|
|
市場調査レポート
商品コード
1090214
RNA治療薬の世界市場:成長機会Global RNA Therapeutics Growth Opportunities |
||||||
| RNA治療薬の世界市場:成長機会 |
|
出版日: 2022年05月31日
発行: Frost & Sullivan
ページ情報: 英文 51 Pages
納期: 即日から翌営業日
|
- 全表示
- 概要
- 目次
核酸系治療法における科学の進歩は、世界のバイオ医薬品業界に大きな影響を与えています。RNA(リボ核酸)治療薬市場は、「治療不可能」な経路を標的とすることから、著しい臨床的な進歩を遂げることが期待されています。技術的な優位性と、競争力を高めるためにRNA治療薬の臨床的に優れたポートフォリオを構築する必要性の高まりは、RNA治療薬業界のステークホルダー間の戦略的パートナーシップを促進すると予想されます。
当レポートでは、世界のRNA治療薬市場について調査分析し、戦略的必須要因、促進要因、抑制要因、主要動向、収益予測、競合環境、成長機会等に関する情報を提供しています。
目次
戦略的必須要因
- 成長がますます困難になるのはなぜか?
- 戦略的必須要因 8(TM)
- RNA治療薬業界における上位3つの戦略的必須要因の影響
- 成長機会がGrowth Pipeline Engine (TM)を促進
成長機会分析
- 分析の範囲
- セグメンテーション
- 調査手法
- 重要なポイント
- 成長指標
- 成長の促進要因
- 成長の抑制要因
- 主要動向
- 主要な疾患の適応症
- 2021年のパイプラインスナップショット
- バリューチェーン-主要なステークホルダー
- 混乱を引き起こしている著名なステークホルダー
- メーカーの地域スナップショット
- SMの製造と供給ニーズを満たすための戦略
- サプライチェーンにおけるステークホルダーの進化する役割
- RNA治療薬のバイオ製造における全体的な変化
- 流通と供給のフレームワーク
- 主要なパートナーシップテーマ
- 注目すべきパートナーシップ
- 注目すべきmRNA製造パートナーシップ:地域別-欧州
- 注目すべきmRNA製造パートナーシップ:地域別-米国
- 予測の前提条件
- 収益予測
- 収益予測分析
- 収益予測:製品タイプ別
- 開発中の主要なモダリティ
- 競合環境
- 市場シェア分析
成長機会ユニバース
- 成長機会1-腫瘍特有を標的とした治療薬の需要を満たすために免疫腫瘍学への注目が高まる
- 成長機会2-RNA治療薬の安定性の問題に対処するためにLNPデリバリーを最適化
- 成長機会3-バイオ医薬品企業をサポートするためにCDMO機能を拡大
- 図表リスト
- 免責事項
Future Growth Potential Driven by Co-Development Programs and Robust Clinical Trials for Diverse Disease Areas
Scientific advancements in nucleic acid-based therapies have significantly impacted the global biopharmaceutical industry. The ribonucleic acid (RNA) therapeutics market is expected to witness remarkable clinical progress as therapies target "undruggable" pathways. Technologically advanced platforms are integrated into RNA therapeutics bioprocessing to overcome stability issues. Technological advantages, coupled with the growing need to build clinically superior portfolios in RNA therapeutics for a competitive edge, are anticipated to drive strategic partnerships among stakeholders in the RNA therapeutics industry.
Spurred by the COVID-19 pandemic, biopharmaceutical companies are likely to prioritize microRNA, small interfering RNA, and antisense oligonucleotides as promising therapeutic modalities during the forecast period. Midsize and large companies have ramped up the production of starting materials and the final formulation of RNA therapeutics, which is likely to propel stakeholders to seek synergistic partnerships. In the industry's transition to personalized therapeutics, RNA developers are capitalizing on the high precision of RNA to treat untapped chronic disease areas such as cystic fibrosis, solid tumors, and spinal muscular atrophy. They engage in co-development programs to improve the stability profile of RNA therapeutics by optimizing drug delivery carriers, such as lipid nanoparticles.
Scaling up the production of starting materials, including plasmid DNA, oligonucleotides, and delivery materials, to ensure continuous manufacturing is anticipated to reduce the operational timeframe of RNA therapeutics production. Technology transfer and the outsourcing of crucial operations to contract development and manufacturing organizations (CDMO) catalyze the development of cost-effective and efficient models for RNA therapeutics.
This Frost & Sullivan research service provides an overview of the global RNA therapeutics industry from 2021 to 2027, including emerging trends, growth drivers, and growth opportunities.
The research service highlights the following:
- RNA therapeutics market size, including key segments from 2022 to 2027
- Advancements in bioprocessing, modalities, and technologies that are shaping the market
- Current and future R&D, investment outlook, and trends
Table of Contents
Strategic Imperatives
- Why is it Increasingly Difficult to Grow?
- The Strategic Imperative 8™
- The Impact of the Top 3 Strategic Imperatives on the RNA Therapeutics Industry
- Growth Opportunities Fuel the Growth Pipeline Engine™
Growth Opportunity Analysis
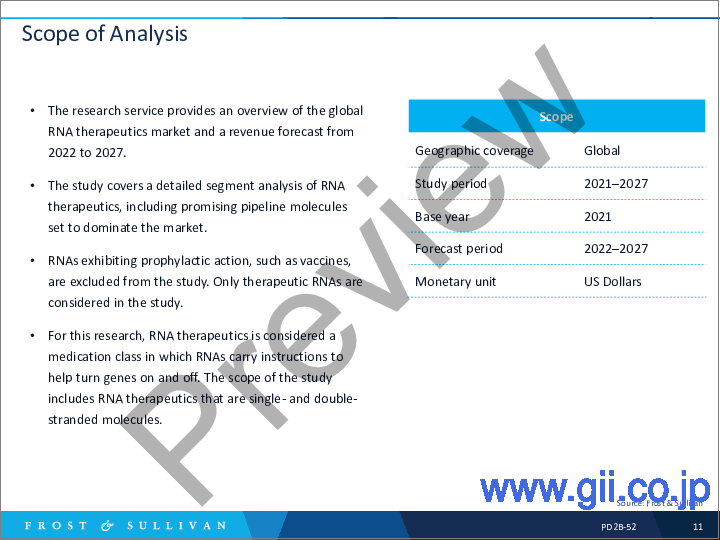
- Scope of Analysis
- Segmentation
- Research Methodology
- Key Takeaways
- Growth Metrics
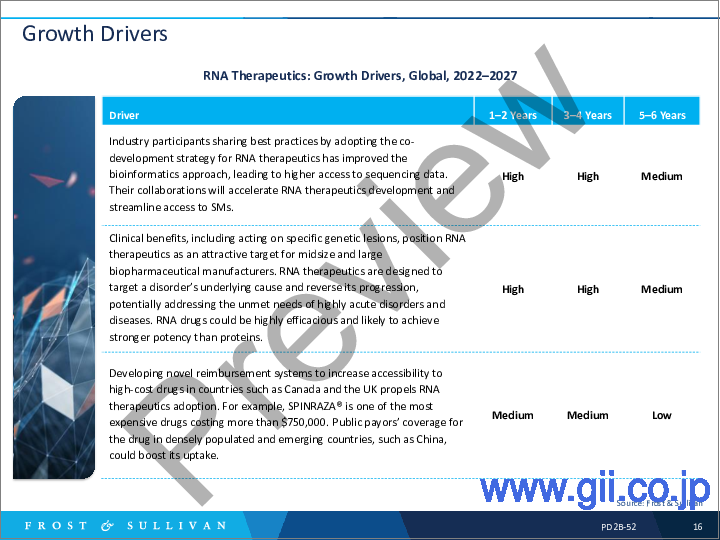
- Growth Drivers
- Growth Restraints
- Key Trends
- Key Disease Indications
- Pipeline Snapshot for 2021
- Value Chain-Key Stakeholders
- Value Chain-Key Stakeholders (continued)
- Notable Stakeholders Driving Disruption
- Regional Snapshot of Manufacturers
- Strategies to Meet SM Manufacturing and Supply Needs
- Evolving Role of Stakeholders in the Supply Chain
- Evolving Role of Stakeholders in the Supply Chain (continued)
- Holistic Changes in RNA Therapeutics Biomanufacturing
- Distribution and Supply Framework
- Key Partnership Themes
- Notable Partnerships
- Notable Partnerships (continued)
- Notable mRNA Manufacturing Partnerships by Region-Europe
- Notable mRNA Manufacturing Partnerships by Region-US
- Forecast Assumptions
- Revenue Forecast
- Revenue Forecast Analysis
- Percent Revenue Forecast by Product Type
- Key Modalities Under Development
- Competitive Environment
- Market Share Analysis
Growth Opportunity Universe
- Growth Opportunity 1-Increase Focus on Immuno-oncology to Meet the Demand for Tumor-specific Targeted Therapeutics
- Growth Opportunity 1-Increase Focus on Immuno-oncology to Meet the Demand for Tumor-specific Targeted Therapeutics (continued)
- Growth Opportunity 2-Optimize LNP Delivery to Address the Stability Issues of RNA Therapeutics
- Growth Opportunity 2-Optimize LNP Delivery to Address the Stability Issues of RNA Therapeutics (continued)
- Growth Opportunity 3-Expand CDMO Capabilities to Support Biopharmaceutical Companies
- Growth Opportunity 3-Expand CDMO Capabilities to Support Biopharmaceutical Companies (continued)
- Growth Opportunity 3-Expand CDMO Capabilities to Support Biopharmaceutical Companies (continued)
- List of Exhibits
- Legal Disclaimer