and commercialized) type of product (hydrogels / ECMs, micropatterned surfaces, solid scaffolds, and microcarriers), source of scaffold (human based, chemical based, animal based, plant based, and polymer based), and fabrication material. In addition, it presents details of the companies involved in the development of scaffold based products, providing information on their year of establishment, company size, and location of headquarters.
Chapter 8 presents information on around 40 scaffold free products that are either commercialized or under development. It features detailed analysis of these products based on a number of relevant parameters, such as status of development (under development, developed and not commercialized, and commercialized), type of product (attachment resistant surfaces, suspension systems and microfluidic systems), type of material (human based, animal based, plant based and polymer based), and material used of fabrication. In addition, it presents details of the companies involved in the development of scaffold free products, providing information on their year of establishment, company size, and location of headquarters.
Chapter 9 presents information on around 90 3D bioreactors that are either commercialized or under development. It features detailed analyses of these products based on a number of relevant parameters, such as type of 3D bioreactor (single-use, perfusion, fed-batch, and fixed-bed), status of development (under development, developed and not commercialized, and commercialized), typical working volume, scale of operation (lab scale, pre-clinical / clinical scale and commercial scale), type of manufacturing process (batch-continuous, fed-batch and continuous), type of cell culture system (mammalian cell, insect cell, microbial cell, and plant cell), type of molecule processed (vaccine, monoclonal antibody, recombinant protein, stem cell, cell therapy and gene therapy), and application area (drug discovery / toxicity testing, stem cell research, regenerative medicine / tissue engineering and cancer research). In addition, it presents details of the companies involved in the development of 3D bioreactors, providing information on their year of establishment, company size, and location of headquarters.
Chapter 10 presents information on the key application areas (cancer research, drug discovery and toxicity screening, stem cell research, tissue engineering and regenerative medicine) for which various 3D cell culture products are being developed / used.
Chapter 11 features elaborate profiles of prominent players engaged in the development of scaffold based products (offering at least five hydrogel / ECM products). Each company profile includes a brief overview of the company, details on its product portfolio, recent developments and an informed future outlook.
Chapter 12 features elaborate profiles of prominent players engaged in the development of scaffold free products (offering at least three scaffold free cell culture products). Each company profile includes a brief overview of the company, details on its product portfolio, recent developments and an informed future outlook.
Chapter 13 features elaborate profiles of prominent players that engaged in the development of 3D bioreactors (offering at least two bioreactors). Each company profile includes a brief overview of the company, details on its product portfolio, recent developments and an informed future outlook.
Chapter 14 features an analysis of the investments made in the period between 2016-2022, including instances of seed financing, venture capital financing, debt financing, grants / awards, capital raised from IPOs and subsequent offerings, at various stages of development in small and mid-sized companies (established after 2005; with less than 200 employees) that are engaged in the development of 3D cell culture products.
Chapter 15 features an analysis of the various partnerships related to 3D cell culture products, that have been established since 2015, based on several parameters, such as year of agreement, type of partnership (product development and commercialization agreements, product integration and utilization agreements, product licensing agreement, research and development agreements, distribution agreements, acquisitions, joint venture and other agreements), 3D cell culture format (scaffold based products, scaffold free products and 3D bioreactor), type of product (hydrogels / ECMs, micropatterned surfaces, solid scaffolds, microcarriers, attachment resistant surfaces, suspension systems and microfluidic systems), and most active players. It also provides the regional distribution of players involved in the collaborations.
Chapter 16 provides an in-depth analysis of over 6,400 patents that have been filed / granted for 3D cell culture products, between 2016-2021, based on parameters, such as type of patent, publication year, issuing authority involved, CPC symbols, type of applicant, emerging focus areas, leading patent assignees (in terms of number of patents filed / granted), patent characteristics and geography. It also includes a detailed patent valuation analysis.
Chapter 17 features an analysis of more than 3,800 peer-reviewed scientific articles related to 3D cell culture and its technologies, published since 2019, based on several parameters, based on several parameters, such as year of publication, emerging focus areas, most popular authors, and most popular journals (in terms of number of articles published in the given time period and journal impact factor), top publisher and type of funding institute.
Chapter 18 features an insightful competitiveness analysis of 3D bioreactors, taking into consideration the supplier power (based on the year of establishment of the 3D bioreactors developer) and key features of bioreactors, such as scale of operation (lab scale, pre-clinical/clinical scale and commercial scale), type of molecule supported (vaccine, monoclonal antibody, recombinant protein, stem cell, cell therapy and gene therapy), type of cell culture supported (mammalian cell, insect cell, microbial cell, and plant cell) and application area (drug discovery / toxicity testing, stem cell research, regenerative medicine / tissue engineering and cancer research).
Chapter 19 is a case study providing an overview on the current market landscape of 3D cell culture products for organoids and organ-on-chips, along with analysis based on parameters, such as development status, and area of application. In addition, it presents details of the developer companies, along with information on their year of establishment, company size, and location of headquarters.
Chapter 20 presents an insightful market forecast analysis, highlighting the likely growth of 3D cell culture systems market, till 2035. In order to provide an informed future outlook, our projections have been segmented on the basis of [A] 3D cell culture format (scaffold based systems, scaffold free systems, and 3D bioreactors), [B] type of product (hydrogels / ECMs, micropatterned surfaces, solid scaffolds, microcarriers, attachment resistant surfaces, suspension systems, and microfluidic systems), [C] area of application (cancer research, drug discovery / toxicity testing, stem cell research, and regenerative medicine / tissue engineering), [D] purpose (research use and therapeutic use), [E] key geographical regions (North America, Europe, Asia-Pacific, Latin America, MENA and rest of the world), and [F] leading product developers.
Chapter 21 presents insights from the survey conducted for this study. We contacted over 150 stakeholders involved in the development of 3D cell culture systems. The participants, who were primarily Founder / CXO / Senior Management level representatives of their respective companies, helped us develop a deeper understanding on the nature of their products / services and the associated commercial potential.
Chapter 22 is a summary of the overall report. It presents a list of key takeaways and our independent opinions on the current market scenario.
Chapter 23 is a collection of interview transcripts of the discussions held with various stakeholders in the industry. We have presented details of interviews held with Brigitte Angres (Co-founder, Cellendes), Bill Anderson (President and CEO, Synthecon), anonymous (President and CEO, Anonymous), anonymous (Co-founder and Vice President, Anonymous), Scott Brush (Vice President, BRTI Life Sciences), Malcolm Wilkinson (Managing Director, Kirkstall), Ryder Clifford (Director, QGel) and Simone Carlo Rizzi (Chief Scientific Officer, QGel), Tanya Yankelevich (Director, Xylyx Bio), Jens Kelm (Chief Scientific Officer, InSphero), Walter Tinganelli (Group Leader, GSI), and Darlene Thieken (Project Manager, Nanofiber Solutions), Andrea Picon (Director, Business Development, FlexCell International), Frank Junker (Chief Business Officer, InSphero) and Mamun Rahman (Manager, Business Development, MBL International)
Chapter 24 is an appendix, that contains tabulated data and numbers for all the figures provided in the report.
Chapter 25 is an appendix, that provide the list of companies and organizations mentioned in the report.
TABLE OF CONTENTS
1. PREFACE
- 1.1. Scope of the Report
- 1.2. Research Methodology
- 1.3. Key Questions Answered
- 1.4. Chapter Outlines
2. EXECUTIVE SUMMARY
3. INTRODUCTION
- 3.1. Chapter Overview
- 3.2. Types of Cell Cultures
- 3.2.1. Primary Cell Cultures
- 3.2.2. Cell Lines
- 3.3. Morphology of Cells in Culture
- 3.4. 2D Cell Cultures vs 3D Cell Cultures
- 3.5. Overview of 3D Cell Culturing
- 3.5.1. Components of the Extracellular Matrix (ECM)
- 3.5.2. In Vitro Cell Culturing
- 3.5.3. Selection of Culture Format
- 3.6. Establishment and Maintenance of Cell Cultures
- 3.6.1. Isolating Cells from Tissues
- 3.6.2. Maintaining Cells in Culture
- 3.6.3. Sub-Culturing / Passaging
- 3.6.4. Cryogenic Storage
- 3.7. Requirements for Maintaining Healthy Cell Cultures
- 3.7.1. Safety Guidelines in a Cell Culture Facility
- 3.7.2. Cell Culture Health and Optimal Conditions for Growth
- 3.7.3. Cross Contamination in Cell Cultures
- 3.7.4. Methods to Prevent Contamination
- 3.8. Applications of 3D Cell Culture Systems
- 3.8.1. Model Systems
- 3.8.2. Drug Discovery and Preclinical Research
- 3.8.3. Cancer Research
- 3.8.4. Virology Research
- 3.8.5. Genetic Engineering and Gene Therapy Research
- 3.9. Advantages and Limitations of 3D Cell Culture Systems
- 3.10. Future Perspectives
4. CLASSIFICATION OF 3D CELL CULTURE SYSTEMS
- 4.1. 3D Cell Culture Classification
- 4.2. Scaffold Based 3D Cell Cultures
- 4.2.1. Hydrogels / ECM Analogs
- 4.2.2. Solid Scaffolds
- 4.2.3. Micropatterned Surfaces
- 4.2.4. Microcarriers
- 4.3. Scaffold Free 3D Cell Cultures
- 4.3.1. Attachment Resistant Surfaces
- 4.3.2. Suspension Culture Systems
- 4.3.2.1. Hanging Drop Plates
- 4.3.2.2. Magnetic Levitation and 3D Bioprinting
- 4.3.3. Microfluidic Surfaces and Organs-on-Chips
- 4.3.4. 3D Bioreactors
- 4.4. Organoids
5. FABRICATION OF 3D MATRICES AND SCAFFOLDS
- 5.1. Chapter Overview
- 5.2. Methods for Fabricating Porous Scaffolds
- 5.2.1. Particulate Leaching
- 5.2.2. Solvent Casting
- 5.2.3. Emulsion Templating
- 5.2.4. Gas Foaming
- 5.2.5. Melt Molding
- 5.2.6. Microsphere Sintering
- 5.3. Methods for Fabricating Fibrous Scaffolds
- 5.3.1. Electrospinning
- 5.3.2. Phase Separation
- 5.3.3. Self-Assembly
- 5.3.4. Fiber Mesh and Fiber Bonding
- 5.4. Methods for Fabricating Hydrogels
- 5.4.1. Gelation
- 5.4.2. Solvent Casting and Particulate Leaching
- 5.4.3. Gas Foaming
- 5.4.4. Freeze Drying
- 5.4.5. Co-polymerization / Crosslinking
- 5.4.6. Microfluidics
- 5.5. Methods for Fabricating Custom Scaffolds
- 5.5.1. Stereo-Lithography
- 5.5.2. 3D Bioprinting and Selective Laser Sintering (SLS)
- 5.5.3. Fused Deposition Modeling
- 5.5.4. Membrane Lamination
- 5.5.5. Rapid Prototyping / Solid Free-Form Technique
- 5.6. Methods for Fabricating Microspheres
- 5.6.1. Solvent Evaporation
- 5.6.2. Single and Double Emulsification
- 5.6.3. Particle Aggregation
- 5.7. Methods for Fabricating Native Scaffolds
- 5.7.1. Decellularization
6. 3D CELL CULTURE SYSTEMS: DEVELOPER LANDSCAPE
- 6.1. Chapter Overview
- 6.2. 3D Cell Culture System Developers: Overall Market Landscape
- 6.2.1. Analysis by Year of Establishment
- 6.2.2. Analysis by Company Size
- 6.2.3. Analysis by Location of Headquarters
- 6.2.4. Analysis by 3D Cell Culture Format
- 6.2.5. Analysis by Type of Product
- 6.2.6. Analysis by 3D Cell Culture Format and Location of Headquarters
- 6.2.7. Analysis by Company Size and Type of Product
- 6.2.8. Analysis by Location of Headquarters
- 6.3. 3D Cell Cultures: List of Service Providers
- 6.4. 3D Cell Cultures: List of Affiliated Assays, Kits and Reagents
7. MARKET LANDSCAPE: SCAFFOLD BASED PRODUCTS
- 7.1. Chapter Overview
- 7.2. Scaffold Based Products: Overall Market Landscape
- 7.2.1. Analysis by Status of Development
- 7.2.2. Analysis by Type of Product
- 7.2.3. Analysis by Source of Scaffold
- 7.2.4. Analysis by Material Used for Fabrication
- 7.2.5. Analysis by Type of Product and Source of Scaffold
- 7.2.6. Analysis by Type of Product and Material Used for Fabrication
- 7.3. Scaffold Based Products: Developer Landscape
- 7.3.1. Analysis by Year of Establishment
- 7.3.2. Analysis by Company Size
- 7.3.3. Analysis by Company Size and Type of Product
- 7.3.4. Analysis by Location of Headquarters
- 7.3.5. Leading Developers: Analysis by Number of Scaffold Based Products
8. MARKET LANDSCAPE: SCAFFOLD FREE PRODUCTS
- 8.1. Chapter Overview
- 8.2. Scaffold Free Products: Overall Market Landscape
- 8.2.1. Analysis by Status of Development
- 8.2.2. Analysis by Type of Product
- 8.2.3. Analysis by Material Used for Fabrication
- 8.2.4. Analysis by Material Used for Scaffold
- 8.2.5. Analysis by Type of Product and Material Used for Fabrication
- 8.3. Scaffold Free Products: Developer Landscape
- 8.3.1. Analysis by Year of Establishment
- 8.3.2. Analysis by Company Size
- 8.3.3. Analysis by Company Size and Type of Product
- 8.3.4. Analysis by Location of Headquarters
- 8.3.5. Leading Developers: Analysis by Number of Scaffold Free Products
9. MARKET LANDSCAPE: 3D BIOREACTORS
- 9.1. Chapter Overview
- 9.2. 3D Bioreactors: Overall Market Landscape
- 9.2.1. Analysis by Type of 3D Bioreactor
- 9.2.2. Analysis by Status of Development
- 9.2.3. Analysis by Working Volume
- 9.2.4. Analysis by Scale of Operation
- 9.2.5. Analysis by Manufacturing Process
- 9.2.6. Analysis by Type of Cell Culture System
- 9.2.7. Analysis by Type of Molecule Processed
- 9.2.8. Analysis by Area of Application
- 9.3. 3D Bioreactors: Developer Landscape
- 9.3.1. Analysis by Year of Establishment
- 9.3.2. Analysis by Company Size
- 9.3.3. Analysis by Location of Headquarters
- 9.3.4. Leading Developers: Analysis by Number of 3D Bioreactors
10. KEY APPLICATION AREAS
- 10.1. Chapter Overview
- 10.2. 3D Cell Culture Systems in Cancer Research
- 10.2.1. Need for 3D Culture Systems in Cancer Research
- 10.2.1.1. Cancer Drug Screening with 3D Culture Systems
- 10.2.1. Need for 3D Culture Systems in Cancer Research
- 10.3. 3D Cell Culture Systems in Drug Discovery and Toxicity Screening
- 10.3.1. Drug Development Studies
- 10.3.2. Toxicity Screening
- 10.3.2.1. 3D Liver Models
- 10.3.2.2. Other 3D Models
- 10.4. 3D Cell Culture Systems in Stem Cell Research
- 10.4.1. 3D Culture Systems in Stem Cell Differentiation
- 10.4.2. In Vitro 3D Microenvironment to Induce Embryoid Body Formation
- 10.5. 3D Cell Cultures in Regenerative Medicine and Tissue Engineering
- 10.6. 3D Cell Culture Systems: Analysis by Key Application Areas
- 10.6.1. 3D Cell Culture Systems: Analysis by Key Application Areas and 3D Cell Culture Format
- 10.6.1.1. Scaffold Based 3D Products: Analysis by Key Application Areas
- 10.6.1.2. Scaffold Free 3D Products: Analysis by Key Application Areas
- 10.6.1.3. 3D Bioreactors: Analysis by Key Application Areas
- 10.6.1. 3D Cell Culture Systems: Analysis by Key Application Areas and 3D Cell Culture Format
11. COMPANY PROFILES: SCAFFOLD BASED PRODUCTS (HYDROGEL / ECM DEVELOPERS)
- 11.1. Chapter Overview
- 11.1.1. 3D Biotek
- 11.1.1.1. Company Overview
- 11.1.1.2. Product Portfolio
- 11.1.1.3. Recent Developments and Future Outlook
- 11.1.2. Advanced BioMatrix
- 11.1.2.1. Company Overview
- 11.1.2.2. Product Portfolio
- 11.1.2.3. Recent Development and Future Outlook
- 11.1.3. Alphabioregen
- 11.1.3.1. Company Overview
- 11.1.3.2. Product Portfolio
- 11.1.3.3. Recent Developments and Future Outlook
- 11.1.4. Corning Life Sciences
- 11.1.4.1. Company Overview
- 11.1.4.2. Product Portfolio
- 11.1.4.3. Recent Developments and Future Outlook
- 11.1.5. REPROCELL
- 11.1.5.1. Company Overview
- 11.1.5.2. Product Portfolio
- 11.1.5.3. Recent Developments and Future Outlook
- 11.1.1. 3D Biotek
12. COMPANY PROFILES: SCAFFOLD FREE PRODUCTS (ORGAN-ON-CHIPS DEVELOPERS)
- 12.1. Chapter Overview
- 12.1.1. CN Bio Innovations
- 12.1.1.1. Company Overview
- 12.1.1.2. Financial Information
- 12.1.1.3. Product Portfolio
- 12.1.1.4. Recent Developments and Future Outlook
- 12.1.2. Emulate
- 12.1.2.1. Company Overview
- 12.1.2.2. Financial Information
- 12.1.2.3. Product Portfolio
- 12.1.2.4. Recent Developments and Future Outlook
- 12.1.3. InSphero
- 12.1.3.1. Company Overview
- 12.1.3.2. Financial Information
- 12.1.3.3. Product Portfolio
- 12.1.3.4. Recent Developments and Future Outlook
- 12.1.4. MIMETAS
- 12.1.4.1. Company Overview
- 12.1.4.2. Financial Information
- 12.1.4.3. Product Portfolio
- 12.1.4.4. Recent Developments and Future Outlook
- 12.1.5. TissUse
- 12.1.5.1. Company Overview
- 12.1.5.2. Product Portfolio
- 12.1.5.3. Recent Developments and Future Outlook
- 12.1.1. CN Bio Innovations
13. COMPANY PROFILES: 3D BIOREACTORS
- 13.1. Chapter Overview
- 13.2. BISS TGT
- 13.2.1. Company Overview
- 13.2.2. Product Portfolio
- 13.2.3. Recent Developments and Future Outlook
- 13.3. Celartia
- 13.3.1. Company Overview
- 13.3.2. Product Portfolio
- 13.3.3. Recent Developments and Future Outlook
- 13.4. Cell Culture
- 13.4.1. Company Overview
- 13.4.2. Product Portfolio
- 13.4.3. Recent Developments and Future Outlook
- 13.5. EBERS
- 13.5.1. Company Overview
- 13.5.2. Product Portfolio
- 13.5.3. Recent Developments and Future Outlook
- 13.6. Flexcell International
- 13.6.1. Company Overview
- 13.6.2. Product Portfolio
- 13.6.3. Recent Developments and Future Outlook
- 13.7. PBS Biotech
- 13.7.1. Company Overview
- 13.7.2. Product Portfolio
- 13.7.3. Recent Developments and Future Outlook
- 13.8. Synthecon
- 13.8.1. Company Overview
- 13.8.2. Product Portfolio
- 13.8.3. Recent Developments and Future Outlook
14. FUNDING AND INVESTMENT ANALYSIS
- 14.1. Chapter Overview
- 14.2. Types of Funding
- 14.3. 3D Cell Culture Systems: Funding and Investment Analysis
- 14.3.1. Analysis by Number of Funding Instances
- 14.3.2. Analysis by Amount Invested
- 14.3.3. Analysis by Type of Funding
- 14.3.4. Analysis by 3D Cell Culture Format
- 14.3.5. Analysis by Type of Product
- 14.3.6. Analysis by Geography
- 14.3.7. Most Active Players: Analysis by Number of Funding Instances
- 14.3.8. Most Active Players: Analysis by Amount of Funding
- 14.3.9. Most Active Investors: Analysis by Number of Instances
- 14.4 Summary of Funding and Investments
15. PARTNERSHIPS AND COLLABORATIONS
- 15.1. Chapter Overview
- 15.2. Partnership Models
- 15.3. 3D Cell Culture Systems: List of Partnerships and Collaborations
- 15.3.1. Analysis by Year of Partnership
- 15.3.2. Analysis by Type of Partnership
- 15.3.2.1. Analysis by Year of Partnership and Type of Partnership
- 15.3.2.2. Analysis by Company Size and Type of Partnership
- 15.3.3. Analysis by Type of Partner
- 15.3.3.1. Analysis by Year of Partnership and Type of Partner
- 15.3.3.2. Analysis by Type of Partnership and Type of Partner
- 15.3.4. Analysis by 3D Cell Culture Format
- 15.3.4.1. Analysis by Year of Partnership and 3D Cell Culture Format
- 15.3.4.2. Analysis by Type of Partnership and 3D Cell Culture Format
- 15.3.5. Analysis by Type of Product
- 15.3.5.1. Analysis by Year of Partnership and Type of Product
- 15.3.5.2. Analysis by Type of Partnership and Type of Product
- 15.3.6. Most Active Players: Analysis by Number of Partnerships
- 15.3.7. Regional Analysis
- 15.3.8. Intercontinental and Intracontinental Agreements
16. PATENT ANALYSIS
- 16.1. Chapter Overview
- 16.2. Scope and Methodology
- 16.3. 3D Cell Culture Systems: Patent Analysis
- 16.3.1. Analysis by Type of Patent
- 16.3.2. Analysis by Publication Year
- 16.3.3. Analysis by Type of Patent and Publication Year
- 16.3.3. Analysis by Issuing Authority
- 16.3.4. Analysis by CPC Symbols
- 16.3.5. Analysis by Type of Applicant
- 16.3.6. Word Cloud Analysis: Emerging Focus Areas
- 16.3.7. Leading Industry Players: Analysis by Number of Patents
- 16.3.8. Leading Non-Industry Players: Analysis by Number of Patents
- 16.4. 3D Cell Culture Systems: Patent Valuation Analysis
- 16.5. Leading Patents: Analysis by Number of Citations
17. PUBLICATION ANALYSIS
- 17.1. 3D Cell Culture Systems: Publication Analysis
- 17.2. Assumptions and Key Parameters
- 17.3. Methodology
- 17.3.1. Analysis by Year of Publication
- 17.3.2. Word Cloud Analysis: Emerging Focus Areas
- 17.3.3. Top Authors: Analysis by Number of Publications
- 17.3.4. Key Journals: Analysis by Number of Publications
- 17.3.5. Key Publishers: Analysis by Number of Publications
- 17.3.6. Leading Funding Institutes: Analysis by Number of Publications
18. PRODUCT COMPETITIVENESS ANALYSIS
- 18.1. Chapter Overview
- 18.2. Assumptions / Key Parameters
- 18.3. Methodology
- 18.4. Product Competitiveness Analysis: 3D Bioreactors
- 18.4.1. Companies Headquartered in North America
- 18.4.2. Companies Headquartered in Europe
- 18.4.3. Companies Headquartered in Asia-Pacific and Rest of the World
19. CASE STUDY: ORGANIDS AND ORGAN-ON-CHIPS
- 19.1. Chapter Overview
- 19.2. Organoids and Organ-on-Chips: List of Products
- 19.2.1. Analysis by Status of Development
- 19.2.2. Analysis by Application Area
- 19.3. Organoids and Organ-on-Chips: List of Product Developers
- 19.3.1. Analysis by Year of Establishment
- 19.3.2. Analysis by Company Size
- 19.3.3. Analysis by Location of Headquarters
20. MARKET FORECAST
- 20.1. Chapter Overview
- 20.2. Key Assumptions and Forecast Methodology
- 20.3. Global 3D Cell Culture Market, 2022-2035
- 20.4. Global 3D Cell Culture Market: Distribution by Business Segment
- 20.4.1. 3D Cell Culture Systems Market, 2022-2035
- 20.4.2. 3D Cell Culture Consumables Market, 2022-2035
- 20.4.3. 3D Cell Culture Services Market, 2022-2035
- 20.5. Global 3D Cell Culture Systems Market: Distribution by 3D Cell Culture Format
- 20.5.1. 3D Cell Culture Systems Market for Scaffold Based Products, 2022-2035
- 20.5.2. 3D Cell Culture Systems Market for Scaffold Free Products, 2022-2035
- 20.5.3. 3D Cell Culture Systems Market for 3D Bioreactors, 2022-2035
- 20.6. Global 3D Cell Culture Systems Market: Distribution by Type of Product
- 20.6.1. 3D Cell Culture Systems Market for Attachment Resistant Surfaces, 2022-2035
- 20.6.2. 3D Cell Culture Systems Market for Hydrogels / ECMs, 2022-2035
- 20.6.3 3D Cell Culture Systems Market for Micropatterned Surface, 2022-2035
- 20.6.4. 3D Cell Culture Systems Market for Microcarriers, 2022-2035
- 20.6.5. 3D Cell Culture Systems Market for Microfluidic Systems, 2022-2035
- 20.6.6. 3D Cell Culture Systems Market for Solid Scaffolds, 2022-2035
- 20.6.7. 3D Cell Culture Systems Market for Suspension Culture Systems, 2022-2035
- 20.7. Global 3D Cell Culture Systems Market: Distribution by Area of Application
- 20.7.1. 3D Cell Culture Systems Market for Cancer Research, 2022-2035
- 20.7.2 3D Cell Culture Systems Market for Drug Discovery and Toxicity Testing, 2022-2035
- 20.7.3. 3D Cell Culture Systems Market for Stem Cell Research, 2022-2035
- 20.7.4. 3D Cell Culture Systems Market for Regenerative Medicine and Tissue Engineering, 2022-2035
- 20.8. Global 3D Cell Culture Systems Market: Distribution by Purpose
- 20.8.1. 3D Cell Culture Systems Market for Research Use, 2022-2035
- 20.8.2 3D Cell Culture Systems Market for Therapeutic Use, 2022-2035
- 20.9. Global 3D Cell Culture Systems Market: Distribution by Geography
- 20.9.1. 3D Cell Culture Systems Market in North America, 2022-2035
- 20.9.2 3D Cell Culture Systems Market in Europe, 2022-2035
- 20.9.3. 3D Cell Culture Systems Market in Asia-Pacific, 2022-2035
- 20.9.4. 3D Cell Culture Systems Market in Latin America, 2022-2035
- 20.9.5. 3D Cell Culture Systems Market in Middle East and North Africa, 2022-2035
- 20.9.6. 3D Cell Culture Systems Market in Rest of the World, 2022-2035
21. SURVEY ANALYSIS
- 21.1. Chapter Overview
- 21.2. Overview of Respondents
- 21.2.1. Designation of Respondents
- 21.3. Survey Insights
- 21.3.1. 3D Cell Culture Format
- 21.3.2. Type of Product(s) Offered
- 21.3.3. Status of Development of Product(s)
- 21.3.4. Source of 3D Cultured Cells
- 21.3.5. Method Used for Fabrication
- 21.3.6. Area(s) of Application
- 21.3.7. Services Offered for 3D Cell Cultures
- 21.3.8. Current and Future Market Opportunity
22. CONCLUSION
23. EXECUTIVE INSIGHTS
- 23.1. Chapter Overview
- 23.2. Cellendes
- 23.2.1. Company Snapshot
- 23.2.2. Interview Transcript: Brigitte Angres, Co-founder
- 23.3. Synthecon
- 23.3.1. Company Snapshot
- 23.3.2. Interview Transcript: Bill Anderson, President and Chief Executive Officer
- 23.4. Anonymous
- 23.4.1. Interview Transcript: Anonymous, President and Chief Executive Officer
- 23.5. Anonymous
- 23.5.1. Interview Transcript: Anonymous, Co-founder and Vice President
- 23.6. BRTI Life Sciences
- 23.6.1. Company Snapshot
- 22.6.2. Interview Transcript: Scott Brush, Vice President
- 23.7. Kirkstall
- 23.7.1. Company Snapshot
- 23.7.2. Interview Transcript: Malcolm Wilkinson, Non-Executive Director
- 23.8. QGel
- 23.8.1. Company Snapshot
- 23.8.2. Interview Transcript: Ryder Clifford, Chief Executive Officer and Simone Carlo Rizzi, Chief Scientific Officer
- 23.9. Xylyx Bio
- 23.9.1. Company Snapshot
- 23.9.2. Interview Transcript: Tanya Yankelevich, Former Director of Product Management and Business Development
- 23.10. InSphero
- 23.10.1. Company Snapshot
- 23.10.2. Interview Transcript: Jens Kelm, Former Chief Scientific Officer
- 23.11. GSI
- 23.11.1. Company Snapshot
- 23.11.2. Interview Transcript: Walter Tinganelli, Group Leader, Clinical Radiobiology
- 23.12. Nanofiber Solutions
- 23.12.1. Company Snapshot
- 23.12.2. Interview Transcript: Darlene Thieken, Former Project Manager
- 23.13. FlexCell International
- 23.13.1. Company Snapshot
- 23.13.2. Interview Transcript: Andrea Picon, Director of Business Development
- 23.14. InSphero
- 23.14.1. Company Snapshot
- 23.14.2. Interview Transcript: Frank Junker, Chief Business officer
- 23.15. MBL International
- 23.15.1. Company Snapshot
- 23.15.2. Interview Transcript: Mamun, Rahman, Manger, Business Development
24. APPENDIX I: TABULATED DATA
25. APPENDIX II: LIST OF COMPANIES AND ORGANIZATIONS
List Of Figures
- Figure 3.1 Classification of Cell Cultures
- Figure 3.2 Types of Cell Cultures
- Figure 3.3 Key Components of ECM
- Figure 3.4 Factors Influencing the Selection of 3D Cell Culture Systems
- Figure 3.5 Methods Used for Isolation of Cells from Tissues
- Figure 3.6 Methods Used for Cryogenic Storage of Cell Cultures
- Figure 3.7 Biosafety Levels for Cell Cultures
- Figure 3.8 Key Applications of Cell Cultures
- Figure 3.9 Shapes of 3D Spheroids Generated via 3D Cell Culture Systems
- Figure 3.10 Advantages and Limitations of 3D Cell Culture Systems
- Figure 4.1 Classification of 3D Cell Culture Systems
- Figure 4.2 Natural Components of ECM Used for Fabrication of Scaffolds
- Figure 4.3 Advantages and Disadvantages of Hydrogels
- Figure 4.4 Advantages of Microcarriers
- Figure 4.5 Techniques Used for Formation of 3D Spheroids
- Figure 4.6 Structures of Spinner Flask and Rotating Wall Bioreactors
- Figure 6.1 3D Cell Culture System Developers: Distribution by Year of Establishment
- Figure 6.2 3D Cell Culture System Developers: Distribution by Company Size
- Figure 6.3 3D Cell Culture System Developers: Distribution by Location of Headquarters
- Figure 6.4 3D Cell Culture System Developers: Distribution by 3D Cell Culture Format
- Figure 6.5 3D Cell Culture System Developers: Distribution by Type of Product
- Figure 6.6 Heat Map Representation: Distribution by 3D Cell Culture Format and Location of Headquarters
- Figure 6.7 Tree Map Representation: Distribution by Company Size and Type of Product
- Figure 6.8 World Map Representation: Distribution by Location of Headquarters
- Figure 7.1 Scaffold Based Products: Distribution by Status of Development
- Figure 7.2 Scaffold Based Products: Distribution by Type of Product
- Figure 7.3 Scaffold Based Products: Distribution by Source of Scaffold
- Figure 7.4 Scaffold Based Products: Distribution by Material Used for Fabrication
- Figure 7.5 Scaffold Based Products: Distribution by Type of Product and Source of Scaffold
- Figure 7.6 Scaffold Based Products: Distribution by Type of Product and Material Used for Fabrication
- Figure 7.7 Scaffold Based Product Developers: Distribution by Year of Establishment
- Figure 7.8 Scaffold Based Product Developers: Distribution by Company Size
- Figure 7.9 Scaffold Based Product Developers: Distribution by Location of Headquarters
- Figure 7.10 Leading Developers: Distribution by Number of Scaffold Based Products
- Figure 7.11 Tree Map Representation: Distribution by Company Size and Type of Product
- Figure 8.1 Scaffold Free Products: Distribution by Status of Development
- Figure 8.2 Scaffold Free Products: Distribution by Type of Product
- Figure 8.3 Scaffold Free Products: Distribution by Method Used for Fabrication
- Figure 8.4 Scaffold Free Products: Distribution by Material Used for Fabrication
- Figure 8.5 Scaffold Free Products: Distribution by Type of Product and Material Used for Fabrication
- Figure 8.6 Scaffold Free Product Developers: Distribution by Year of Establishment
- Figure 8.7 Scaffold Free Product Developers: Distribution by Company Size
- Figure 8.8 Scaffold Free Product Developers: Distribution by Location of Headquarters
- Figure 8.9 Leading Developers: Distribution by Number of Scaffold Free Products
- Figure 8.10 Tree Map Representation: Distribution by Company Size and Type of Product
- Figure 9.1 3D Bioreactors: Distribution by Type of 3D Bioreactor
- Figure 9.2 3D Bioreactors: Distribution by Status of Development
- Figure 9.3 3D Bioreactors: Distribution by Working Volume
- Figure 9.4 3D Bioreactors: Distribution by Scale of Operation
- Figure 9.5 3D Bioreactors: Distribution by Manufacturing Process
- Figure 9.6 3D Bioreactors: Distribution by Type of Cell Culture System
- Figure 9.7 3D Bioreactors: Distribution by Type of Molecule Processed
- Figure 9.8 3D Bioreactors: Distribution by Area of Application
- Figure 9.9 3D Bioreactor Developers: Distribution by Year of Establishment
- Figure 9.10 3D Bioreactor Developers: Distribution by Company Size
- Figure 9.11 3D Bioreactor Developers: Distribution by Location of Headquarters
- Figure 9.12 Leading Developers: Distribution by Number of 3D Bioreactors
- Figure 10.1 Key Application Areas of 3D Cell Culture Systems
- Figure 10.2 3D Cell Culture Systems in Cancer Research
- Figure 10.3 3D Cell Culture Systems in Drug Discovery and Toxicity Screening
- Figure 10.4 Methods to Generate Embryoid Bodies
- Figure 10.5 Top-Down and Bottom-Up Approaches for Tissue Engineering
- Figure 10.6 3D Cell Culture Systems: Distribution by Key Application Areas
- Figure 10.7 3D Cell Culture Systems: Distribution by Key Application Areas and 3D Cell Culture Format
- Figure 10.8 Scaffold Based 3D Products: Distribution by Key Application Areas
- Figure 10.9 Scaffold Free 3D Products: Distribution by Key Application Areas
- Figure 10.10 3D Bioreactors: Distribution by Key Application Areas
- Figure 13.1 Key Features of 3D Perfusion Bioreactors
- Figure 13.2 MagDrive and AirDrive Mechanisms for PBS Bioreactors
- Figure 13.3 Advantages of Rotary Cell Culture System (RCCS)
- Figure 14.1 Funding and Investments: Distribution of Recipient Companies by Year of Establishment and Type of Funding, 2015 - 2021
- Figure 14.2 Funding and Investments: Cumulative Number of Funding Instances by Year, 2015 - 2021
- Figure 14.3 Funding and Investments: Cumulative Amount Invested, 2015 - 2021 (USD Million)
- Figure 14.4 Funding and Investments: Distribution of Instances by Type of Funding, 2015 -2021
- Figure 14.5 Funding and Investments: Year-Wise Distribution of Instances and Type of Funding, 2015 - 2021
- Figure 14.6 Funding and Investments: Distribution by Amount Invested and Type of Funding, 2015 - 2021 (USD Million)
- Figure 14.7 Funding and Investments: Year-Wise Distribution of Amount Invested and Type of Funding, 2015 - 2021
- Figure 14.8 Funding and Investments: Distribution by Number of Instances and Amount Invested by 3D Cell Culture Format, 2015 - 2021
- Figure 14.9 Funding and Investments: Distribution by Number of Instances and Amount Invested by Type of Product, 2015 - 2021
- Figure 14.10 Funding and Investments: Distribution by Geography
- Figure 14.11 Funding and Investments: Regional Distribution by Total Amount Invested, 2015 - 2021
- Figure 14.12 Most Active Players: Distribution by Number of Funding Instances, 2015 -2021
- Figure 14.13 Most Active Players: Distribution by Amount Raised, 2015 - 2021 (USD Million)
- Figure 14.14 Most Active Investors: Distribution by Number of Funding Instances, 2015 -2021
- Figure 14.15 Funding and Investment Summary, 2015 - 2021 (USD Million)
- Figure 15.1 Partnerships and Collaborations: Cumulative Year-Wise Trend, 2015 - 2021
- Figure 15.2 Partnerships and Collaborations: Distribution by Type of Partnership
- Figure 15.3 Partnerships and Collaborations: Distribution by Year of Partnership and Type of Partnership
- Figure 15.4 Partnerships and Collaborations: Distribution by Company Size and Type of Partnership
- Figure 15.5 Partnerships and Collaborations: Distribution by Type of Partner
- Figure 15.6 Partnerships and Collaborations: Distribution by Year of Partnership and Type of Partner
- Figure 15.7 Partnerships and Collaborations: Distribution by Type of Partnership and Type of Partner
- Figure 15.8 Partnerships and Collaborations: Distribution by 3D Cell Culture Format
- Figure 15.9 Partnerships and Collaborations: Distribution by Year of Partnership and 3D Cell Culture Format
- Figure 15.10 Partnerships and Collaborations: Distribution by Type of Partnership and 3D Cell Culture Format
- Figure 15.11 Partnerships and Collaborations: Distribution by Type of Product
- Figure 15.12 Partnerships and Collaborations: Distribution by Year of Partnership and Type of Product
- Figure 15.13 Partnerships and Collaborations: Distribution by Type of Partnership and Type of Product
- Figure 15.14 Most Active Players: Distribution by Number of Partnerships
- Figure 15.15 Partnerships and Collaborations: Regional Distribution
- Figure 15.16 Partnerships and Collaborations: Distribution by Intercontinental and Intracontinental Agreements
- Figure 16.1 Patent Analysis: Distribution by Type of Patent
- Figure 16.2 Patent Analysis: Cumulative Distribution by Publication Year, 2016 - Q1 2022
- Figure 16.3 Patent Analysis: Distribution of Granted Patents by Publication Year, 2016 - Q1 2022
- Figure 16.4 Patent Analysis: Distribution of Filed Patents Publication Year, 2016 - Q1 2022
- Figure 16.5 Patent Analysis: Distribution by Type of Patent and Publication Year, 2016 - Q1 2022
- Figure 16.6 Patent Analysis: Distribution by Issuing Authority
- Figure 16.7 Patent Analysis: Distribution by CPC Symbols
- Figure 16.8 Patent Analysis: Cumulative Year-wise Distribution by Type of Applicant, 2016 - Q1 2022
- Figure 16.9 Word Cloud Analysis: Distribution by Emerging Focus Area
- Figure 16.10 Leading Industry Players: Distribution by Number of Patents
- Figure 16.11 Leading Non-Industry Players: Distribution by Number of Patents
- Figure 16.12 Patent Analysis: Distribution by Patent Age, 2002-2022
- Figure 16.13 Patent Analysis: Distribution by Relatuve Valuation
- Figure 17.1 Publication Analysis: Distribution by Year of Publication
- Figure 17.2 Word Cloud Analysis: Emerging Focus Areas
- Figure 17.3 Top Authors: Distribution by Number of Publications
- Figure 17.4 Key Journals: Distribution by Number of Publications
- Figure 17.5 Key Publishers: Distribution by Number of Publications
- Figure 17.6 Leading Funding Institutes: Distribution by Number of Publications
- Figure 18.1 Competitiveness Analysis: 3D Bioreactors Developers based in North America
- Figure 18.2 Competitiveness Analysis: 3D Bioreactors Developers based in Europe
- Figure 18.3 Competitiveness Analysis: 3D Bioreactors Developers based in Asia-Pacific and Rest of the World
- Figure 19.1 Organoids and Organ-on-Chips: Distribution by Status of Development
- Figure 19.2 Organoids and Organ-on-Chips: Distribution by Application Area
- Figure 19.3 Organoids and Organ-on-Chips Developers: Distribution by Year of Establishment
- Figure 19.4 Organoids and Organ-on-Chips Developers: Distribution by Company Size
- Figure 19.5 Organoids and Organ-on-Chips Developers: Distribution by Location of Headquarters
- Figure 20.1 Global 3D Cell Culture Market, 2022-2035 (USD Million)
- Figure 20.2 Global 3D Cell Culture Market: Distribution by Business Segment, 2022 and 2035
- Figure 20.3 3D Cell Culture Systems Market, 2022-2035 (USD Million)
- Figure 20.4 3D Cell Culture Consumables Market, 2022-2035 (USD Million)
- Figure 20.5 3D Cell Culture Services Market, 2022-2035 (USD Million)
- Figure 20.6. Global 3D Cell Culture Systems Market: Distribution by 3D Cell Culture Format, 2022-2035
- Figure 20.7 3D Cell Culture Systems Market for Scaffold Based Products, 2022-2035 (USD Million)
- Figure 20.8 3D Cell Culture Systems Market for Scaffold Free Products, 2022-2035 (USD Million)
- Figure 20.9 3D Cell Culture Systems Market for 3D Bioreactors, 2022-2035 (USD Million)
- Figure 20.10 Global 3D Cell Culture Systems Market: Distribution by Type of Product, 2022 and 2035
- Figure 20.11 3D Cell Culture Systems Market for Attachment Resistant Surfaces, 2022-2035 (USD Million)
- Figure 20.12 3D Cell Culture Systems Market for Hydrogels / ECMs, 2022-2035 (USD Million)
- Figure 20.13 3D Cell Culture Systems Market for Micropatterned Surface, 2022-2035 (USD Million)
- Figure 20.14 3D Cell Culture Systems Market for Microcarriers, 2022-2035 (USD Million)
- Figure 20.15 3D Cell Culture Systems Market for Microfluidic Systems, 2022-2035 (USD Million)
- Figure 20.16 3D Cell Culture Systems Market for Solid Scaffolds, 2022-2035 (USD Million)
- Figure 20.17 3D Cell Culture Systems Market for Suspension Cultures, 2022-2035 (USD Million)
- Figure 20.18 Global 3D Cell Culture Systems Market: Distribution by Area of Application, 2022 and 2035
- Figure 20.19 3D Cell Culture Systems Market for Cancer Research, 2022-2035 (USD Million)
- Figure 20.20 3D Cell Culture Systems Market for Drug Discovery and Toxicity Testing, 2022-2035 (USD Million)
- Figure 20.21 3D Cell Culture Systems Market for Stem Cell Research, 2022-2035 (USD Million)
- Figure 20.22 3D Cell Culture Systems Market for Regenerative Medicine and Tissue Engineering, 2022-2035 (USD Million)
- Figure 20.23 Global 3D Cell Culture Systems Market: Distribution by Purpose, 2022 and 2035
- Figure 20.24 3D Cell Culture Systems Market for Research Use, 2022-2035 (USD Million)
- Figure 20.25 3D Cell Culture Systems Market for Therapeutic Use, 2022-2035 (USD Million)
- Figure 20.26 Global 3D Cell Culture Systems Market: Distribution by Geography, 2022 and 2035
- Figure 20.27 3D Cell Culture Systems Market in North America, 2022-2035 (USD Million)
- Figure 20.28 3D Cell Culture Systems Market in Europe, 2022-2035 (USD Million)
- Figure 20.29 3D Cell Culture Systems Market in Asia-Pacific, 2022-2035 (USD Million)
- Figure 20.30 3D Cell Culture Systems Market in Latin America, 2022-2035 (USD Million)
- Figure 20.31 3D Cell Culture Systems Market in Middle East and North Africa (MENA), 2022-2035 (USD Million)
- Figure 20.32 3D Cell Culture Systems Market in Rest of the World, 2022-2035 (USD Million)
- Figure 20.33 Global 3D Cell Culture Systems Market: Distribution by Leading Players, 2022
- Figure 20.34 Global 3D Cell Culture Systems Market: Conservative, Base and Optimistic Scenarios, 2022, 2028 and 2035 (USD Million)
- Figure 21.1 Survey Insights: Distribution of Respondents by Year of Establishment of the Company
- Figure 21.2 Survey Insights: Distribution of Respondents by Company Size
- Figure 21.3 Survey Insights: Distribution of Respondents by Location of Company Headquarters (Region-Wise)
- Figure 21.4 Survey Insights: Distribution of Respondents by Location of Company Headquarters (Country-Wise)
- Figure 21.5 Survey Insights: Distribution of Respondents by Designation and Seniority Level
- Figure 21.6 Survey Insights: Distribution by Focus Area
- Figure 21.7 Survey Insights: Distribution by Type of 3D Cell Culture Products Offered
- Figure 21.8 Survey Insights: Distribution by Status of Development of Product(s)
- Figure 21.9 Survey Insights: Distribution by Method of Fabrication Used
- Figure 21.10 Survey Insights: Distribution by Source of Cultured Cells
- Figure 21.11 Survey Insights: Distribution by Key Applications
- Figure 21.12 Survey Insights: Distribution by 3D Cell Culture Services Offered
- Figure 21.13 Survey Insights: Distribution by Current and Future Market Opportunity, 2022 and 2035
- Figure 22.1 Concluding Remarks: Overall Market Landscape of 3D Cell Culture Systems Market
- Figure 22.2 Concluding Remarks: Funding and Investments
- Figure 22.3 Concluding Remarks: Partnerships and Collaborations
- Figure 22.4 Concluding Remarks: Patent Analysis
- Figure 22.5 Concluding Remarks: Publication Analysis
- Figure 22.6 Concluding Remarks: Market Sizing and Opportunity Analysis




