|
|
市場調査レポート
商品コード
1077364
合成生物学:DNAクローニング市場 - クローニング手法タイプ別、エンドユーザー別Synthetic Biology: DNA Cloning Market by Type of Cloning Method (Blunt End Cloning, Ligase Independent Cloning, PCR Cloning, Seamless Cloning, TA Cloning and Other Methods), End-Users (Academic and Research Institutes, Pharmaceutical |
||||||
|
● お客様のご希望に応じて、既存データの加工や未掲載情報(例:国別セグメント)の追加などの対応が可能です。 詳細はお問い合わせください。 |
|||||||
| 合成生物学:DNAクローニング市場 - クローニング手法タイプ別、エンドユーザー別 |
|
出版日: 2022年05月23日
発行: Roots Analysis
ページ情報: 英文 203 Pages
納期: 即日から翌営業日
|
- 全表示
- 概要
- 図表
- 目次
ハイライトの例
目次
第1章 序文
第2章 エグゼクティブサマリー
第3章 イントロダクション
- 章の概要
- DNAクローニングイントロダクション
- DNAクローニングの方法
- DNAクローニングキット
- DNAクローニング試薬
- DNAクローニングツール
- DNAクローニングに関連する課題
- DNAクローニングの応用
- 今後の展望
第4章 市場の市場情勢:DNAクローニングキット
- 章の概要
- DNAクローニングキット:全体的な市場情勢
- キットコンポーネント別分析
- クローン化されたフラグメントのタイプ別分析
- DNAクローニングキット:開発者情勢
第5章 市場情勢:DNA市場情勢試薬
- 章の概要
- DNAクローニング試薬:市場情勢
- DNAクローニング試薬:開発者の情勢
第6章 DNAクローニングキットおよび試薬:企業プロファイル
- 章の概要
- Thermo Fisher Scientific
- Merck
- Takara Bio
- Vazyme
- GenScript
- Promega
- Agilent Technologies
- Bio-Rad
第7章 出版物の分析
第8章 助成金分析
第9章 特許分析
第10章 市場予測と機会分析
- 章の概要
- 予測調査手法と主要な仮定
- 世界のDNAクローニングキット市場、2022年~2035年
第11章 ケーススタディ:DNAクローニングサービスプロバイダー
- 章の概要
- DNAクローニングサービスプロバイダー:企業リスト
第12章 DNAクローニングの進化
- 章の概要
- 合成生物学におけるAIのイントロダクション
- 結論
第13章 結論
第14章 エグゼクティブインサイト
第15章 付録1:集計データ
第16章 付録2:企業と組織のリスト
List Of Tables
- Table 4.1. DNA Cloning Kits: Information on Kit Components and Number of Reactions
- Table 4.2. DNA Cloning Kits: Information on Cloning Method and Cloning Time
- Table 4.3. DNA Cloning Kits: Information on Overlaps Recognized and Type of Fragments Cloned
- Table 4.4. DNA Cloning Kits: Information on Efficacy, Kit Shelf Life and Kit Price
- Table 4.5. DNA Cloning Kit Providers: List of Players
- Table 5.1. DNA Cloning Reagents: Information on Unit Size, Reagent Components and Concentration
- Table 5.2. DNA Cloning Reagents: Information on Product Overhangs, Exonuclease Activity, Storage Temperature and Reagent Price
- Table 5.3. DNA Cloning Reagent Providers: List of Players
- Table 6.1. DNA Cloning Kit and Reagent Providers: List of Companies Profiled
- Table 6.2. Thermo Fisher Scientific: Company Snapshot
- Table 6.3. DNA Cloning Kit Portfolio
- Table 6.4. DNA Cloning Reagent Portfolio
- Table 6.5. Recent Developments and Future Outlook
- Table 6.6. Merck: Company Snapshot
- Table 6.7. DNA Cloning Kit Portfolio
- Table 6.8. DNA Cloning Reagent Portfolio
- Table 6.9. Recent Developments and Future Outlook
- Table 6.10. Takara Bio: Company Snapshot
- Table 6.11. DNA Cloning Kit Portfolio
- Table 6.12. DNA Cloning Reagent Portfolio
- Table 6.13. Recent Developments and Future Outlook
- Table 6.14. Vazyme: Company Snapshot
- Table 6.15. DNA Cloning Kit Portfolio
- Table 6.16. GenScript: Company Snapshot
- Table 6.17. DNA Cloning Kit Portfolio
- Table 6.18. Recent Developments and Future Outlook
- Table 6.19. Promega: Company Snapshot
- Table 6.20. DNA Cloning Reagent Portfolio
- Table 6.21. Agilent Technologies: Company Snapshot
- Table 6.22. DNA Cloning Reagent Portfolio
- Table 6.23. Recent Developments and Future Outlook
- Table 6.24. Bio-Rad: Company Snapshot
- Table 6.25. DNA Cloning Reagent Portfolio
- Table 6.26. Recent Developments and Future Outlook
- Table 9.1. Patent Analysis: Prominent CPC Symbols
- Table 9.2. Patent Analysis: List of Top CPC Symbols
- Table 9.3. Patent Analysis: Most Popular CPC Symbols
- Table 9.4. Patent Analysis: Summary of Benchmarking Analysis
- Table 9.5. Patent Analysis: Categorization based on Weighted Valuation Scores
- Table 9.6. Patent Portfolio: List of Leading Patents (by Highest Relative Valuation)
- Table 9.7. Patent Portfolio: List of Leading Patents (by Number of Citations)
- Table 11.1. DNA Cloning Service Providers: List of Players
- Table 14.1. SBS Genentech: Company Snapshot
- Table 14.2. Canvax Biotech: Company Snapshot
- Table 15.1. DNA Cloning Kits: Distribution by Kit Components
- Table 15.2. DNA Cloning Kits: Distribution by Number of Reactions
- Table 15.3. DNA Cloning Kits: Distribution by Type of Cloning Method Used
- Table 15.4. DNA Cloning Kits: Distribution by Cloning Time
- Table 15.5. DNA Cloning Kits: Distribution by Overlaps Recognized
- Table 15.6. DNA Cloning Kits: Distribution by Type of Fragment(s) Cloned
- Table 15.7. DNA Cloning Kits: Distribution by Efficacy
- Table 15.8. DNA Cloning Kits: Distribution by Kit Shelf Life
- Table 15.9. DNA Cloning Kits: Distribution by Kit Price
- Table 15.10. DNA Cloning Kit Providers: Distribution by Year of Establishment
- Table 15.11. DNA Cloning Kit Providers: Distribution by Company Size
- Table 15.12. DNA Cloning Kit Providers: Distribution by Region of Headquarters
- Table 15.13. DNA Cloning Kit Providers: Distribution by Location of Headquarters
- Table 15.14. DNA Cloning Kit Providers: Distribution by Company Size and Region of Headquarters
- Table 15.15. Leading Players: Distribution by Number of DNA Cloning Kits
- Table 15.16. DNA Cloning Reagents: Distribution by Unit Size
- Table 15.17. DNA Cloning Reagents: Distribution by Concentration (units/µl)
- Table 15.18. DNA Cloning Reagents: Distribution by Reagent Components
- Table 15.19. DNA Cloning Reagents: Distribution by Exonuclease Activity
- Table 15.20. DNA Cloning Reagents: Distribution by Product Overhangs
- Table 15.21. DNA Cloning Reagents: Distribution by Storage Temperature
- Table 15.22. DNA Cloning Reagents: Distribution by Reagent Price
- Table 15.23. DNA Cloning Reagent Providers: Distribution by Year of Establishment
- Table 15.24. DNA Cloning Reagent Providers: Distribution by Company Size
- Table 15.25. DNA Cloning Reagent Providers: Distribution by Region of Headquarters
- Table 15.26. DNA Cloning Reagent Providers: Distribution by Location of Headquarters
- Table 15.27. DNA Cloning Reagent Providers: Distribution by Company Size and Region of Headquarters
- Table 15.28. Leading Players: Distribution by Number of Products
- Table 15.29. Thermo Fisher Scientific: Annual Revenues, 2017-2021 (USD Billion)
- Table 15.30. Merck: Annual Revenues, 2017- 2021 (EUR Billion)
- Table 15.31. Takara Bio: Annual Revenues, 2017-9M 2022 (JPY Billion)
- Table 15.32. GenScript: Annual Revenues, 2016- 2021 (USD Billion)
- Table 15.33. Agilent Technologies: Annual Revenues, 2017- 1M 2022 (USD Billion)
- Table 15.34. Bio-Rad: Annual Revenues, 2017- 2021 (USD Billion)
- Table 15.35. Publication Analysis: Cumulative Year-wise Trend, 2018-2022 (till January)
- Table 15.36. Publication Analysis: Distribution by Type of Article
- Table 15.37. Publication Analysis: Distribution by Type of Publication
- Table 15.38. Popular Publishers: Distribution by Number of Publications
- Table 15.39. Popular Journals: Distribution by Number of Publications
- Table 15.40. Popular Journals: Distribution by Journal Impact Factor
- Table 15.41. Publication Analysis: Distribution by Geography
- Table 15.42. Grant Analysis: Cumulative Year-Wise Trend, 2017-2022 (till January)
- Table 15.43. Grant Analysis: Distribution by Amount Awarded (USD Million), 2017-2022 (till January)
- Table 15.44. Grant Analysis: Distribution by Administering Institute Center
- Table 15.45. Grant Analysis: Distribution by Support Period
- Table 15.46. Grant Analysis: Distribution by Type of Grant Application
- Table 15.47. Grant Analysis: Distribution by Purpose of Grant Award
- Table 15.48. Grant Analysis: Distribution of Amount Awarded by Purpose of Grant
- Table 15.49. Grant Analysis: Distribution by Activity Code
- Table 15.50. Grant Analysis: Distribution by Study Section
- Table 15.51. Popular Program Officers: Distribution by Number of Grants
- Table 15.52. Popular Recipient Organizations: Distribution by Number of Grants
- Table 15.53. Popular Recipient Organizations: Distribution by Amount Awarded (USD Million)
- Table 15.54. Grant Analysis: Distribution by Location of Recipient Organizations
- Table 15.55. Patent Analysis: Distribution by Type of Patent
- Table 15.56. Patent Analysis: Cumulative Distribution by Publication Year, 2017-2021
- Table 15.57. Patent Analysis: Cumulative Distribution by Application Year, 2017-2021
- Table 15.58. Patent Analysis: Distribution by Geographical Location
- Table 15.59. Patent Analysis: Cumulative Year-wise Distribution by Type of Organization, 2017-2021
- Table 15.60. Leading Industry Players: Distribution by Number of Patents
- Table 15.61. Leading Non-Industry Players: Distribution by Number of Patents
- Table 15.62. Leading Individual Assignees: Distribution by Number of Patents
- Table 15.63. Leading Players: Benchmarking by Patent Characteristics (CPC Symbols)
- Table 15.64. Patent Analysis: Year-wise Distribution of Patents by Age, 2001-2021
- Table 15.65. DNA Cloning: Patent Valuation Analysis
- Table 15.66. Global DNA Cloning Kits Market, 2022-2035: Conservative, Base and Optimistic Scenarios (USD Million)
- Table 15.67. DNA Cloning Kits Market, 2022 and 2035: Distribution by Type of Cloning Method Used
- Table 15.68. DNA Cloning Kits Market for Blunt End Cloning, 2022-2035: Conservative, Base and Optimistic Scenarios (USD Million)
- Table 15.69. DNA Cloning Kits Market for Ligase Independent Cloning, 2022-2035: Conservative, Base and Optimistic Scenarios (USD Million)
- Table 15.70. DNA Cloning Kits Market for PCR Cloning, 2022-2035: Conservative, Base and Optimistic Scenarios (USD Million)
- Table 15.71. DNA Cloning Kits Market for Seamless Cloning, 2022-2035: Conservative, Base and Optimistic Scenarios (USD Million)
- Table 15.72. DNA Cloning Kits Market for TA Cloning, 2022-2035: Conservative, Base and Optimistic Scenarios (USD Million)
- Table 15.73. DNA Cloning Kits Market for Other Methods, 2022-2035: Conservative, Base and Optimistic Scenarios (USD Million)
- Table 15.74. DNA Cloning Kits Market for Academic and Research Institutes, 2022-2035: Conservative, Base and Optimistic Scenarios (USD Million)
- Table 15.75. DNA Cloning Kits Market for Pharmaceutical and Biotechnology Companies, 2022-2035: Conservative, Base and Optimistic Scenarios (USD Million)
- Table 15.76. DNA Cloning Kits Market for Hospitals and Clinics, 2022-2035: Conservative, Base and Optimistic Scenarios (USD Million)
- Table 15.77. DNA Cloning Kits Market for Other End-users, 2022-2035: Conservative, Base and Optimistic Scenarios (USD Million)
- Table 15.78. DNA Cloning Kits Market, 2022 and 2035: Distribution by Geography (USD Million)
- Table 15.79. DNA Cloning Kits Market in North America, 2022-2035: Conservative, Base and Optimistic Scenarios (USD Million)
- Table 15.80. DNA Cloning Kits Market in Europe, 2022-2035: Conservative, Base and Optimistic Scenarios (USD Million)
- Table 15.81. DNA Cloning Kits Market in Asia-Pacific, 2022-2035: Conservative, Base and Optimistic Scenarios (USD Million)
- Table 15.82. DNA Cloning Kits Market in Middle East and North Africa, 2022-2035: Conservative, Base and Optimistic Scenarios (USD Million)
- Table 15.83. DNA Cloning Kits Market in Latin America, 2022-2035: Conservative, Base and Optimistic Scenarios (USD Million)
- Table 15.84. DNA Cloning Kits Market in Rest of the World, 2022-2035: Conservative, Base and Optimistic Scenarios (USD Million)
- Table 15.85. DNA Cloning Service Providers: Distribution by Year of Establishment
- Table 15.86. DNA Cloning Service Providers: Distribution by Company Size
- Table 15.87. DNA Cloning Service Providers: Distribution by Region of Headquarters
- Table 15.88. DNA Cloning Service Providers: Distribution by Location of Headquarters
- Table 15.89. DNA Cloning Service Providers: Distribution by Company Size and Region of Headquarters
List Of Companies
The following companies and organizations have been mentioned in the report:
- 1. AbFrontier
- 2. Applied Biological Materials
- 3. ABP Biosciences
- 4. Addgene
- 5. Agilent Technologies
- 6. America Diagnostics
- 7. Amid Biosciences
- 8. AMSBIO
- 9. Applied Biosystems
- 10. AstraZeneca
- 11. Biocompare
- 12. Bio-Fab Research
- 13. Bioline (acquired by Meridian Bioscience)
- 14. Biomiga
- 15. Bioneer
- 16. Bionics
- 17. Bio-Rad Laboratories
- 18. BioServ
- 19. BIOSS Centre for Biological Signalling Studies
- 20. Blue Heron Biotech (acquired by Eurofins Genomics)
- 21. Broad Institute
- 22. Boston University Medical Campus (BUMC)
- 23. Cambridge Bioscience
- 24. Canvax Biotech
- 25. Cellecta
- 26. Cellomics Technology
- 27. Charles River Laboratories
- 28. Chromous Biotech
- 29. Codex DNA
- 30. Columbia University
- 31. Columbia University Irving Medical Center (CUIMC)
- 32. Commonwealth Scientific and Industrial Research Organisation (CSIRO)
- 33. Creative Biogene
- 34. Creative Biolabs
- 35. Credora
- 36. Curia (formerly AMRI)
- 37. Cytiva
- 38. Dana-Farber Cancer Institute
- 39. Dow AgroSciences
- 40. Duke University
- 41. DuPont
- 42. Elpis Biotech
- 43. Enzynomics
- 44. Epoch Life Science
- 45. Eurofins
- 46. Exonbio
- 47. Fisher Scientific
- 48. Fraser International College (FIC)
- 49. Fred Hutchinson Cancer Research Center
- 50. Friedrich Miescher Institute for Biomedical Research
- 51. GCC Biotech
- 52. Gemini Biosciences
- 53. GENAXXON bioscience
- 54. GeneCust
- 55. Genei
- 56. geneOmbio Technologies
- 57. General Biosystems
- 58. Genentech
- 59. GENEWIZ
- 60. GenScript
- 61. Harvard University
- 62. Hologic
- 63. IBA Lifesciences
- 64. Innoprot
- 65. Intact Genomics
- 66. Invitrogen
- 67. J. Craig Venter Institute (JCVI)
- 68. Jackson Laboratory
- 69. Johns Hopkins University
- 70. Labtoo
- 71. Lamda Biotech
- 72. Leland Stanford Junior University
- 73. Massachusetts Institute of Technology
- 74. MedGenome
- 75. Merck
- 76. Meridian Bioscience
- 77. Molecular Cloning Laboratories (MCLAB)
- 78. Molecular Diagnostic Services
- 79. National Cancer Institute (NCI)
- 80. National Center for Advancing Translational Sciences (NCATS)
- 81. National Center for Complementary and Integrative Health (NCCIH)
- 82. National Human Genome Research Institute (NHGRI)
- 83. National Institute for Health and Medical Research (INSERM)
- 84. National Institute of Allergy and Infectious Diseases (NIAID)
- 85. National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS)
- 86. National Institute of Biomedical Imaging and Bioengineering (NIBIB)
- 87. National Institute of Child Health and Human Development (NICHD)
- 88. National Institute of Dental and Craniofacial Research (NIDCR)
- 89. National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK)
- 90. National Institute of Environmental Health Sciences (NIEHS)
- 91. National Institute of General Medical Sciences (NIGMS)
- 92. National Institute of Mental Health (NIMH)
- 93. National Institute of Neurological Disorders and Stroke (NINDS)
- 94. National Institute on Aging (NIA)
- 95. National Institute on Deafness and Other Communication Disorders (NIDCD)
- 96. National Institute on Drug Abuse (NIDA)
- 97. National Institute on Minority Health and Health Disparities (NIMHD)
- 98. New England Biolabs
- 99. Novozymes
- 100. National Heart, Lung, and Blood Institute (NHLBI)
- 101. Novozymes
- 102. Nuclear Energy Institute (NEI)
- 103. NZYTech
- 104. Office of the Director (OD)
- 105. OriCiro Genomics
- 106. OriGene Technologies
- 107. Patheon
- 108. Pioneer
- 109. Precision BioSciences
- 110. Promega
- 111. Protein Ark
- 112. QIAGEN
- 113. Quintara Biosciences
- 114. Roche
- 115. SBS Genetech
- 116. SciGenom Labs
- 117. Sentebiolab
- 118. Signosis
- 119. Stanford University
- 120. Synbio Technologies
- 121. Synovance
- 122. Takara Bio
- 123. Texas A&M Health Science Center
- 124. Thermo Fisher Scientific
- 125. Trenzyme
- 126. Twist Bioscience
- 127. Unity Lab Services
- 128. University of Alabama
- 129. University of California
- 130. University of Helsinki
- 131. University of Minnesota
- 132. University of North Carolina
- 133. University of Pennsylvania
- 134. University of Queensland
- 135. University of Vienna
- 136. University of Washington
- 137. Vazyme
- 138. VectorBuilder
- 139. Veterans Affairs (VA)
- 140. WZ Biosciences
- 141. Yale University
- 142. Zymerge
Title:
Synthetic Biology:
DNA Cloning Market by Type of Cloning Method (Blunt End Cloning, Ligase Independent Cloning, PCR Cloning, Seamless Cloning, TA Cloning and Other Methods), End-Users (Academic and Research Institutes, Pharmaceutical and Biotechnology Companies, Hospitals and Clinics, and Other End-Users) and Key Geographical Regions (North America, Europe, Asia Pacific, Latin America, and Middle East and North Africa): Industry Trends and Global Forecasts, 2022-2035.
Example Highlights:

Overview
The field of gene cloning remained a largely unexplored area until 1973, when A. C. Y. Chang, H. W. Boyer, R. B. Helling and Stanley N. Cohen reported that individual genes can be cloned and isolated by cleaving DNA enzymatically into DNA fragments. Over time, the evolution of genome engineering techniques has allowed for alterations in the genome of microorganisms, thereby enabling the production of substances having various research and therapeutic applications. DNA cloning, which is a highly regulated method, is widely acknowledged and employed in many laboratories throughout the world. Specifically, during the COVID-19 pandemic, several well-known pharmaceutical and biopharmaceutical companies, as well as synthetic biology market players, have stepped forward and contributed to the research and development of a variety of products, such as test kits, treatment solutions, and vaccines to combat the coronavirus infection using synthetic biology. Based on the requirement, a variety of approaches, such as traditional cloning, PCR cloning, ligation independent cloning, seamless cloning and recombinational cloning, can be used to clone the DNA. Despite several advancements in the field of synthetic biology, the DNA cloning process is associated with various challenges, such as requirement of large amounts of expensive vectors for cloning, different reagents and longer time duration for the completion of process.
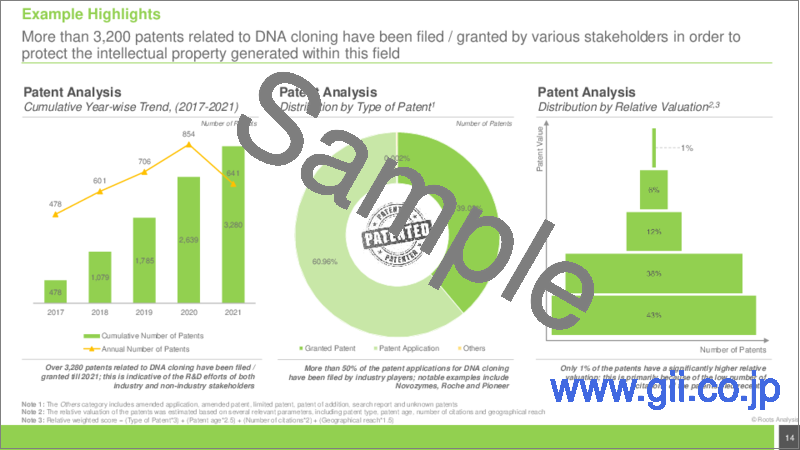
In order to overcome these drawbacks, innovators in the pharmaceutical industry have undertaken several efforts for identifying and developing ways to improve the process of DNA cloning. Among other alternatives, the use of DNA cloning kits has emerged as a viable option for various drug developers and academic / research institutes to overcome the challenges associated with the traditional DNA cloning methods. Presently, more than 250 DNA cloning kits are available in the market. These cloning kits are affordable, easy to use and produce high quality results in short duration. Further, more than 3,200 patents and 4,000 research articles have been published for DNA cloning technologies in the past few years; this is indicative of the innovative efforts of the stakeholders engaged in this domain. Driven by the increasing demand for gene therapies and the introduction of novel and advanced DNA cloning technologies, the DNA cloning market is anticipated to witness steady growth in the coming years.
Scope of the Report
The "Synthetic Biology: DNA Cloning Market, 2022-2035: Distribution by Type of Cloning Method (Blunt End Cloning, Ligase Independent Cloning, PCR Cloning, Seamless Cloning, TA Cloning and Other Methods), End-Users (Academic and Research Institutes, Pharmaceutical and Biotechnology Companies, Hospitals and Clinics, and Other End-Users) and Key Geographical Regions (North America, Europe, Asia Pacific, Latin America, and Middle East and North Africa): Industry Trends and Global Forecasts, 2022-2035" report features an extensive study of the current market landscape and future potential of DNA cloning kits and reagents over the next decade. The study presents an in-depth analysis, highlighting the capabilities of various stakeholders engaged in this domain, across different geographies. In addition to other elements, the report includes:
- A detailed assessment of the current market landscape of DNA cloning kits, featuring information on the kit components (enzyme mix, vector, buffer, ligase and primer), number of reactions (between 1-35, between 36-50 and more than 50), type of cloning method used (ligation independent cloning, TA cloning, blunt cloning and in-fusion seamless cloning), type of fragment(s) cloned (multiple fragments, long fragments and short oligonucleotides), cloning time (5 minutes, between 10-30 minutes, between 31-60 minutes and more than 60 minutes), overlaps recognized, efficacy (80-100%, between >90->99%, >=98%)and kit shelf life (between 1-10 months, between 11-20 months, more than 20 months) and kit price (1-500 USD, 501-1500 USD, 1501-2500 USD, more than 2500 USD). In addition to this, the chapter features information on DNA cloning kit providers and a detailed analysis based on several relevant parameters, such as year of establishment, company size, region of headquarters, location of headquarters, company size and region of headquarters and leading players (in terms of number of products).
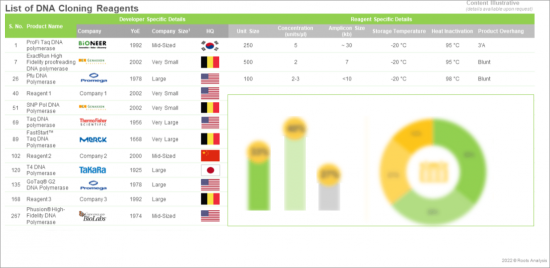
- A detailed assessment of the current market landscape of DNA cloning regents, featuring information on reagent unit size (less than 500, 500-2,000 and more than 2,000), reagent concentration (less than 5 units/µl, 5-50 units/µl and more than 50 units/µl) reagent components (polymerase, buffers and additional components), exonuclease activity (5'->3' exonuclease activity and 3'-> 5' exonuclease activity), product overhangs (3'A, blunt, 3', 5', 3'/blunt, 3'A/ blunt, 3'-dA and 5'/3'), storage temperature (-20°C,-30°C to -10 °C,-5 °C to -30 °C and -25 °C to -15 °C) and reagent price (less than USD 100, USD 100-500 and more than USD 500). In addition, the chapter features analysis related to DNA cloning reagents providers based on parameters, such as year of establishment, company size, region of headquarters, location of headquarters, company size and region of headquarters and leading players (in terms of number of products).
- Elaborate profiles of prominent players offering DNA cloning kits and reagents (shortlisted based on number of products), based in North America, Europe and Asia Pacific. Each profile features a brief overview of the company, details related to its financials (if available), DNA cloning kit portfolio, DNA cloning reagent portfolio, recent developments and an informed future outlook.
- An analysis of over 4,400 peer-reviewed scientific articles related to DNA cloning, published since 2018, based on several parameters, such as year of publication, type of article, type of publication, emerging focus areas, most popular publishers, most popular authors, and most popular journals.
- An analysis of more than 1,200 grants related to DNA cloning, since 2017, based on several parameters, such as year of grant, amount of grant, administrating institute center, support period, type of grant application, purpose of grant, activity code, study section awarded, emerging focus areas, most popular program officers, popular recipient organizations, popular recipient organizations and geographical distribution of recipient organizations.
- An in-depth analysis of over 3,400 patents that have been filed / granted for DNA cloning, between 2017-2021, based on multiple parameters, such as type of patent, publication year, application year, geography, CPC symbols, emerging focus areas, issuing authority involved, type of applicant, leading industry players, leading non-industry players, leading patent assignees, patent benchmarking analysis, patent characteristics and geography, patent age. It also includes a detailed patent valuation analysis and information on the leading patents.
- A case study on the DNA cloning service providers, featuring information and detailed analysis based on their year of establishment, company size, location of headquarters, region of headquarters and company size and region of headquarters.
- A case study on the general overview of advancements in DNA cloning, covering details related to the current and future trends in the domain.
One of the key objectives of the report was to estimate the future growth potential of synthetic biology: DNA cloning (kits and reagents) market, over the coming decade. We have provided informed estimates on the financial evolution of the market for the period 2022-2035. For this purpose, we have segmented the future opportunity across types of cloning methods (blunt end cloning, ligase independent cloning, PCR cloning, seamless cloning, TA cloning and other methods), end-users (academic and research institutes, pharmaceutical and biotechnology companies, hospitals and clinics, and other end-users) and key geographical regions (North America, Europe, Asia Pacific, Latin America, and Middle East and North Africa). In order to account for uncertainties and to add robustness to our model, we have provided three market forecast scenarios, portraying the conservative, base and optimistic tracks of the anticipated industry's growth.
The opinions and insights presented in the report were influenced by survey inputs from various experienced stakeholders in the industry. The report features detailed insights of respondents who participated in the study (in alphabetical order):
- Jesús C. Morales (Business Development Manager, Canvax Biotech)
- Stephen Lan (Vice President, SBS Genetech)
All actual figures have been sourced and analyzed from publicly available information forums and primary research discussions. Financial figures mentioned in this report are in USD, unless otherwise specified.
Key Questions Answered:
- Who are the key players engaged in the development of DNA cloning kits and reagents?
- What is the focus area of various publications related to DNA cloning?
- Which research institutes have received relatively more grants for projects related to DNA cloning?
- How has the intellectual property landscape of DNA cloning evolved over the last several years?
- Which region(s) are likely to occupy the maximum market share in DNA cloning kits market?
- Which factors are likely to influence the evolution of this market?
- How is the current and future market opportunity related to DNA cloning kits likely to be distributed across key market segments?
- Who are the key players offering services related to DNA cloning?
Chapter Outlines
Chapter 2 is an executive summary of the key insights captured during our research. It offers a high-level view on the likely evolution of the DNA cloning (kits and reagents) market in the mid to long term.
Chapter 3 provides a general overview of DNA cloning including information on the methods of DNA cloning. It also provides details on the types of DNA cloning kits and reagents (PCR cloning kits, TOPO cloning kits). In addition, the chapter discusses various advantages, applications, associated challenges and future perspectives of DNA cloning.
Chapter 4 provides an overview of the current market landscape of DNA cloning kits, based on several relevant parameters, such as information on the kit components (enzyme mix, vector, buffer, ligase and primer), number of reactions (between 1-35, between 36-50 and more than 50), type of cloning methods used (ligation independent, TA, blunt and in-fusion seamless cloning), type of fragment(s) cloned (multiple fragments, long fragments and short oligonucleotides), cloning time (5 minutes, between 10-30 minutes, between 31-60 minutes and more than 60 minutes), overlaps recognized, efficacy (80-100%, between >90->99%, >=98%) and kit shelf life (between 1-10 months, between 11-20 months, more than 20 months) and kit price (1-500 USD, 501-1500 USD, 1501-2500 USD and more than 2500 USD). In addition, the chapter features information on DNA cloning kit providers and a detailed analysis based on several relevant parameters, such as year of establishment, company size, region of headquarters, location of headquarters, company size and region of headquarters and leading players (in terms of number of products).
Chapter 5 provides an overview of the current market landscape of DNA cloning reagents, based on several relevant parameters, such as reagent unit size (less than 500, 500-2,000 and more than 2,000), reagent concentration (less than 5 units/µ, 5-50 units/ and more than 50 units/µ) reagent components (polymerase, buffers and additional components), exonuclease activity (5'->3' exonuclease activity and 3'-> 5' exonuclease activity), product overhangs (3'A, blunt, 3', 5', 3'/blunt, 3'A/ blunt, 3'-dA and 5'/3'), storage temperature (-20°C,-30°C to -10 °C,-5 °C to -30 °C and -25 °C to -15 °C) and reagent price (less than USD 100, USD 100-500 and more than USD 500). In addition, the chapter features analysis related to DNA cloning reagents providers based on parameters, such as year of establishment, company size, region of headquarters, location of headquarters, company size and region of headquarters and leading players (in terms of number of products).
Chapter 6 includes elaborate profiles of prominent players offering DNA cloning kits and reagents (shortlisted based on number of products), based in North America, Europe and Asia Pacific. Each profile features a brief overview of the company, details related to its financials (if available), DNA cloning kit portfolio, DNA cloning reagent portfolio, recent developments and an informed future outlook.
Chapter 7 features a detailed review of over 4,400 peer-reviewed scientific articles related to DNA cloning, published since 2018, based on several parameters, such as year of publication, type of article, type of publication, emerging focus areas, most popular publishers, most popular authors publishers, and most popular journals.
Chapter 8 provides an in-depth analysis of grants that have been awarded to various research institutes for projects related to DNA cloning, during 2017-2022, on the basis of important parameters, such as year of grant, amount of grant, administrating institute center, support period, type of grant application, purpose of grant, activity code, study section awarded, emerging focus areas, most popular program officers, popular recipient organizations, popular recipient organizations. This chapter also includes geographical distribution of recipient organizations.
Chapter 9 features an in-depth analysis of the patents that have been filed / granted for DNA cloning, between 2017-2021, on the basis of various relevant parameters, such as type of patent, publication year, application year, geography, CPC symbols, emerging focus areas, issuing authority involved, type of applicant, leading industry players, leading non- industry players, leading patent assignees, patent benchmarking analysis, patent characteristics and geography, patent age In addition, it includes a detailed patent valuation analysis and leading patents.
Chapter 10 presents an insightful market forecast analysis, highlighting the future potential of the DNA cloning kits market till the year 2035. In order to provide a detailed future outlook, our projections have been segmented across types of cloning methods (blunt end cloning, ligase independent cloning, PCR cloning, seamless cloning, TA cloning and other methods), End-User (academic and research institutes, pharmaceutical and biotechnology companies, hospitals and clinics and other end-users), key geographical regions (North America, Europe and Asia-Pacific, Latin America and, Middle East and North Africa).
Chapter 11 presents a case study of the DNA cloning service providers. The chapter features information and detailed analysis on their year of establishment, company size, location of headquarters, region of headquarters and company size and region of headquarters.
Chapter 12 is a case study on the evolution of DNA cloning, providing an includes overview on the advancements in DNA cloning with the innovation of Xenobots- Novel Living Machines. It also discusses various applications of xenobots along with its ethical issues and future perspectives.
Chapter 13 is a summary of the overall report, presenting the insights on the market trends and the likely evolution of the DNA cloning kits and reagents market.
Chapter 14 is a collection of survey inputs from various experienced stakeholders in the industry. In this chapter, we have presented detailed insights of respondents who participated in the study.
Chapter 15 is an appendix that contains tabulated data and numbers for all the figures in the report.
Chapter 16 is an appendix that provides the list of companies and organizations mentioned in the report.
TABLE OF CONTENTS
1. PREFACE
- 1.1. Scope of the Report
- 1.2. Research Methodology
- 1.3. Key Questions Answered
- 1.4. Chapter Outlines
2. EXECUTIVE SUMMARY
3. INTRODUCTION
- 3.1. Chapter Overview
- 3.2. Introduction to DNA Cloning
- 3.3. Methods of DNA Cloning
- 3.3.1. Restriction Enzyme Based Cloning
- 3.3.2. PCR Cloning
- 3.3.3. Ligation Independent Cloning
- 3.3.4. TA Cloning
- 3.3.5. Seamless Cloning
- 3.3.6. Recombinational Cloning
- 3.4. DNA Cloning Kits
- 3.4.1. PCR Cloning Kits
- 3.4.2. TOPO Cloning Kits
- 3.5. DNA Cloning Reagents
- 3.5.1. Taq Polymerase
- 3.5.2. Hot-Start DNA Polymerase
- 3.5.3. Processivity-Enhancing Domain
- 3.6. DNA Cloning Tools
- 3.6.1. Advantages of Cloning tools
- 3.7. Challenges associated with DNA Cloning
- 3.8. Applications of DNA Cloning
- 3.8.1. Medicinal Applications
- 3.8.2. Agricultural Applications
- 3.8.3. Gene Therapy
- 3.8.4. Genomic Library
- 3.9. Future Perspectives
4. MARKET LANDSCAPE: DNA CLONING KITS
- 4.1. Chapter Overview
- 4.2. DNA Cloning Kits: Overall Market Landscape
- 4.2.1 Analysis by Kit Components
- 4.2.2. Analysis by Number of Reactions
- 4.2.3. Analysis by Type of Cloning Method Used
- 4.2.4 Analysis by Type of Fragment(s) Cloned
- 4.2.5. Analysis by Cloning Time
- 4.2.6. Analysis by Overlaps Recognized
- 4.2.7. Analysis by Efficacy
- 4.2.8. Analysis by Kit Shelf Life
- 4.2.9. Analysis by Kit Price
- 4.3. DNA Cloning Kits: Developer Landscape
- 4.3.1. Analysis by Year of Establishment
- 4.3.2. Analysis by Company Size
- 4.3.3. Analysis by Region of Headquarters
- 4.3.4. Analysis by Location of Headquarters
- 4.3.5. Analysis by Company Size and Region of Headquarters
- 4.3.6. Leading Players: Analysis by Number of DNA Cloning Kits
5. MARKET LANDSCAPE: DNA CLONING REAGENTS
- 5.1. Chapter Overview
- 5.2. DNA Cloning Reagents: Overall Market Landscape
- 5.2.1. Analysis by Unit Size
- 5.2.2. Analysis by Reagent Concentration
- 5.2.3. Analysis by Reagent Components
- 5.2.4. Analysis by Exonuclease Activity
- 5.2.5. Analysis by Product Overhangs
- 5.2.6. Analysis by Storage Temperature
- 5.2.7. Analysis by Reagent Price
- 5.3. DNA Cloning Reagents: Developer Landscape
- 5.3.1. Analysis by Year of Establishment
- 5.3.2. Analysis by Company Size
- 5.3.3. Analysis by Region of Headquarters
- 5.3.4. Analysis by Location of Headquarters
- 5.3.5. Analysis by Company Size and Region of Headquarters
- 5.3.6. Leading Players: Analysis by Number of DNA Cloning Reagents
6. DNA CLONING KITS AND REAGENTS: COMPANY PROFILES
- 6.1. Chapter Overview
- 6.2. Thermo Fisher Scientific
- 6.2.1. Company Overview
- 6.2.2. Financial Information
- 6.2.3. DNA Cloning Kit Portfolio
- 6.2.4. DNA Cloning Reagent Portfolio
- 6.2.5. Recent Developments and Future Outlook
- 6.3. Merck
- 6.3.1. Company Overview
- 6.3.2. Financial Information
- 6.3.3. DNA Cloning Kit Portfolio
- 6.3.4. DNA Cloning Reagent Portfolio
- 6.3.5. Recent Developments and Future Outlook
- 6.4. Takara Bio
- 6.4.1. Company Overview
- 6.4.2. Financial Information
- 6.4.3. DNA Cloning Kit Portfolio
- 6.4.4. DNA Cloning Reagent Portfolio
- 6.4.5. Recent Developments and Future Outlook
- 6.5. Vazyme
- 6.5.1. Company Overview
- 6.5.2. DNA Cloning Kit Portfolio
- 6.5.3. Recent Developments and Future Outlook
- 6.6. GenScript
- 6.6.1. Company Overview
- 6.6.2. Financial Information
- 6.6.3. DNA Cloning Kit Portfolio
- 6.6.4. Recent Developments and Future Outlook
- 6.7. Promega
- 6.7.1. Company Overview
- 6.7.2. DNA Cloning Reagent Portfolio
- 6.7.3. Recent Developments and Future Outlook
- 6.8. Agilent Technologies
- 6.8.1. Company Overview
- 6.8.2. Financial Information
- 6.8.3. DNA Cloning Reagent Portfolio
- 6.8.4. Recent Developments and Future Outlook
- 6.9. Bio-Rad
- 6.9.1. Company Overview
- 6.9.2. Financial Information
- 6.9.3. DNA Cloning Reagent Portfolio
- 6.9.4. Recent Developments and Future Outlook
7. PUBLICATION ANALYSIS
- 7.1. Chapter Overview
- 7.2. Scope and Methodology
- 7.2.1. Analysis by Year of Publication
- 7.2.2. Analysis by Type of Article
- 7.2.3. Analysis by Type of Publication
- 7.2.4. Word Cloud: Emerging Focus Areas
- 7.2.5. Popular Publishers: Analysis by Number of Publications
- 7.2.6. Popular Journals: Analysis by Number of Publications
- 7.2.7. Popular Journals: Analysis of Journal Impact Factor
- 7.2.8. Analysis by Geography
8. GRANT ANALYSIS
- 8.1. Chapter Overview
- 8.2. Scope and Methodology
- 8.2.1. Analysis by Year of Grant Award
- 8.2.2. Analysis by Amount Awarded
- 8.2.3. Analysis by Administering Institute Center
- 8.2.4. Analysis by Support Period
- 8.2.5. Analysis by Administering Institute Center and Support Period
- 8.2.6. Analysis by Type of Grant Application
- 8.2.7. Analysis by Purpose of Grant
- 8.2.8. Analysis by Purpose of Grant and Amount Awarded
- 8.2.9. Analysis by Activity Code
- 8.2.10. Analysis by Study Section Involved
- 8.2.11. Word Cloud: Emerging Focus Areas
- 8.2.12. Prominent Program Officers: Analysis by Number of Grants
- 8.2.13. Popular Recipient Organizations: Analysis by Number of Grants
- 8.2.14. Popular Recipient Organizations: Analysis by Amount Awarded
- 8.2.15. Grant Analysis: Geographical Distribution of Recipient Organizations
9. PATENT ANALYSIS
- 9.1. Chapter Overview
- 9.2. Scope and Methodology
- 9.2.1. Analysis by Type of Patent
- 9.2.2. Analysis by Publication Year
- 9.2.3. Analysis by Application Year
- 9.2.4. Analysis by Geography
- 9.2.5. Analysis by CPC Symbols
- 9.2.6. Word Cloud: Emerging Focus Areas
- 9.2.7. Analysis by Type of Organization
- 9.2.8. Leading Industry Players: Analysis by Number of Patents
- 9.2.9. Leading Non-Industry Players: Analysis by Number of Patents
- 9.2.10. Leading Patent Assignees: Analysis by Number of Patents
- 9.2.11. DNA Cloning: Patent Benchmarking Analysis
- 9.2.12. Analysis by Patent Characteristics
- 9.2.13. Analysis by Patent Age
- 9.2.14. DNA Cloning: Patent Valuation Analysis
- 9.2.15 Leading Patents by Number of Citations
10. MARKET FORECAST AND OPPORTUNITY ANALYSIS
- 10.1. Chapter Overview
- 10.2. Forecast Methodology and Key Assumptions
- 10.3. Global DNA Cloning Kits Market, 2022-2035
- 10.3.1. DNA Cloning Kits Market, 2022- 2035: Distribution by Type of Cloning Method
- 10.3.1.1. DNA Cloning Kits Market for Blunt End Cloning, 2022-2035
- 10.3.1.2. DNA Cloning Kits Market for Ligase Independent Cloning, 2022-2035
- 10.3.1.3. DNA Cloning Kits Market for PCR Cloning, 2022-2035
- 10.3.1.4. DNA Cloning Kits Market for Seamless Cloning, 2022-2035
- 10.3.1.5. DNA Cloning Kits Market for TA Cloning, 2022-2035
- 10.3.1.6. DNA Cloning Kits Market for Other Methods, 2022-2035
- 10.3.2. DNA Cloning Kits Market, 2022 and 2035: Distribution by End User
- 10.3.2.1. DNA Cloning Kits Market for Academic and Research Institutes, 2022-2035
- 10.3.2.2. DNA Cloning Kits Market for Pharmaceutical and Biotechnology Companies, 2022-2035
- 10.3.2.3. DNA Cloning Kits Market for Hospitals and Clinics, 2022-2035
- 10.3.2.4. DNA Cloning Kits Market for Other End-users, 2022-2035
- 10.3.3. DNA Cloning Kits Market: Distribution by Key Geographical Regions, 2022-2035
- 10.3.3.1. DNA Cloning Kits Market in North America, 2022-2035
- 10.3.3.2. DNA Cloning Kits Market in Europe, 2022-2035
- 10.3.3.3. DNA Cloning Kits Market in Asia-Pacific, 2022-2035
- 10.3.3.4. DNA Cloning Kits Market in Latin America, 2022-2035
- 10.3.3.5. DNA Cloning Kits Market in Middle East and North Africa, 2022-2035
- 10.3.1. DNA Cloning Kits Market, 2022- 2035: Distribution by Type of Cloning Method
11. CASE STUDY: DNA CLONING SERVICE PROVIDERS
- 11.1. Chapter Overview
- 11.2 DNA Cloning Service Providers: List of Players
- 11.2.1. Analysis by Year of Establishment
- 11.2.2. Analysis by Company Size
- 11.2.3. Analysis by Region of Headquarters
- 11.2.4. Analysis by Location of Headquarters
- 11.2.5. Analysis by Company Size and Region of Headquarters
12. EVOLUTION OF DNA CLONING
- 12.1. Chapter Overview
- 12.2. Introduction of AI in Synthetic Biology
- 12.2.1. Xenobots- Novel Living Machines
- 12.2.2. Applications of Xenobots
- 12.2.3. Ethical Issues
- 12.2.4. Future of Xenobots
- 12.3. Conclusion
13. CONCLUDING REMARKS
14. EXECUTIVE INSIGHTS
- 14.1. Chapter Overview
- 14.2. SBS Genetech
- 14.2.1. Company Snapshot
- 14.2.2. Interview Transcript: Stephen Lan, Vice President
- 14.3. Canvax Biotech
- 14.3.1. Company Snapshot
- 14.3.2. Interview Transcript: Jesús C. Morales, Business Development Manager
15. APPENDIX 1: TABULATED DATA
16. APPENDIX 2: LIST OF COMPANIES AND ORGANIZATIONS
List Of Figures
- Figure 2.1. Executive Summary: Market Forecast
- Figure 2.2. Executive Summary: DNA Cloning Kits Market Landscape
- Figure 2.3. Executive Summary: DNA Cloning Reagents Market Landscape
- Figure 2.4. Executive Summary: Publications Analysis
- Figure 2.5. Executive Summary: Grant Analysis
- Figure 2.6. Executive Summary: Patent Analysis
- Figure 3.1. Mechanism of Restriction Enzyme Based Cloning
- Figure 3.2. Mechanism of PCR Cloning
- Figure 3.3. Mechanism of Ligation-Independent Cloning
- Figure 3.4. Mechanism of TA Cloning
- Figure 3.5. Mechanism of Seamless Cloning
- Figure 3.6. Mechanism of Recombinational Cloning
- Figure 3.7. Applications of DNA Cloning
- Figure 4.1. DNA Cloning Kits: Distribution by Kit Components
- Figure 4.2. DNA Cloning Kits: Distribution by Number of Reactions
- Figure 4.3. DNA Cloning Kits: Distribution by Type of Cloning Method Used
- Figure 4.4. DNA Cloning Kits: Distribution by Type of Fragment(s) Cloned
- Figure 4.5. DNA Cloning Kits: Distribution by Cloning Time
- Figure 4.6. DNA Cloning Kits: Distribution by Overlaps Recognized
- Figure 4.7. DNA Cloning Kits: Distribution by Efficacy
- Figure 4.8. DNA Cloning Kits: Distribution by Kit Shelf Life
- Figure 4.9. DNA Cloning Kits: Distribution by Kit Price
- Figure 4.10. DNA Cloning Kit Providers: Distribution by Year of Establishment
- Figure 4.11. DNA Cloning Kit Providers: Distribution by Company Size
- Figure 4.12. DNA Cloning Kit Providers: Distribution by Region of Headquarters
- Figure 4.13. DNA Cloning Kit Providers: Distribution by Location of Headquarters
- Figure 4.14. DNA Cloning Kit Providers: Distribution by Company Size and Region of Headquarters
- Figure 4.15. Leading Players: Distribution by Number of DNA Cloning Kits
- Figure 5.1. DNA Cloning Reagents: Distribution by Unit Size
- Figure 5.2. DNA Cloning Reagents: Distribution by Reagent Concentration (units/µl)
- Figure 5.3. DNA Cloning Reagents: Distribution by Reagent Components
- Figure 5.4. DNA Cloning Reagents: Distribution by Exonuclease Activity
- Figure 5.5. DNA Cloning Reagents: Distribution by Product Overhangs
- Figure 5.6. DNA Cloning Reagents: Distribution by Storage Temperature
- Figure 5.7. DNA Cloning Reagents: Distribution by Reagent Price
- Figure 5.8. DNA Cloning Reagent Providers: Distribution by Year of Establishment
- Figure 5.9. DNA Cloning Reagent Providers: Distribution by Company Size
- Figure 5.10. DNA Cloning Reagent Providers: Distribution by Region of Headquarters
- Figure 5.11. DNA Cloning Reagent Providers: Distribution by Location of Headquarters
- Figure 5.12. DNA Cloning Reagent Providers: Distribution by Company Size and Region of Headquarters
- Figure 5.13. Leading Players: Distribution by Number of Reagents
- Figure 6.1. Thermo Fisher Scientific: Annual Revenues, 2017-2021 (USD Billion)
- Figure 6.2. Merck: Annual Revenues, 2017- 2021 (EUR Billion)
- Figure 6.3. Takara Bio: Annual Revenues, 2017-9M 2022 (JPY Billion)
- Figure 6.4. GenScript: Annual Revenues, 2016- 2021 (USD Billion)
- Figure 6.5. Agilent Technologies: Annual Revenues, 2017- 1M 2022 (USD Billion)
- Figure 6.6. Bio-Rad: Annual Revenues, 2017- 2021 (USD Billion)
- Figure 7.1. Publication Analysis: Cumulative Year-wise Trend, 2018-2022 (till January)
- Figure 7.2. Publication Analysis: Distribution by Type of Article
- Figure 7.3. Publication Analysis: Distribution by Type of Publication
- Figure 7.4. Word Cloud: Key focus Areas
- Figure 7.5. Popular Publishers: Distribution by Number of Publications
- Figure 7.6. Popular Journals: Distribution by Number of Publications
- Figure 7.7. Popular Journals: Distribution by Journal Impact Factor
- Figure 7.8. Publication Analysis: Distribution by Geography
- Figure 8.1. Grant Analysis: Cumulative Year-Wise Trend, 2017-2022 (till January)
- Figure 8.2. Grant Analysis: Distribution by Amount Awarded (USD Million), 2017-2022 (till January)
- Figure 8.3. Grant Analysis: Distribution by Administering Institute Center
- Figure 8.4. Grant Analysis: Distribution by Support Period
- Figure 8.5. Grant Analysis: Distribution by Administering Institute Center and Support Period
- Figure 8.6. Grant Analysis: Distribution by Type of Grant Application
- Figure 8.7. Grant Analysis: Distribution by Purpose of Grant Award
- Figure 8.8. Grant Analysis: Distribution of Amount Awarded by Purpose of Grant
- Figure 8.9. Grant Analysis: Distribution by Activity Code
- Figure 8.10. Grant Analysis: Distribution by Study Section
- Figure 8.11. Word Cloud: Emerging Focus Areas
- Figure 8.12. Popular program officers: Distribution by Number of Grants
- Figure 8.13. Popular Recipient Organizations: Distribution by Number of Grants
- Figure 8.14. Popular Recipient Organization: Distribution by Amount Awarded (USD Million)
- Figure 8.15. Grant Analysis: Distribution by Location of Recipient Organizations
- Figure 9.1. Patent Analysis: Distribution by Type of Patent
- Figure 9.2. Patent Analysis: Cumulative Distribution by Publication Year, 2017-2021
- Figure 9.3. Patent Analysis: Cumulative Distribution by Application Year, 2017-2022
- Figure 9.4. Patent Analysis: Distribution by Geographical Location
- Figure 9.5. Patent Analysis: Distribution by CPC Symbols
- Figure 9.6. Word Cloud: Emerging Focus Areas
- Figure 9.7. Patent Analysis: Cumulative Year-wise Distribution by Type of Organization, 2017-2021
- Figure 9.8. Leading Industry Players: Distribution by Number of Patents
- Figure 9.9. Leading Non-Industry Players: Distribution by Number of Patents
- Figure 9.10. Leading Individual Assignees: Distribution by Number of Patents
- Figure 9.11. Leading Players: Benchmarking by Patent Characteristics (CPC Symbols)
- Figure 9.12. Patent Analysis: Year-wise Distribution of Patents by Age, 2001-2021
- Figure 9.13. DNA Cloning: Patent Valuation Analysis
- Figure 10.1. Global DNA Cloning Kits Market, 2022-2035 (USD Million)
- Figure 10.2. DNA Cloning Kits: Likely Growth Scenarios
- Figure 10.3. DNA Cloning Kits Market: Distribution by Type of Cloning Method, 2022 and 2035
- Figure 10.4. DNA Cloning Kits Market for Blunt End Cloning, 2022-2035 (USD Million)
- Figure 10.5. DNA Cloning Kits Market for Ligase Independent Cloning, 2022-2035 (USD Million)
- Figure 10.6. DNA Cloning Kits Market for PCR Cloning, 2022-2035 (USD Million)
- Figure 10.7. DNA Cloning Kits Market for Seamless Cloning, 2022-2035 (USD Million)
- Figure 10.8. DNA Cloning Kits Market for TA Cloning, 2022-2035 (USD Million)
- Figure 10.9. DNA Cloning Kits Market for Other Methods, 2022-2035
- Figure 10.10. DNA Cloning Kits Market: Distribution by End User, 2022- 2035 (USD Million)
- Figure 10.11. DNA Cloning Kits Market for Academic and Research Institutes, 2022-2035 (USD Million)
- Figure 10.12. DNA Cloning Kits Market for Pharmaceutical and Biotechnology Companies, 2021-2035 (USD Million)
- Figure 10.13. DNA Cloning Kits Market for Hospitals and Clinics, 2022-2035 (USD Million)
- Figure 10.14. DNA Cloning Kits Market for Other End-users, 2022-2035 (USD Million)
- Figure 10.15. DNA Cloning Kits: Distribution by Geography, 2022 and 2035
- Figure 10.16. DNA Cloning Kits Market in North America, 2022-2035 (USD Million)
- Figure 10.17. DNA Cloning Kits Market in Europe, 2022-2035 (USD Million)
- Figure 10.18. DNA Cloning Kits Market in Asia Pacific, 2022-2035 (USD Million)
- Figure 10.19. DNA Cloning Kits Market in Latin America, 2022-2035 (USD Million)
- Figure 10.20. DNA Cloning Kits Market in Middle East and North Africa, 2022-2035 (USD Million)
- Figure 11.1. DNA Cloning Service Providers: Distribution by Year of Establishment
- Figure 11.2. DNA Cloning Service Providers: Distribution by Company Size
- Figure 11.3. DNA Cloning Service Providers: Distribution by Region of Headquarters
- Figure 11.4. DNA Cloning Service Providers: Distribution by Location of Headquarters
- Figure 11.5. DNA Cloning Service Providers: Distribution by Company Size and Region of Headquarters
- Figure 12.1. Future Applications of Xenobots
- Figure 13.1. Concluding Remarks: DNA Cloning Kits Market Landscape
- Figure 13.2. Concluding Remarks: DNA Cloning Reagents Market Landscape
- Figure 13.3. Concluding Remarks:Grant Analysis
- Figure 13.4. Concluding Remarks: Publications Analysis
- Figure 13.5. Concluding Remarks: Patent Analysis
- Figure 13.6. Concluding Remarks: Market Forecast and Opportunity Analysis