|
|
市場調査レポート
商品コード
1160818
腫瘍溶解性ウイルス免疫療法の世界市場・治験の考察 (2028年)Global Oncolytic Virus Immunotherapy Market & Clinical Trials Insight 2028 |
||||||
| 腫瘍溶解性ウイルス免疫療法の世界市場・治験の考察 (2028年) |
|
出版日: 2022年11月01日
発行: KuicK Research
ページ情報: 英文
納期: 即日から翌営業日
|
- 全表示
- 概要
- 図表
- 目次
世界のがん免疫療法市場は、幅広い活用領域を包含しており、その中でも、腫瘍溶解性ウイルス免疫療法は、市場全体の発展において重要な役割を担っています。研究者たちは、宿主細胞を標的とするウイルスの利点を導入することで、基本的な免疫療法戦略を修正することに成功しています。宿主細胞に取り込まれるウイルスの優れた能力を超えて、がん治療薬におけるウイルスの治療的導入は、長期にわたってがん治療薬市場全体で何らかの形で欠けていた膨大な量の機会を提供しています。今後、幅広い種類のがんに対する腫瘍溶解性ウイルス治療が承認されれば、製薬業界は強化され、これまでどのがん治療薬も記録したことのない売上高に到達すると考えられます。
当レポートでは、世界の腫瘍溶解性ウイルス免疫療法の市場・治験動向について分析し、製品の概要や主な用途、全体的な市場規模の動向見通し、現在治験中の製品の概略 (全180種以上)、主要製品の入手可能性・投与量・価格水準の見通し、治験の全体的な進展状況、昨今の資本取引の動き、主要企業のプロファイル、といった情報を取りまとめてお届けいたします。
目次
第1章 腫瘍溶解性ウイルスのイ概略:創世記から生物遺伝学への道のり
第2章 悪性腫瘍におけるウイルス療法:概略
- がんと対峙する腫瘍溶解性ウイルス
- 腫瘍細胞を標的とするアプローチ
- 腫瘍溶解性ウイルスの作用機序
第3章 腫瘍溶解性ウイルス免疫療法の概要
- 抗腫瘍免疫応答の刺激
- がんワクチンとしての腫瘍溶解性ウイルス
第4章 がんに対する腫瘍溶解性ウイルス免疫療法の可能性
- 乳がん
- 肺がん
- 前立腺がん
- 黒色腫 (メラノーマ)
- 脳腫瘍
- 血液がん
- 大腸がん
- 膀胱がん
- 膵臓がん
- 頭頸部がん
第5章 世界の腫瘍溶解性ウイルス免疫療法市場:概要
- 腫瘍溶解性ウイルスのアーケードに向けての序文
- 承認された腫瘍溶解性ウイルスの市場的側面
第6章 Imlygic (Talimogene laherparepvec):臨床・価格設定に関する考察
- 臨床概要
- 入手可能性、投与量、価格の分析
第7章 Oncorine (H101):臨床・価格設定に関する考察
- 臨床概要
- 入手可能性、投与量、価格の分析
第8章 Delytact (DS 1647):臨床・価格設定に関する考察
- 臨床概要
- 入手可能性、投与量、価格の分析
第9章 世界の腫瘍溶解性ウイルス免疫療法の臨床パイプライン:概要
- 相別 (フェーズ別)
- 企業別
- 国別
- 適応症別
- 患者セグメント別
第10章 世界の腫瘍溶解性ウイルスの臨床パイプライン:企業別・適応症別・段階別
- 研究
- 前臨床
- 第I相
- 第I/II相
- 第II相
- 第III相
- 登録済み
第11章 腫瘍溶解性ウイルス療法市場を前進させるための戦略的提携・協力、企業合併・買収 (M&A)
第12章 世界の腫瘍溶解性ウイルス免疫療法市場力学
- 有利な市場パラメータ
- 成長阻害剤
第13章 世界の腫瘍溶解性ウイルス免疫療法市場の将来の見通し
第14章 競合情勢
- AdCure Bio
- Beijing SyngenTech
- BioVex Inc.
- Calidi Biotherapeutics
- Genelux Corporation
- Immvira Pharma
- Jennerex Biotherapeutics
- KaliVir
- Lokon Pharma
- Merck
- Oncolys BioPharma
- Oncorus
- PsiOxus Therapeutics
- Seneca Therapeutics
- Shanghai Sunway Biotech
- Takara Bio
- Targovax
- TILT Biotherapeutics
- Transgene
- Virogin Biotech
List of Figures
- Figure 1-1: Demonstration of Categorization of Oncolytic Viruses
- Figure 1-2: Illustration of Major Events in Clinical Virotherapy
- Figure 2-1: Potential Advantages of Oncolytic Viruses over Conventional Therapies
- Figure 2-2: The Diagrammatic Representation of Entry of Virions into Cancerous cells
- Figure 2-3: Route of Anti-tumoral Efficacy of Oncolytic Viruses
- Figure 2-4: Mechanism of Tumor Destruction by Oncolytic Viruses
- Figure 2-5: Categorization Followed by Oncolytic Viruses for Tumor Selectivity
- Figure 3-1: Demonstration of Phases of Immune Responses Elicited by the Oncolytic Viruses
- Figure 3 2: Categorization of Oncolytic Viruses over the Basis of their Behavior
- Figure 3 3: Diagrammatic Representation of Oncolysis Shown by Reovirus
- Figure 3 4: Mechanism of Oncolytic Behavior of Adenoviruses
- Figure 4-1: Selectively Killing of Prostate Cancer Cells by Oncolytic M-Protein Strains of VSV
- Figure 4-2: Proposed Treatment of Oncolytic Viruses for Hematological Malignancies
- Figure 5-1: Global - Oncolytic Virus Immunotherapy Market Opportunity (US$ Million), 2021 -2028
- Figure 6-1: Imlygic - US Patent Expiration Year
- Figure 6-2: Imlygic - Price of 1 Million PUF/ml & 100 Million PUF/ml Injectable Suspension (US$), November'2022
- Figure 6-3: Imlygic - Dose for Initial Treatment Cycle & Subsequent Treatment Cycle (Million PFU/ ml), November'2022
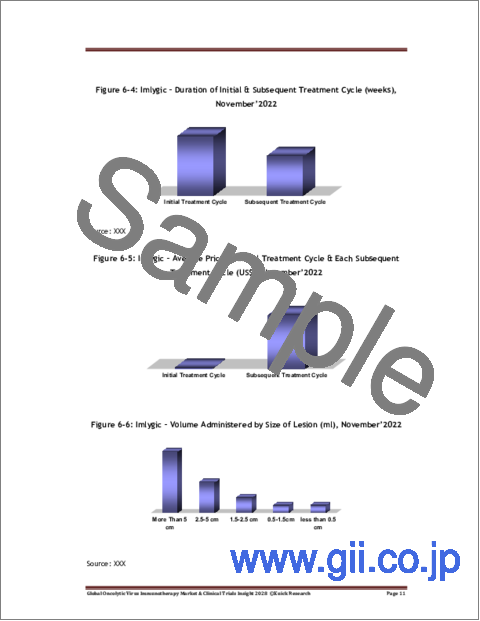
- Figure 6-4: Imlygic - Duration of Initial & Subsequent Treatment Cycle (weeks), November'2022
- Figure 6-5: Imlygic - Average Price of Initial Treatment Cycle & Each Subsequent Treatment Cycle (US$), November'2022
- Figure 6-6: Imlygic - Volume Administered by Size of Lesion (ml), November'2022
- Figure 7-1: Oncorine - Cost of Single Dose & Single Treatment Course (US$), September'2020
- Figure 7-2: Oncorine - Duration of Treatment Cycle by Type of Cancer (Days), November'2022
- Figure 8-1: Delytact - Initial & Present Price (US$), November'2022
- Figure 8-2: Delytact - Designations
- Figure 8-3: Japan - Malignant Gliomas Incidences, 2022-2026
- Figure 9-1: Oncolytic Virus Immunotherapy Clinical Trials by Phase (Numbers), 2022 till 2028
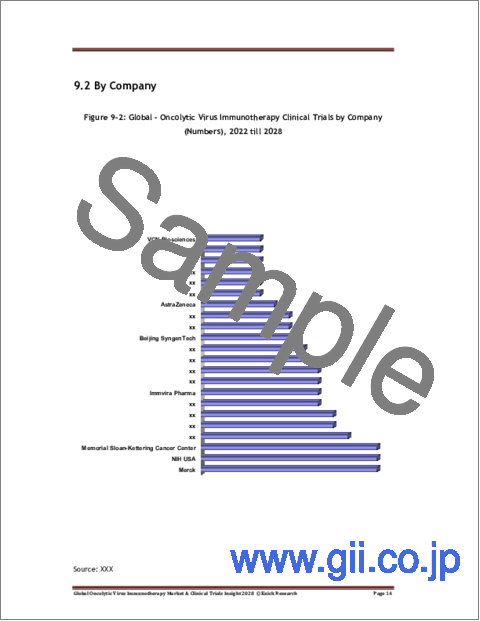
- Figure 9-2: Global - Oncolytic Virus Immunotherapy Clinical Trials by Company (Numbers), 2022 till 2028
- Figure 9-3: Global - Oncolytic Virus Immunotherapy Clinical Trials by Country (Numbers), 2022 till 2028
- Figure 9-4: Global - Oncolytic Virus Immunotherapy Clinical Trials by Indication (Numbers), 2022 till 2028
- Figure 9-5: Global - Oncolytic Virus Immunotherapy Clinical Trials by Patient Segment (Numbers), 2022 till 2028
- Figure 12-1: Major Drivers for the Oncolytic Virus Therapies
- Figure 12-2: Major Challenges Faced by Oncolytic Viral Therapies
“Global Oncolytic Virus Immunotherapy Market & Clinical Trials Insight 2028” Report Overview:
- Global Oncolytic Virus Immunotherapy Therapy Market Overview
- Global Oncolytic Virus Immunotherapy Therapy Market Opportunity: > USD 1 Billion By 2028
- Insight On More Than 180 Oncolytic Virus Immunotherapies In Clinical Trials
- Patent Information On More Than 60 Therapies in Clinical Trials
- IMLYGIC, Oncorine, Delytact: Availability, Dosage & Price Analysis
- Oncolytic Virus Immunotherapy Clinical Pipeline By Country, Phase, Indication, Organization, Patient Segment
- Oncolytic Virus Immunotherapy Application By 10 Cancer
- Recent Strategic Partnerships, Collaborations, Mergers & Acquisitions
Global cancer immunotherapy market encompasses broad range of applications, in which oncolytic virus immunotherapy is playing a vital role in the overall development. The therapy involves a very basic thread of using virus for targeting cancer cells and to harness the natural as well as destructive power of the immune system against the cancer cells. In a very short period of time, the clinical outcome associated with the therapy has laid down foundation for the over-achieving future of the therapy. The therapy market is estimated to get recognized as a necessity in the overall cancer therapeutics market where old and traditional therapies available are not successful in declining the increasing mortality rate across the globe.
Researchers have been successful in modifying the basic immunotherapy strategy by introducing the benefits of virus in targeting the host cells. Beyond the excellent capacity of the virus to get incorporated into the host cell, the therapeutic introduction of virus in the cancer therapeutics is providing enormous amounts of opportunities which were somehow lacking in the overall cancer therapeutics market for prolonged period of time. The future approval of the oncolytic virus therapy against wide range of cancer types is believed to strengthen the pharmaceutical industry to reach the revenue sales that have not been recorded for any of the cancer therapy drugs.
The strong link of the therapy with clinical pipeline and the future timeframe which is about to justify the applications of the therapy are observed to over-come the challenges which the researchers have been facing in terms of resistance development, off-targeting and adverse side effects. Amid the growth of other cancer therapies in the cancer therapeutics market, oncolytic virus therapy soon is going to deliver significant revolution and growth. The unimaginable applications of the therapy and the orientation of the government bodies towards expanding the clinical trial market of the same is estimated to develop the market as a dominant one for millions of patients who haven't received any benefits from the available therapies. The entry of the oncolytic virus therapy into the market horizon is estimated to provide enormous healthcare opportunities to the patients who have been struggling with cancer for a prolonged period of time.
As per “Global Oncolytic Virus Immunotherapy Market & Clinical Trials Insight 2028” report findings, the overall appreciation that the therapy has achieved in such shorter time period is poised to incline the therapy towards substantial growth. In the coming time period, the therapy market is believed to deliver promising trends and opportunities for the researchers working in different bio-pharmaceutical companies and research centers, with hundreds of driving forces driving the market through various complications. On the regulatory side, the legislations of developed pharmaceutical countries are also inclining towards boosting the researchers to expand the market applications and drive the competition in the right direction, where appropriate funds for the clinical research activities is acting as the catalyst for the therapy. The unlimited applications found within the therapy and the clinical platform for the same is estimated to move the therapy towards success at an unprecedented rate in the coming years.
Table of Contents
1. Introduction to Oncolytic Virus: Trail From Genesis To Biogenetics
2. Primer of Virotherapy in Malignancies
- 2.1. Oncolytic Viruses Towards Cancer
- 2.2. Approaches for Targeting Tumor Cells
- 2.3. Viral Oncolysis Mechanism of Action
3. Oncolytic Viruses Immunotherapy Overview
- 3.1. Stimulation of Antitumor Immune Responses
- 3.2. Oncolytic Viruses as Cancer Vaccines
4. Potential Oncolytic Virus Immunotherapy for Cancers
- 4.1. Breast Cancer
- 4.2. Lung Cancer
- 4.3. Prostate Cancer
- 4.4. Melanoma
- 4.5. Brain Tumor
- 4.6. Blood Cancer
- 4.7. Colorectal Cancer
- 4.8. Bladder Cancer
- 4.9. Pancreatic Cancer
- 4.10. Head & Neck Cancer
5. Global Oncolytic Virus Immunotherapy Therapy Market Overview
- 5.1. Preface Towards Oncolytic Virus Arcade
- 5.2. Market Aspects of Approved Oncolytic Viruses
6. Imlygic (Talimogene laherparepvec): Clinical & Pricing Insight
- 6.1. Clinical Overview
- 6.2. Availability, Dosage & Price Analysis
7. Oncorine (H101): Clinical & Pricing Insight
- 7.1. Clinical Overview
- 7.2. Availability, Dosage & Price Analysis
8. Delytact (DS 1647): Clinical & Pricing Insight
- 8.1. Clinical Overview
- 8.2. Availability, Dosage & Price Analysis
9. Global Oncolytic Virus Immunotherapy Clinical Pipeline Overview
- 9.1. By Phase
- 9.2. By Company
- 9.3. By Country
- 9.4. By Indication
- 9.5. By Patient Segment
10. Global Oncolytic Viruses Clinical Pipeline By Company, Indication & Phase
- 10.1. Research
- 10.2. Preclinical
- 10.3. Phase-I
- 10.4. Phase-I/II
- 10.5. Phase-II
- 10.6. Phase-III
- 10.7. Registered
11. Strategic Partnerships, Collaborations, Mergers & Acquisitions to Advance the Oncolytic Virus Therapy Market
- 11.1. Estrella Biopharma Signs Deal To Merge With Blank Check Company
- 11.2. ABL & Imugene Announce Partnership to Advance Oncolytic Virus Candidates Into Late Clinical Trial Phase
- 11.3. TILT Biotherapeutics & Merck to Undergo Clinical Trial Collaboration for Refractory Non-Small Cell Lung Cancer
- 11.4. Virogin Biotech & MD Anderson Announce Strategic Collaboration For Treating Advance Cancers
- 11.5. ABL Signs Pact With KaliVir On Oncolytic Viruses
- 11.6. Targovax Announces Clinical Collaboration With Agenus For Melanoma Trial
- 11.7. KaliVir Announces Exclusive Agreement With Roche For Developing Novel Oncolytic Viruses
- 11.8. ImmVira & Roche Enter Cooperation Agreement For Evaluating Novel Combination For Cancer Therapy
- 11.9. Calidi & Edoc Agree On Merger To Revolutionize Oncolytic Viral Therapies
- 11.10. Synthetic Biologics Completes Acquisition of VCN Biosciences
- 11.11. VacV Biotherapeutics Exits Stealth With $3 Million Investment
- 11.12. Targovax Enters Research Collaboration With Karolinska Institutet Professor
- 11.13. Transgene and PersonGen To Collaborate To Evaluate New Combination Therapy Against Solid Tumors
- 11.14. Lokon Pharma Initiates Collaboration With To Develop Novel Cancer Therapeutics
- 11.15. Bionaut Labs and Candel Therapeutics Announce Collaboration For Novel Targeting Mechanism For Oncolytic Virus Delivery
- 11.16. Imugene & Eureka Announce Strategic Collaboration to Accelerate Oncolytic Virus and T-Cell Therapy Combination
- 11.17. CG Oncology Enters Into Clinical Trial Collaboration With Roche For Novel Oncolytic Immunotherapy Combination
- 11.18. Imugene Enters Into Exclusive Strategic Partnership With Celularity To Develop Novel Combination For Treating Solid Tumors
12. Global Oncolytic Virus Immunotherapy Market Dynamics
- 12.1. Favorable Market Parameters
- 12.2. Growth Inhibitors
13. Global Oncolytic Virus Immunotherapy Market Future Outlook
14. Competitive Landscape
- 14.1. AdCure Bio
- 14.2. Beijing SyngenTech
- 14.3. BioVex Inc.
- 14.4. Calidi Biotherapeutics
- 14.5. Genelux Corporation
- 14.6. Immvira Pharma
- 14.7. Jennerex Biotherapeutics
- 14.8. KaliVir
- 14.9. Lokon Pharma
- 14.10. Merck
- 14.11. Oncolys BioPharma
- 14.12. Oncorus
- 14.13. PsiOxus Therapeutics
- 14.14. Seneca Therapeutics
- 14.15. Shanghai Sunway Biotech
- 14.16. Takara Bio
- 14.17. Targovax
- 14.18. TILT Biotherapeutics
- 14.19. Transgene
- 14.20. Virogin Biotech