|
|
市場調査レポート
商品コード
1303039
細胞および遺伝子治療の製造QCの世界市場 (2023-2033年):治療タイプ・提供区分・プロセス・技術・用途・地域別Cell and Gene Therapy Manufacturing QC Market - A Global and Regional Analysis : Focus on Therapy Type, Offering, Process, Technology, Application, and Region - Analysis and Forecast, 2023-2033 |
||||||
|
● お客様のご希望に応じて、既存データの加工や未掲載情報(例:国別セグメント)の追加などの対応が可能です。 詳細はお問い合わせください。 |
|||||||
| 細胞および遺伝子治療の製造QCの世界市場 (2023-2033年):治療タイプ・提供区分・プロセス・技術・用途・地域別 |
|
出版日: 2023年06月29日
発行: BIS Research
ページ情報: 英文 361 Pages
納期: 1~5営業日
|
- 全表示
- 概要
- 図表
- 目次
世界の細胞および遺伝子治療の製造QCの市場規模は、2022年の19億5,000万米ドルから、予測期間中は16.85%のCAGRで推移し、2033年には106億5,000万米ドルの規模に成長すると予測されています。
同市場の成長は、承認された治療法の増加とインフラ要件の増大によって牽引されると予想されています。また、細胞および遺伝子治療の適応症の拡大により、大規模製造とQCの需要が高まっています。治療タイプ別では、細胞治療の部門が2022年の市場をリードしました。CAR-T細胞療法や幹細胞療法などの細胞ベースの治療法の採用が増加しており、同部門の優位性に寄与しています。
当レポートでは、世界の細胞および遺伝子治療の製造QCの市場を調査し、市場概要、規制の枠組み、市場影響因子の分析、市場規模の推移・予測、各種区分・地域/主要国別の詳細分析、競合情勢、主要企業のプロファイルなどをまとめています。
目次
第1章 市場
- 製品の定義
- 市場範囲
- 調査手法
- 市場概要
- 世界市場シナリオ
- 市場のフットプリントと成長の可能性
- 将来性
- COVID-19:市場への影響
第2章 世界の細胞および遺伝子治療の製造QC市場:産業分析
- 規制の枠組み
第3章 世界の細胞および遺伝子治療の製造QC市場:市場力学
- 概要
- 影響分析
- 市場促進要因
- 市場抑制要因
- 市場機会
第4章 世界の細胞および遺伝子治療の製造QC市場:競合情勢
- 概要
- 主要な戦略と展開
- 市場シェア分析
- 成長シェア分析
- サプライチェーン分析
第5章 世界の細胞および遺伝子治療の製造QC市場:治療タイプ別
- 概要
- 細胞治療
- 自己由来
- 同種異系
- その他
- 遺伝子治療
- ウイルスベクター
- 非ウイルスベクター
第6章 世界の細胞および遺伝子治療の製造QC市場:提供区分別
- 概要
- 製品
- 消耗品
- 機器
- ソフトウェア
- サービス
- 安全性試験
- 効力試験
- アイデンティティテスト
- 安定性および遺伝的忠実度の試験
- その他
第7章 世界の細胞および遺伝子治療の製造QC市場:プロセス別
- 概要
- 原料準備
- 上流処理
- 下流処理
- パッケージング
第8章 世界の細胞および遺伝子治療の製造QC市場:技術別
- 概要
- ポリメラーゼ連鎖反応 (PCR)
- フローサイトメトリー
- カブトガニ変形細胞ライセート (LAL)
- 酵素免疫測定法 (ELISA)
- クロマトグラフィー
- 質量分析法
- ウェスタンブロッティング
- 次世代シーケンス (NGS)
- 電気泳動
- その他の技術
第9章 世界の細胞および遺伝子治療の製造QC市場:用途別
- 概要
- 安全性試験
- 効力試験
- アイデンティティテスト
- 安定性および遺伝的忠実度の試験
- その他
第10章 世界の細胞および遺伝子治療の製造QC市場:地域別
- 概要
- 北米
- 欧州
- アジア太平洋
- 中東・アフリカ
- ラテンアメリカ
第11章 企業プロファイル
- 製造業者
- サービス
- 新興企業
List of Figures
- Figure 1:. Cell and Gene Therapy Manufacturing QC Market, $Billion, 2022-2033
- Figure 2:. Global Cell and Gene Therapy Manufacturing QC Market, Impact Analysis
- Figure 3:. Global Cell and Gene Therapy Manufacturing QC Market (by Therapy Type), % Share, 2022 and 2033
- Figure 4:. Global Cell and Gene Therapy Manufacturing QC Market (by Offering), % Share, 2022 and 2033
- Figure 5:. Global Cell and Gene Therapy Manufacturing QC Market (by Process), % Share, 2022 and 2033
- Figure 6:. Global Cell and Gene Therapy Manufacturing QC Market (by Technology), % Share, 2022 and 2033
- Figure 7:. Global Cell and Gene Therapy Manufacturing QC Market (by Application), % Share, 2022 and 2033
- Figure 8:. Global Cell and Gene Therapy Manufacturing QC Market (by Region), Market Snapshot
- Figure 9:. Global Cell and Gene Therapy Manufacturing QC Market Segmentation
- Figure 10:. Global Cell and Gene Therapy Manufacturing QC Market: Research Methodology
- Figure 11:. Primary Research Methodology
- Figure 12:. Bottom-Up Approach (Segment-Wise Analysis)
- Figure 13:. Top-Down Approach (Segment-Wise Analysis)
- Figure 14:. Global Cell and Gene Therapy Manufacturing QC Market Size and Growth Potential (Realistic Scenario), $Billion, 2022-2033
- Figure 15:. Global Cell and Gene Therapy Manufacturing QC Market Size and Growth Potential (Optimistic Scenario), $Billion, 2022-2033
- Figure 16:. Global Cell and Gene Therapy Manufacturing QC Market Size and Growth Potential (Pessimistic Scenario), $Billion, 2022-2033
- Figure 17:. Global Cell and Gene Therapy Manufacturing QC Market, $Billion, 2022-2033
- Figure 18:. Impact of COVID-19 on CGT Developmental Activities
- Figure 19:. Global Cell and Gene Therapy Manufacturing QC Market - Market Dynamics
- Figure 20:. U.S.FDA-Approved Cell and Gene Therapies, 2011-2022
- Figure 21:. Number of Cell and Gene Therapy Developers (by Region)
- Figure 22:. Share of Key Developments, January 2020-April 2023
- Figure 23:. Number of Product and Service Launches (by Company), January 2020 to April 2023
- Figure 24:. Market Share Analysis of the Global Cell and Gene Therapy Manufacturing QC Market (by Company), 2022
- Figure 25:. Growth Share Analysis for Global Cell and Gene Therapy Manufacturing QC Market (by Applications), 2022
- Figure 26:. Supply Chain Analysis
- Figure 27:. Global Cell and Gene Therapy Manufacturing QC Market (by Therapy Type)
- Figure 28:. Global Cell and Gene Therapy Manufacturing QC Market (by Therapy Type), % Share, 2022 and 2033
- Figure 29:. Global Cell and Gene Therapy Manufacturing QC Market (Cell Therapy), $Billion, 2022-2033
- Figure 30:. Global Cell and Gene Therapy Manufacturing QC Market (Autologous), $Billion, 2022-2033
- Figure 31:. Global Cell and Gene Therapy Manufacturing QC Market (Allogeneic), $Billion, 2022-2033
- Figure 32:. Global Cell and Gene Therapy Manufacturing QC Market (Others), $Million, 2022-2033
- Figure 33:. Global Cell and Gene Therapy Manufacturing QC Market (Gene Therapy), $Billion, 2022-2033
- Figure 34:. Several Types of Viral Vectors Used in Gene Therapy
- Figure 35:. Global Cell and Gene Therapy Manufacturing QC Market (Viral Vectors), $Million, 2022-2033
- Figure 36:. Global Cell and Gene Therapy Manufacturing QC Market (Non-Viral Vectors), $Million, 2022-2033
- Figure 37:. Global Cell and Gene Therapy Manufacturing QC Market (by Offering)
- Figure 38:. Global Cell and Gene Therapy Manufacturing QC Market (by Offering), % Share, 2022 and 2033
- Figure 39:. Global Cell and Gene Therapy Manufacturing QC Market (Products), $Billion, 2022-2033
- Figure 40:. Global Cell and Gene Therapy Manufacturing QC Market (Products), % Share, 2022 and 2033
- Figure 41:. Global Cell and Gene Therapy Manufacturing QC Market (Consumables), $Million, 2022-2033
- Figure 42:. Global Cell and Gene Therapy Manufacturing QC Market (Kits and Assays), $Million, 2022-2033
- Figure 43:. Global Cell and Gene Therapy Manufacturing QC Market (Reagents), $Million, 2022-20333
- Figure 44:. Global Cell and Gene Therapy Manufacturing QC Market (Cell Culture Media), $Million, 2022-2033
- Figure 45:. Global Cell and Gene Therapy Manufacturing QC Market (Instruments), $Million, 2022-2033
- Figure 46:. Global Cell and Gene Therapy Manufacturing QC Market (Bioreactors/Fermenters), $Million, 2022-2033
- Figure 47:. Global Cell and Gene Therapy Manufacturing QC Market (PCR Systems), $Million, 2022-2033
- Figure 48:. Global Cell and Gene Therapy Manufacturing QC Market (Chromatography System), $Million, 2022-2033
- Figure 49:. Global Cell and Gene Therapy Manufacturing QC Market (Others), $Million, 2022-2033
- Figure 50:. Global Cell and Gene Therapy Manufacturing QC Market (Software), $Million, 2022-2033
- Figure 51:. Global Cell and Gene Therapy Manufacturing QC Market (Services), $Billion, 2022-2033
- Figure 52:. Global Cell and Gene Therapy Manufacturing QC Market (Services), % Share, 2022 and 2033
- Figure 53:. Global Cell and Gene Therapy Manufacturing QC Market (Safety Testing), $ Billion, 2022-2033
- Figure 54:. Global Cell and Gene Therapy Manufacturing QC Market (Potency Testing), $Million, 2022-2033
- Figure 55:. Global Cell and Gene Therapy Manufacturing QC Market (Identity Testing), $Million, 2022-2033
- Figure 56:. Global Cell and Gene Therapy Manufacturing QC Market (Stability and Genetic Fidelity Testing), $Million, 2022-2033
- Figure 57:. Global Cell and Gene Therapy Manufacturing QC Market (Others), $Million, 2022-2033
- Figure 58:. Global Cell and Gene Therapy Manufacturing QC Market (by Process)
- Figure 59:. Global Cell and Gene Therapy Manufacturing QC Market (by Process), % Share, 2022-2033
- Figure 60:. Global Cell and Gene Therapy Manufacturing QC Market (Raw Material Preparation), $Million, 2022-2033
- Figure 61:. Followed Steps in Upstream Processing
- Figure 62:. Global Cell and Gene Therapy Manufacturing QC Market (Upstream Processing), $Billion, 2022-2033
- Figure 63:. Followed Steps in Downstream Processing
- Figure 64:. Global Cell and Gene Therapy Manufacturing QC Market (Downstream Processing), $Billion, 2022-2033
- Figure 65:. Steps Involved in Packaging Operations
- Figure 66:. Global Cell and Gene Therapy Manufacturing QC Market (Packaging), $Million, 2022-2033
- Figure 67:. Global Cell and Gene Therapy Manufacturing QC Market (by Technology)
- Figure 68:. Global Cell and Gene Therapy Manufacturing QC Market (by Technology), % Share, 2022 and 2033
- Figure 69:. Global Cell and Gene Therapy Manufacturing QC Market (PCR), $Million, 2022-2033
- Figure 70:. Global Cell and Gene Therapy Manufacturing QC Market (Flow Cytometry), $Million,2022-2033
- Figure 71:. Global Cell and Gene Therapy Manufacturing QC Market (LAL), $Million,2022-2033
- Figure 72:. Global Cell and Gene Therapy Manufacturing QC Market (ELISA), $Million, 2022-2033
- Figure 73:. Global Cell and Gene Therapy Manufacturing QC Market (Chromatography), $Million, 2022-2033
- Figure 74:. Global Cell and Gene Therapy Manufacturing QC Market (Mass Spectrometry), $Million, 2022-2033
- Figure 75:. Global Cell and Gene Therapy Manufacturing QC Market (Western Blotting), $Million, 2022-2033
- Figure 76:. Global Cell and Gene Therapy Manufacturing QC Market (NGS), $Million, 2022-2033
- Figure 77:. Global Cell and Gene Therapy Manufacturing QC Market (Electrophoresis), $Million, 2022-2033
- Figure 78:. Global Cell and Gene Therapy Manufacturing QC Market (Other Technologies), $Million, 2022-2033
- Figure 79:. Global Cell and Gene Therapy Manufacturing QC Market (by Application)
- Figure 80:. Global Cell and Gene Therapy Manufacturing QC Market (by Application), % Share, 2022 and 2033
- Figure 81:. Global Cell and Gene Therapy Manufacturing QC Market (Safety Testing), $Billion, 2022-2033
- Figure 82:. Global Cell and Gene Therapy Manufacturing QC Market (by Safety Testing Type), % Share, 2022 and 2033
- Figure 83:. Global Cell and Gene Therapy Manufacturing QC Market (Potency Testing), $Million, 2022-2033
- Figure 84:. Global Cell and Gene Therapy Manufacturing QC Market (by Potency Testing Type), % Share, 2022 and 2033
- Figure 85:. Global Cell and Gene Therapy Manufacturing QC Market (Identity Testing), $Million, 2022-2033
- Figure 86:. Global Cell and Gene Therapy Manufacturing QC Market (by Identity Testing Type), % Share, 2022 and 2033
- Figure 87:. Global Cell and Gene Therapy Manufacturing QC Market (Stability and Genetic Fidelity Testing), $Million, 2022-2033
- Figure 88:. Global Cell and Gene Therapy Manufacturing QC Market (by Identity Testing Type), % Share, 2022 and 2033
- Figure 89:. Global Cell and Gene Therapy Manufacturing QC Market (Others), $Million, 2022-2033
- Figure 90:. Global Cell and Gene Therapy Manufacturing QC Market (by Region), Market Snapshot
- Figure 91:. North America Cell and Gene Therapy Manufacturing QC Market, $Million, 2022-2033
- Figure 92:. North America Cell and Gene Therapy Manufacturing QC Market (by Country), % Share, 2022 and 2033
- Figure 93:. North America Cell and Gene Therapy Manufacturing QC Market (by Offering), $Million, 2022-2033
- Figure 94:. North America Cell and Gene Therapy Manufacturing QC Market (by Therapy Type), $Million, 2022-2033
- Figure 95:. North America Cell and Gene Therapy Manufacturing QC Market (by Process), $Million, 2022-2033
- Figure 96:. North America Cell and Gene Therapy Manufacturing QC Market (by Technology), $Million, 2022-2033
- Figure 97:. North America Cell and Gene Therapy Manufacturing QC Market (by Application), $Million, 2022-2033
- Figure 98:. U.S. Cell and Gene Therapy Manufacturing QC Market, $Million, 2022-2033
- Figure 99:. U.S. Cell and Gene Therapy Manufacturing QC Market (by Offering), $Million, 2022-2033
- Figure 100:. U.S. Cell and Gene Therapy Manufacturing QC Market (by Therapy Type), $Million, 2022-2033
- Figure 101:. U.S. Cell and Gene Therapy Manufacturing QC Market (by Process), $Million, 2022-2033
- Figure 102:. U.S. Cell and Gene Therapy Manufacturing QC Market (by Technology), $Million, 2022-2033
- Figure 103:. U.S. Cell and Gene Therapy Manufacturing QC Market (by Application), $Million, 2022-2033
- Figure 104:. Canada Cell and Gene Therapy Manufacturing QC Market, $Million, 2022-2033
- Figure 105:. Canada Cell and Gene Therapy Manufacturing QC Market (by Offering), $Million, 2022-2033
- Figure 106:. Canada Cell and Gene Therapy Manufacturing QC Market (by Therapy Type), $Million, 2022-2033
- Figure 107:. Canada Cell and Gene Therapy Manufacturing QC Market (by Process), $Million, 2022-2033
- Figure 108:. Canada Cell and Gene Therapy Manufacturing QC Market (by Technology), $Million, 2022-2033
- Figure 109:. Canada Cell and Gene Therapy Manufacturing QC Market (by Application), $Million, 2022-2033
- Figure 110:. Europe Cell and Gene Therapy Manufacturing QC Market, $Million, 2022-2033
- Figure 111:. Europe Cell and Gene Therapy Manufacturing QC Market (by Country), % Share, 2022-2033
- Figure 112:. Europe Cell and Gene Therapy Manufacturing QC Market (by Offering), $Million, 2022-2033
- Figure 113:. Europe Cell and Gene Therapy Manufacturing QC Market (by Therapy Type), $Million, 2022-2033
- Figure 114:. Europe Cell and Gene Therapy Manufacturing QC Market (by Process), $Million, 2022-2033
- Figure 115:. Europe Cell and Gene Therapy Manufacturing QC Market (by Technology), $Million, 2022-2033
- Figure 116:. Europe Cell and Gene Therapy Manufacturing QC Market (by Application), $Million, 2022-2033
- Figure 117:. U.K. Cell and Gene Therapy Manufacturing QC Market, $Million, 2022-2033
- Figure 118:. U.K. Cell and Gene Therapy Manufacturing QC Market (by Offering), $Million, 2022-2033
- Figure 119:. U.K. Cell and Gene Therapy Manufacturing QC Market (by Therapy Type), $Million, 2022-2033
- Figure 120:. U.K. Cell and Gene Therapy Manufacturing QC Market (by Process), $Million, 2022-2033
- Figure 121:. U.K. Cell and Gene Therapy Manufacturing QC Market (by Technology), $Million, 2022-2033
- Figure 122:. U.K. Cell and Gene Therapy Manufacturing QC Market (by Application), $Million, 2022-2033
- Figure 123:. Germany Cell and Gene Therapy Manufacturing QC Market, $Million, 2022-2033
- Figure 124:. Germany Cell and Gene Therapy Manufacturing QC Market (by Offering), $Million, 2022-2033
- Figure 125:. Germany Cell and Gene Therapy Manufacturing QC Market (by Therapy Type), $Million, 2022-2033
- Figure 126:. Germany Cell and Gene Therapy Manufacturing QC Market (by Process), $Million, 2022-2033
- Figure 127:. Germany Cell and Gene Therapy Manufacturing QC Market (by Technology), $Million, 2022-2033
- Figure 128:. Germany Cell and Gene Therapy Manufacturing QC Market (by Application), $Million, 2022-2033
- Figure 129:. France Cell and Gene Therapy Manufacturing QC Market, $Million, 2022-2033
- Figure 130:. France Cell and Gene Therapy Manufacturing QC Market (by Offering), $Million, 2022-2033
- Figure 131:. France Cell and Gene Therapy Manufacturing QC Market (by Therapy Type), $Million, 2022-2033
- Figure 132:. France Cell and Gene Therapy Manufacturing QC Market (by Process), $Million, 2022-2033
- Figure 133:. France Cell and Gene Therapy Manufacturing QC Market (by Technology), $Million, 2022-2033
- Figure 134:. France Cell and Gene Therapy Manufacturing QC Market (by Application), $Million, 2022-2033
- Figure 135:. Italy Cell and Gene Therapy Manufacturing QC Market, $Million, 2022-2033
- Figure 136:. Italy Cell and Gene Therapy Manufacturing QC Market (by Offering), $Million, 2022-2033
- Figure 137:. Italy Cell and Gene Therapy Manufacturing QC Market (by Therapy Type), $Million, 2022-2033
- Figure 138:. Italy Cell and Gene Therapy Manufacturing QC Market (by Process), $Million, 2022-2033
- Figure 139:. Italy Cell and Gene Therapy Manufacturing QC Market (by Technology), $Million, 2022-2033
- Figure 140:. Italy Cell and Gene Therapy Manufacturing QC Market (by Application), $Million, 2022-2033
- Figure 141:. Spain Cell and Gene Therapy Manufacturing QC Market, $Million, 2022-2033
- Figure 142:. Spain Cell and Gene Therapy Manufacturing QC Market (by Offering), $Million, 2022-2033
- Figure 143:. Spain Cell and Gene Therapy Manufacturing QC Market (by Therapy Type), $Million, 2022-2033
- Figure 144:. Spain Cell and Gene Therapy Manufacturing QC Market (by Process), $Million, 2022-2033
- Figure 145:. Spain Cell and Gene Therapy Manufacturing QC Market (by Technology), $Million, 2022-2033
- Figure 146:. Spain Cell and Gene Therapy Manufacturing QC Market (by Application), $Million, 2022-2033
- Figure 147:. Rest-of-Europe Cell and Gene Therapy Manufacturing QC Market, $Million, 2022-2033
- Figure 148:. Rest-of-Europe Cell and Gene Therapy Manufacturing QC Market (by Offering), $Million, 2022-2033
- Figure 149:. Rest-of-Europe Cell and Gene Therapy Manufacturing QC Market (by Therapy Type), $Million, 2022-2033
- Figure 150:. Rest-of-Europe Cell and Gene Therapy Manufacturing QC Market (by Process), $Million, 2022-2033
- Figure 151:. Rest-of-Europe Cell and Gene Therapy Manufacturing QC Market (by Technology), $Million, 2022-2033
- Figure 152:. Rest-of-Europe Cell and Gene Therapy Manufacturing QC Market (by Application), $Million, 2022-2033
- Figure 153:. Asia-Pacific Cell and Gene Therapy Manufacturing QC Market, $Million, 2022-2033
- Figure 154:. Asia-Pacific Cell and Gene Therapy Manufacturing QC Market (by Country), % Share, 2022-2033
- Figure 155:. Asia-Pacific Cell and Gene Therapy Manufacturing QC Market (by Offering), $Million, 2022-2033
- Figure 156:. Asia-Pacific Cell and Gene Therapy Manufacturing QC Market (by Therapy Type), $Million, 2022-2033
- Figure 157:. Asia-Pacific Cell and Gene Therapy Manufacturing QC Market (by Process), $Million, 2022-2033
- Figure 158:. Asia-Pacific Cell and Gene Therapy Manufacturing QC Market (by Technology), $Million, 2022-2033
- Figure 159:. Asia-Pacific Cell and Gene Therapy Manufacturing QC Market (by Application), $Million, 2022-2033
- Figure 160:. China Cell and Gene Therapy Manufacturing QC Market, $Million, 2022-2033
- Figure 161:. China Cell and Gene Therapy Manufacturing QC Market (by Offering), $Million, 2022-2033
- Figure 162:. China Cell and Gene Therapy Manufacturing QC Market (by Therapy Type), $Million, 2022-2033
- Figure 163:. China Cell and Gene Therapy Manufacturing QC Market (by Process), $Million, 2022-2033
- Figure 164:. China Cell and Gene Therapy Manufacturing QC Market (by Technology), $Million, 2022-2033
- Figure 165:. China Cell and Gene Therapy Manufacturing QC Market (by Application), $Million, 2022-2033
- Figure 166:. Japan Cell and Gene Therapy Manufacturing QC Market, $Million, 2022-2033
- Figure 167:. Japan Cell and Gene Therapy Manufacturing QC Market (by Offering), $Million, 2022-2033
- Figure 168:. Japan Cell and Gene Therapy Manufacturing QC Market (by Therapy Type), $Million, 2022-2033
- Figure 169:. Japan Cell and Gene Therapy Manufacturing QC Market (by Process), $Million, 2022-2033
- Figure 170:. Japan Cell and Gene Therapy Manufacturing QC Market (by Technology), $Million, 2022-2033
- Figure 171:. Japan Cell and Gene Therapy Manufacturing QC Market (by Application), $Million, 2022-2033
- Figure 172:. South Korea Cell and Gene Therapy Manufacturing QC Market, $Million, 2022-2033
- Figure 173:. South Korea Cell and Gene Therapy Manufacturing QC Market (by Offering), $Million, 2022-2033
- Figure 174:. South Korea Cell and Gene Therapy Manufacturing QC Market (by Therapy Type), $Million, 2022-2033
- Figure 175:. South Korea Cell and Gene Therapy Manufacturing QC Market (by Process), $Million, 2022-2033
- Figure 176:. South Korea Cell and Gene Therapy Manufacturing QC Market (by Technology), $Million, 2022-2033
- Figure 177:. South Korea Cell and Gene Therapy Manufacturing QC Market (by Application), $Million, 2022-2033
- Figure 178:. Australia Cell and Gene Therapy Manufacturing QC Market, $Million, 2022-2033
- Figure 179:. Australia Cell and Gene Therapy Manufacturing QC Market (by Offering), $Million, 2022-2033
- Figure 180:. Australia Cell and Gene Therapy Manufacturing QC Market (by Therapy Type), $Million, 2022-2033
- Figure 181:. Australia Cell and Gene Therapy Manufacturing QC Market (by Process), $Million, 2022-2033
- Figure 182:. Australia Cell and Gene Therapy Manufacturing QC Market (by Technology), $Million, 2022-2033
- Figure 183:. Australia Cell and Gene Therapy Manufacturing QC Market (by Application), $Million, 2022-2033
- Figure 184:. India Cell and Gene Therapy Manufacturing QC Market, $Million, 2022-2033
- Figure 185:. India Cell and Gene Therapy Manufacturing QC Market (by Offering), $Million, 2022-2033
- Figure 186:. India Cell and Gene Therapy Manufacturing QC Market (by Therapy Type), $Million, 2022-2033
- Figure 187:. India Cell and Gene Therapy Manufacturing QC Market (by Process), $Million, 2022-2033
- Figure 188:. India Cell and Gene Therapy Manufacturing QC Market (by Technology), $Million, 2022-2033
- Figure 189:. India Cell and Gene Therapy Manufacturing QC Market (by Application), $Million, 2022-2033
- Figure 190:. Rest-of-Asia-Pacific Cell and Gene Therapy Manufacturing QC Market, $Million, 2022-2033
- Figure 191:. Rest-of-Asia-Pacific Cell and Gene Therapy Manufacturing QC Market (by Offering), $Million, 2022-2033
- Figure 192:. Rest-of-Asia-Pacific Cell and Gene Therapy Manufacturing QC Market (by Therapy Type), $Million, 2022-2033
- Figure 193:. Rest-of-Asia-Pacific Cell and Gene Therapy Manufacturing QC Market (by Process), $Million, 2022-2033
- Figure 194:. Rest-of-Asia-Pacific Cell and Gene Therapy Manufacturing QC Market (by Technology), $Million, 2022-2033
- Figure 195:. Rest-of-Asia-Pacific Cell and Gene Therapy Manufacturing QC Market (by Application), $Million, 2022-2033
- Figure 196:. Middle East and Africa Cell and Gene Therapy Manufacturing QC Market, $Million, 2022-2033
- Figure 197:. Middle East and Africa Cell and Gene Therapy Manufacturing QC Market (by Country), % Share, 2022-2033
- Figure 198:. Middle East and Africa Cell and Gene Therapy Manufacturing QC Market (by Offering), $Million, 2022-2033
- Figure 199:. Middle East and Africa Cell and Gene Therapy Manufacturing QC Market (by Therapy Type), $Million, 2022-2033
- Figure 200:. Middle East and Africa Cell and Gene Therapy Manufacturing QC Market (by Process), $Million, 2022-2033
- Figure 201:. Middle East and Africa Cell and Gene Therapy Manufacturing QC Market (by Technology), $Million, 2022-2033
- Figure 202:. Middle East and Africa Cell and Gene Therapy Manufacturing QC Market (by Application), $Million, 2022-2033
- Figure 203:. Israel Cell and Gene Therapy Manufacturing QC Market, $Million, 2022-2033
- Figure 204:. Israel Cell and Gene Therapy Manufacturing QC Market (by Offering), $Million, 2022-2033
- Figure 205:. Israel Cell and Gene Therapy Manufacturing QC Market (by Therapy Type), $Million, 2022-2033
- Figure 206:. Israel Cell and Gene Therapy Manufacturing QC Market (by Process), $Million, 2022-2033
- Figure 207:. Israel Cell and Gene Therapy Manufacturing QC Market (by Technology), $Million, 2022-2033
- Figure 208:. Israel Cell and Gene Therapy Manufacturing QC Market (by Application), $Million, 2022-2033
- Figure 209:. Türkiye Cell and Gene Therapy Manufacturing QC Market, $Million, 2022-2033
- Figure 210:. Türkiye Cell and Gene Therapy Manufacturing QC Market (by Offering), $Million, 2022-2033
- Figure 211:. Türkiye Cell and Gene Therapy Manufacturing QC Market (by Therapy Type), $Million, 2022-2033
- Figure 212:. Türkiye Cell and Gene Therapy Manufacturing QC Market (by Process), $Million, 2022-2033
- Figure 213:. Türkiye Cell and Gene Therapy Manufacturing QC Market (by Technology), $Million, 2022-2033
- Figure 214:. Türkiye Cell and Gene Therapy Manufacturing QC Market (by Application), $Million, 2022-2033
- Figure 215:. K.S.A. Cell and Gene Therapy Manufacturing QC Market, $Million, 2022-2033
- Figure 216:. K.S.A. Cell and Gene Therapy Manufacturing QC Market (by Offering), $Million, 2022-2033
- Figure 217:. K.S.A. Cell and Gene Therapy Manufacturing QC Market (by Therapy Type), $Million, 2022-2033
- Figure 218:. K.S.A. Cell and Gene Therapy Manufacturing QC Market (by Process), $Million, 2022-2033
- Figure 219:. K.S.A. Cell and Gene Therapy Manufacturing QC Market (by Technology), $Million, 2022-2033
- Figure 220:. K.S.A. Cell and Gene Therapy Manufacturing QC Market (by Application), $Million, 2022-2033
- Figure 221:. U.A.E. Cell and Gene Therapy Manufacturing QC Market, $Million, 2022-2033
- Figure 222:. U.A.E. Cell and Gene Therapy Manufacturing QC Market (by Offering), $Million, 2022-2033
- Figure 223:. U.A.E. Cell and Gene Therapy Manufacturing QC Market (by Therapy Type), $Million, 2022-2033
- Figure 224:. U.A.E. Cell and Gene Therapy Manufacturing QC Market (by Process), $Million, 2022-2033
- Figure 225:. U.A.E. Cell and Gene Therapy Manufacturing QC Market (by Technology), $Million, 2022-2033
- Figure 226:. U.A.E. Cell and Gene Therapy Manufacturing QC Market (by Application), $Million, 2022-2033
- Figure 227:. South Africa Cell and Gene Therapy Manufacturing QC Market, $Million, 2022-2033
- Figure 228:. South Africa Cell and Gene Therapy Manufacturing QC Market (by Offering), $Million, 2022-2033
- Figure 229:. South Africa Cell and Gene Therapy Manufacturing QC Market (by Therapy Type), $Million, 2022-2033
- Figure 230:. South Africa Cell and Gene Therapy Manufacturing QC Market (by Process), $Million, 2022-2033
- Figure 231:. South Africa Cell and Gene Therapy Manufacturing QC Market (by Technology), $Million, 2022-2033
- Figure 232:. South Africa Cell and Gene Therapy Manufacturing QC Market (by Application), $Million, 2022-2033
- Figure 233:. Rest-of-Middle East and Africa Cell and Gene Therapy Manufacturing QC Market, $Million, 2022-2033
- Figure 234:. Rest-of-Middle East and Africa Cell and Gene Therapy Manufacturing QC Market (by Offering), $Million, 2022-2033
- Figure 235:. Rest-of-Middle East and Africa Cell and Gene Therapy Manufacturing QC Market (by Therapy Type), $Million, 2022-2033
- Figure 236:. Rest-of-Middle East and Africa Cell and Gene Therapy Manufacturing QC Market (by Process), $Million, 2022-2033
- Figure 237:. Rest-of-Middle East and Africa Cell and Gene Therapy Manufacturing QC Market (by Technology), $Million, 2022-2033
- Figure 238:. Rest-of-Middle East and Africa Cell and Gene Therapy Manufacturing QC Market (by Application), $Million, 2022-2033
- Figure 239:. Latin America Cell and Gene Therapy Manufacturing QC Market, $Million, 2022-2033
- Figure 240:. Latin America Cell and Gene Therapy Manufacturing QC Market (by Country), % Share, 2022-2033
- Figure 241:. Latin America Cell and Gene Therapy Manufacturing QC Market (by Offering), $Million, 2022-2033
- Figure 242:. Latin America Cell and Gene Therapy Manufacturing QC Market (by Therapy Type), $Million, 2022-2033
- Figure 243:. Latin America Cell and Gene Therapy Manufacturing QC Market (by Process), $Million, 2022-2033
- Figure 244:. Latin America Cell and Gene Therapy Manufacturing QC Market (by Technology), $Million, 2022-2033
- Figure 245:. Latin America Cell and Gene Therapy Manufacturing QC Market (by Application), $Million, 2022-2033
- Figure 246:. Brazil Cell and Gene Therapy Manufacturing QC Market, $Million, 2022-2033
- Figure 247:. Brazil Cell and Gene Therapy Manufacturing QC Market (by Offering), $Million, 2022-2033
- Figure 248:. Brazil Cell and Gene Therapy Manufacturing QC Market (by Therapy Type), $Million, 2022-2033
- Figure 249:. Brazil Cell and Gene Therapy Manufacturing QC Market (by Process), $Million, 2022-2033
- Figure 250:. Brazil Cell and Gene Therapy Manufacturing QC Market (by Technology), $Million, 2022-2033
- Figure 251:. Brazil Cell and Gene Therapy Manufacturing QC Market (by Application), $Million, 2022-2033
- Figure 252:. Mexico Cell and Gene Therapy Manufacturing QC Market, $Million, 2022-2033
- Figure 253:. Mexico Cell and Gene Therapy Manufacturing QC Market (by Offering), $Million, 2022-2033
- Figure 254:. Mexico Cell and Gene Therapy Manufacturing QC Market (by Therapy Type), $Million, 2022-2033
- Figure 255:. Mexico Cell and Gene Therapy Manufacturing QC Market (by Process), $Million, 2022-2033
- Figure 256:. Mexico Cell and Gene Therapy Manufacturing QC Market (by Technology), $Million, 2022-2033
- Figure 257:. Mexico Cell and Gene Therapy Manufacturing QC Market (by Application), $Million, 2022-2033
- Figure 258:. Rest-of-Latin America Cell and Gene Therapy Manufacturing QC Market, $Million, 2022-2033
- Figure 259:. Rest-of-Latin America Cell and Gene Therapy Manufacturing QC Market (by Offering), $Million, 2022-2033
- Figure 260:. Rest-of-Latin America Cell and Gene Therapy Manufacturing QC Market (by Therapy Type), $Million, 2022-2033
- Figure 261:. Rest-of-Latin America Cell and Gene Therapy Manufacturing QC Market (by Process), $Million, 2022-2033
- Figure 262:. Rest-of-Latin America Cell and Gene Therapy Manufacturing QC Market (by Technology), $Million, 2022-2033
- Figure 263:. Rest-of-Latin America Cell and Gene Therapy Manufacturing QC Market (by Application), $Million, 2022-2033
- Figure 264:. Total Number of Companies Profiled
- Figure 265:. Bio-Techne Corporation: Product Portfolio
- Figure 266:. Bio-Techne Corporation: Overall Financials, $Million, 2020-2022
- Figure 267:. Bio-Techne Corporation: Revenue (by Segment), $Million, 2020-2022
- Figure 268:. Bio-Techne Corporation: Revenue (by Region), $Million, 2020-2022
- Figure 269:. bioMérieux SA: Product Portfolio
- Figure 270:. bioMérieux SA: Overall Financials, $Million, 2020-2022
- Figure 271:. bioMérieux SA: Revenue (by Segment), $Million, 2020-2022
- Figure 272:. bioMérieux SA: Revenue (by Region), $Million, 2020-2022
- Figure 273:. Danaher. (Cytiva): Product Portfolio
- Figure 274:. Danaher. (Cytiva): Overall Financials, $Million, 2020-2022
- Figure 275:. Danaher. (Cytiva): Revenue (by Segment), $Million, 2020-2022
- Figure 276:. Danaher. (Cytiva): Revenue (by Region), $Million, 2020-2022
- Figure 277:. Danaher. (Cytiva): R&D Expenditure, $Million, 2019-2021
- Figure 278:. F. Hoffmann-La Roche Ltd: Product Portfolio
- Figure 279:. F. Hoffmann-La Roche Ltd: Overall Financials, $Million, 2020-2022
- Figure 280:. F. Hoffmann-La Roche Ltd: Revenue (by Segment), $Million, 2020-2022
- Figure 281:. F. Hoffmann-La Roche Ltd: Revenue (by Region), $Million, 2020-2022
- Figure 282:. F. Hoffmann-La Roche Ltd: R&D Expenditure, $Million, 2020-2022
- Figure 283:. Lonza.: Product Portfolio
- Figure 284:. Lonza.: Overall Financials, $Million, 2020-2022
- Figure 285:. Lonza.: Revenue (by Segment), $Million, 2021and 2022
- Figure 286:. Miltenyi Biotec B.V. & Co. KG: Product Portfolio
- Figure 287:. Sartorius AG: Product Portfolio
- Figure 288:. Sartorius AG: Overall Financials, $Million, 2020-2022
- Figure 289:. Sartorius AG: Revenue (by Segment), $Million, 2020-2022
- Figure 290:. Sartorius AG: Revenue (by Region), $Million, 2020-2022
- Figure 291:. Thermo Fisher Scientific Inc.: Product Portfolio
- Figure 292:. Thermo Fisher Scientific Inc.: Overall Financials, $Million, 2020-2022
- Figure 293:. Thermo Fisher Scientific Inc.: Revenue (by Segment), $Million, 2020-2022
- Figure 294:. Thermo Fisher Scientific Inc.: Revenue (by Region), $Million, 2020-2022
- Figure 295:. Thermo Fisher Scientific Inc.: R&D Expenditure, 2020-2022
- Figure 296:. WuXi AppTec: Product Portfolio
- Figure 297:. WuXi AppTec: Overall Financials, $Million, 2020-2022
- Figure 298:. WuXi AppTec: Revenue (by Segment), $Million, 2021 and 2022
- Figure 299:. AGC Biologics.: Product Portfolio
- Figure 300:. Charles River Laboratories International, Inc.: Product Portfolio
- Figure 301:. Charles River Laboratories International, Inc.: Overall Financials, $Million, 2020-2022
- Figure 302:. Charles River Laboratories International, Inc: Revenue (by Segment), $Million, 2020-2022
- Figure 303:. Charles River Laboratories International, Inc.: Revenue (by Region), $Million, 2020-2022
- Figure 304:. Catalent, Inc: Product Portfolio
- Figure 305:. Catalent, Inc: Overall Financials, $Million, 2020-2022
- Figure 306:. Catalent, Inc: Revenue (by Segment), $Million, 2020-2022
- Figure 307:. Catalent, Inc: Revenue (by Region), $Million, 2020-2022
- Figure 308:. Eurofins Scientific: Product Portfolio
- Figure 309:. Eurofins Scientific.: Overall Financials, $Million, 2020-2022
- Figure 310:. Eurofins Scientific.: Revenue (by Segment), $Million, 2020-2022
- Figure 311:. Eurofins Scientific.: Revenue (by Region), $Million, 2020-2022
- Figure 312:. Merck KGaA: Product Portfolio
- Figure 313:. Merck KGaA: Overall Financials, $Million, 2020-2022
- Figure 314:. Merck KGaA: Revenue (by Segment), $Million, 2020-2022
- Figure 315:. Merck KGaA: Revenue (by Region), $Million, 2020-2022
List of Tables
- Table 1:. Key Questions Answered in the Report
- Table 2:. Global Regulatory Scenario: Cell and Gene Therapy Manufacturing QC Market
- Table 3:. Impact Analysis, Market Drivers
- Table 4:. Investments in Cell and Gene Therapy Development, 2021-2022
- Table 5:. North America Cell and Gene Therapy Manufacturing QC Market, Impact Analysis
- Table 6:. Europe Cell and Gene Therapy Manufacturing QC Market Dynamics, Impact Analysis
- Table 7:. Asia-Pacific Cell and Gene Therapy Manufacturing QC Market Dynamics, Impact Analysis
- Table 8:. Approved Cell and Gene Therapies, Japan
- Table 9:. Latin America Cell and Gene Therapy Manufacturing QC Market Dynamics, Impact Analysis
- Table 10:. Middle East and Africa Cell and Gene Therapy Manufacturing QC Market Dynamics, Impact Analysis
“Global Cell and Gene Therapy Manufacturing QC Market to Reach $10.65 Billion by 2033.”
Industry Overview
The global cell and gene therapy manufacturing QC market was valued at $1.95 billion in 2022 and is anticipated to reach $10.65 billion by 2033, witnessing a CAGR of 16.85% during the forecast period 2023-2033. The growth in the global cell and gene therapy manufacturing QC market is expected to be driven by the increased number of approved therapies and growing infrastructure requirements. In addition, expansion in target indications for cell and gene-based therapies creates a demand for large-scale manufacturing and QC.
Market Lifecycle Stage
The global cell and gene therapy manufacturing QC market is in progressing phase. The cell and gene therapy manufacturing QC market is experiencing rapid growth due to the increasing adoption of innovative therapies such as CAR T-cells and others. Robust quality control processes are essential to ensure the safety, efficacy, and consistency of cell and gene-based treatments. The FDA has approved more than 25 cell and gene-based therapies in the last 10 years. These factors are expected to drive the demand for consumables, instruments, and software solutions required for manufacturing cell and gene therapy, thereby augmenting the growth of the cell and gene therapy manufacturing QC market.
Impact
The field of medicine is transformed with the commercialization of cell and gene therapies. With the advent of time and introduction of new technologies, cell and gene therapy areas are flourishing. There is constant ongoing research for the development of novel cell and gene therapies. According to the American Society of Gene and Cell Therapy (ASGCT), as of February 2023, there are more than 2,000 clinical trials in the pipeline. The robust clinical pipeline for novel cell and gene entities is expected to create a lucrative opportunity for QC and boost the growth of the cell and gene therapy manufacturing QC market. In addition, the entry of several established players, such as Lonza., Merck KGaA, Charles River Laboratories International Inc., Eurofins Scientific, and others, is expected to aid the market growth.
The field of cell and gene therapy manufacturing QC is witnessing several trends and advancements that are expected to have a significant impact on the market. Some of the trends include the adoption of automation QC processes, advanced analytical technologies, process analytical testing (PAT) and quality risk management (QRM), and others. These trends and advancements in cell and gene therapy manufacturing QC are expected to drive improvements in product quality, manufacturing efficiency, regulatory compliance, and patient safety. They are expected to play a crucial role in supporting the growth and success of the cell and gene therapy industry, making QC an integral part of the development and commercialization of these advanced therapies.
Impact of COVID-19
In December 2019, Wuhan, a city in the Hubei region of China, was the site of the first detection of the COVID-19 outbreak. Following the classification of COVID-19 as novel pneumonia due to a cluster of unexplained pneumonia cases, efforts to pinpoint the culprit causing the outbreak and outline its genomic sequence got underway right once. The virus has already spread to every country on the globe, and researchers, governments, and business leaders are working to find answers to the crisis at a scale and speed that has never been seen. Testing for SARS-CoV-2 in the populace is one of the main steps that has been put into place globally, among many other measures used to stop the spread of the disease. The most crucial benefit of testing is that it offers evidence of illness, enabling those who are tested and those they have come into contact with to take the required precautions, including quarantining, to minimize community exposure.
The COVID-19 pandemic has substantially interrupted social, economic, and political activity around the world due to its unparalleled size and intensity. As a result, the cell and gene therapy (CGT) sector, which has historically struggled with tremendous complexity in the supply of materials, production, and logistical operations, has been disrupted by COVID-19.
The research, production, clinical development, and market introduction of cell and gene-based therapies (CGTs) for diseases unrelated to COVID-19 have all been significantly disrupted as a result of the COVID-19 pandemic. A lack of manufacturing material supplies, challenges with clinical studies, and a delay in the creation of regulatory dossiers are all significant reasons for the manufacturing of cell and gene therapy. This has emphasized the significance of tackling the difficulties in CGTs' supply chain and production to increase resilience during the crisis.
To prevent CGTs' market access from being significantly disrupted, manufacturing resilience, digitalization, telemedicine, value-based pricing, and creative payment systems may be progressively tapped.
Market Segmentation:
Segmentation 1: by Therapy Type
- Cell Therapy
- Gene Therapy
Based on therapy type, the cell therapy segment dominated the global cell and gene therapy manufacturing QC market in FY2022. The increasing adoption of cell-based therapies, such as CAR-T cell therapy and stem cell therapy, contributed to the prominence of the cell therapy segment. Cell therapy is a type of medical treatment that involves the transplantation or infusion of live, healthy cells into a patient's body to treat or cure a disease or disorder. The goal of cell therapy is to restore or replace damaged or malfunctioning cells with healthy ones or to use the therapeutic properties of the transplanted cells to stimulate the body's own natural healing processes.
Segmentation 2: by Offering
- Products
- Services
Based on offering, the services segment dominated the global cell and gene therapy manufacturing QC market in FY2022. This segment encompassed a range of vital services, including quality control testing, analytical services, process development, validation, and regulatory compliance. Within the services segment, there is a further division into various categories, including safety testing, potency testing, sterility testing, identity testing, stability and genetic fidelity testing, and others.
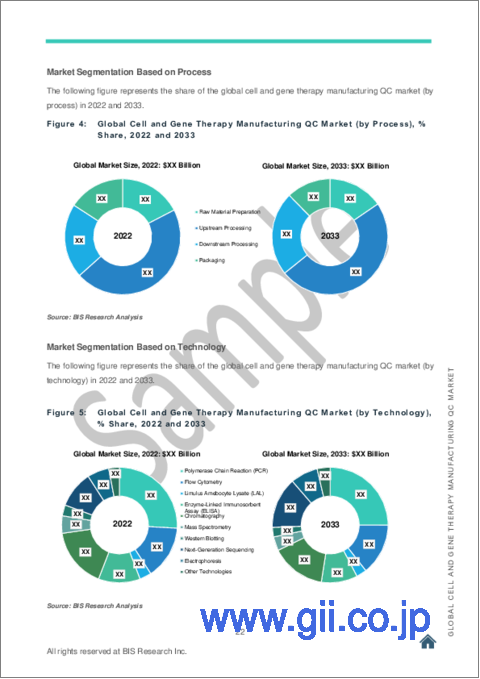
Segmentation 3: by Process
- Raw Material Preparation
- Upstream Processing
- Downstream Processing
- Packaging
Based on process, the global cell and gene therapy manufacturing QC market was dominated by the upstream processing segment in FY2022. The dominance of the upstream processing segment can be attributed to several factors, including advancements in bioprocessing technologies, optimized cell culture media, and improved bioreactor systems.
Segmentation 4: by Application
- Safety Testing
- Potency Testing
- Identity Testing
- Stability and Genetic Fidelity Testing
- Others
Based on application, the safety testing segment accounted for the largest share of the global cell and gene therapy manufacturing QC market in FY2022. This segment encompasses a range of tests, including sterility testing, endotoxin testing, and among others. Furthermore, some of the key players, such as Lonza., Thermo Fisher Scientific Inc., Charles River Laboratories International, Inc., Eurofins Scientific, and Merck KGaA, offer safety testing, potency testing, and other services in the global cell and gene therapy manufacturing QC market.
Segmentation 5: by Technology
- Polymerase Chain Reaction (PCR)
- Flow Cytometry
- Limulus Amebocyte Lysate (LAL)
- Enzyme-Linked Immunosorbent Assay (ELISA)
- Chromatography
- Mass Spectrometry
- Western Blotting
- Next-Generation Sequencing (NGS)
- Electrophoresis
- Other Technologies
Based on technology, the global cell and gene therapy manufacturing QC market is dominated by the PCR segment in FY2022. It plays a vital role in various QC (quality control) processes, including gene expression analysis, viral vector detection, and genetic stability assessment. PCR is used in a variety of tasks in cell and gene therapy manufacturing, including detecting contaminants, measuring gene expression and viral vector integration, and assessing gene editing success. Different types of PCR systems, such as real-time PCR and digital PCR, are used depending on the specific application and process requirements.
Segmentation 6: by Region
- North America
- Europe
- Asia-Pacific
- MEA
- LATAM
North America cell and gene therapy manufacturing QC market is expected to have the highest market share in 2022 and is currently the leading contributor to the market. However, the Asia-Pacific region, constituting several emerging economies, is expected to register the highest CAGR of 18.01% during the forecast period 2023-2033.
Recent Developments in the Global Cell and Gene Therapy Manufacturing QC Market
- In March 2023, Thermo Fisher Scientific Inc. collaborated with Arsenal Biosciences to assist in the clinical manufacturing of autologosus T-cell therapies.
This partnership combined Thermo Fisher's cell therapy manufacturing expertise with Arsenal Biosciences' innovative technologies, with the goal of advancing the development and accessibility of personalized T-cell therapies.
- In January 2023, Sartorius AG collaborated with Roosterbio Inc. to enhance their downstream purification methods in the field of exosome development.
- In February 2023, Charles River Laboratories International, Inc. partnered with Purespring Therapeutics. This partnership would advance gene therapies for kidney diseases and provide innovative treatment options for patients by leveraging Charles River's eXpDNA plasmid platform.
- In January 2023, Bio-Techne Corporation launched MauriceFlex, a new product under its ProteinSimple brand. MauriceFlex is a versatile system that facilitates protein charge variant fractionation along with routine cIEF (capillary isoelectric focusing) and CE-SDS (capillary electrophoresis-sodium dodecyl sulfate) assays. This innovative system offered a comprehensive solution for protein characterization, streamlining workflows in protein analysis.
- In January 2023, Bio-Techne Corporation launched RNAscope plus assay to advance its gene therapy development.
- In April 2023, Danaher. (Cytiva) launched X-platform bioreactors, which aim to streamline single-use upstream bioprocessing operations. These versatile bioreactors can be used for producing monoclonal antibodies, protein-based drugs, cell and gene therapies, and viral vectors. They offer flexibility and efficiency in bioprocessing, facilitating the development and manufacturing of various therapeutic products.
Demand - Drivers and Limitations
Market Demand Drivers:
- Growing Cell and Gene Therapy Production Leading to Increased Demand for Quality Control (QC)Testing
- Increasing Number of Approvals Leading to an Upsurge in Demand for Cell and Gene Therapies QC Testing
- Rise in Investment for the Development of Cell and Gene Therapies Increasing the Demand for QC Products and Services
Market Restraints:
- Limited Adoption of Cell and Gene Therapy due to High Manufacturing and QC Costs
Market Opportunities:
- Continuous Entry of New Market Participants in Cell and Gene Therapies to Create an Opportunity for the Expansion of Manufacturing Facilities and QC Testing Services
- Introduction of Technologically Advanced Products in QC Testing for Cell and Gene Therapies
How can this report add value to an organization?
- Workflow/Innovation Strategy: The cell and gene therapy manufacturing QC market (by offering) has been segmented into products and services. Moreover, the study provides the reader with a detailed understanding of the different applications of cell and gene therapy manufacturing QC in raw material preparation, upstream processing, downstream processing, and packaging.
- Growth/Marketing Strategy: Cell and gene therapy manufacturing QC is being used for raw material preparation, upstream processing, downstream processing, and packaging. Various companies are providing products and services to aid in the manufacturing and QC of various cell and gene therapies, which is also the key strategy for market players to excel in the current cell and gene therapy manufacturing QC market.
- Competitive Strategy: Key players in the global cell and gene therapy manufacturing QC market have been analyzed and profiled in the study, including manufacturers involved in new product launches, acquisitions, expansions, and strategic collaborations. Moreover, a detailed competitive benchmarking of the players operating in the global cell and gene therapy manufacturing QC market has been done to help the reader understand how players stack against each other, presenting a clear market landscape. Additionally, comprehensive competitive strategies such as partnerships, agreements, and collaborations will aid the reader in understanding the untapped revenue pockets in the market.
Key Market Players and Competition Synopsis
Cell and gene-based therapies have sparked efforts to promote treatments such as chimeric antigen receptor (CAR)-T cell therapy has gained significant attention in the field of oncology for its potential to treat certain types of cancer. The need to cater to the clinical and commercial production of cell-based therapies has increased the demand for quality control (QC) testing during the manufacturing of cell and gene-based therapies. The increased demand for treatments in the healthcare sector is fuelling the expansion of the cell and gene therapy manufacturing QC market and helping the market to spread out across different regions.
Key Companies Profiled:
|
|
Table of Contents
1. Markets
- 1.1. Product Definition
- 1.1.1. Product Definition
- 1.1.2. Inclusion and Exclusion Criteria
- 1.2. Market Scope
- 1.2.1. Scope of the Work
- 1.2.2. Key Questions Answered in the Report
- 1.3. Research Methodology
- 1.3.1. Global Cell and Gene Therapy Manufacturing QC Market
- 1.3.2. Data Sources
- 1.3.2.1. Primary Data Sources
- 1.3.2.2. Secondary Data Sources
- 1.3.3. Market Estimation Model
- 1.3.4. Criteria for Company Profiling
- 1.4. Market Overview
- 1.4.1. Global Market Scenario
- 1.4.1.1. Realistic Growth Scenario
- 1.4.1.2. Optimistic Scenario
- 1.4.1.3. Pessimistic Scenario
- 1.4.2. Market Footprint and Growth Potential
- 1.4.3. Future Potential
- 1.4.4. COVID-19 Impact on Market
- 1.4.4.1. Impact on Research and Clinical Operations
- 1.4.4.2. COVID-19 Impact: Current Scenario of the Market
- 1.4.4.3. Pre- and Post-COVID-19 Impact Assessment
- 1.4.4.3.1. Pre-COVID-19 Phase
- 1.4.4.3.2. Post-COVID-19 Phase
- 1.4.1. Global Market Scenario
2. Global Cell and Gene Therapy Manufacturing QC Market: Industry Analysis
- 2.1. Regulatory Framework
- 2.1.1. Chemistry, Manufacturing, and Control (CMC) Requirements by the Food and Drug Administration (FDA)
- 2.1.1.1. Product Testing
- 2.1.1.2. Microbial Testing
- 2.1.1.3. Identity
- 2.1.1.4. Purity
- 2.1.1.5. Potency
- 2.1.1.6. Viability
- 2.1.1.7. Cell Number or Dose
- 2.1.2. Quality Aspects of Cell and Gene Therapy Products by the European Medicines Agency (EMA)
- 2.1.2.1. Characterization
- 2.1.2.2. Identity Testing
- 2.1.2.3. Purity Testing
- 2.1.3. Current Good Manufacturing Practice (CGMP) Regulations
- 2.1.3.1. U.S.
- 2.1.3.2. Europe
- 2.1.4. Global Regulatory Framework: Cell and Gene Therapy Manufacturing QC Market
- 2.1.1. Chemistry, Manufacturing, and Control (CMC) Requirements by the Food and Drug Administration (FDA)
3. Global Cell and Gene Therapy Manufacturing QC Market: Market Dynamics
- 3.1. Overview
- 3.2. Impact Analysis
- 3.3. Market Drivers
- 3.3.1. Growing Cell and Gene Therapy Production Leading to Increased Demand for Quality Control (QC)Testing
- 3.3.2. Increasing Number of Approvals Leading to an Upsurge in Demand for Cell and Gene Therapies QC Testing
- 3.3.3. Rise in Investment for the Development of Cell and Gene Therapies Increasing the Demand for QC Products and Services
- 3.4. Market Restraints
- 3.4.1. Limited Adoption of Cell and Gene Therapy due to High Manufacturing and QC Costs
- 3.5. Market Opportunities
- 3.5.1. Continuous Entry of New Market Participants in Cell and Gene Therapies to Create an Opportunity for the Expansion of Manufacturing Facilities and QC Testing Services
- 3.5.2. Introduction of Technologically Advanced Products in QC Testing for Cell and Gene Therapies
4. Global Cell and Gene Therapy Manufacturing QC Market: Competitive Landscape
- 4.1. Overview
- 4.2. Key Strategies and Developments
- 4.2.1. Synergistic Activities
- 4.2.2. Product and Service Launches
- 4.2.3. Mergers and Acquisitions
- 4.2.4. Product Approvals
- 4.3. Market Share Analysis
- 4.4. Growth Share Analysis
- 4.4.1. Growth Share Analysis (by Application)
- 4.5. Supply Chain Analysis
- 4.5.1. Key Entities in Supply Chain
5. Global Cell and Gene Therapy Manufacturing QC Market (by Therapy Type)
- 5.1. Overview
- 5.2. Cell Therapy
- 5.2.1. Autologous
- 5.2.2. Allogeneic
- 5.2.3. Others
- 5.3. Gene Therapy
- 5.3.1. Viral Vectors
- 5.3.2. Non-Viral Vectors
6. Global Cell and Gene Therapy Manufacturing QC Market (by Offering)
- 6.1. Overview
- 6.2. Products
- 6.2.1. Consumables
- 6.2.1.1. Kits and Assays
- 6.2.1.2. Reagents
- 6.2.1.3. Cell Culture Media and Supplements
- 6.2.2. Instruments
- 6.2.2.1. Bioreactors/Fermenters
- 6.2.2.2. PCR Systems
- 6.2.2.3. Chromatography System
- 6.2.2.4. Others
- 6.2.3. Software
- 6.2.1. Consumables
- 6.3. Services
- 6.3.1. Safety Testing
- 6.3.2. Potency Testing
- 6.3.3. Identity Testing
- 6.3.4. Stability and Genetic Fidelity Testing
- 6.3.5. Others
7. Global Cell and Gene Therapy Manufacturing QC Market (by Process)
- 7.1. Overview
- 7.2. Raw Material Preparation
- 7.3. Upstream Processing
- 7.4. Downstream Processing
- 7.5. Packaging
8. Global Cell and Gene Therapy Manufacturing QC Market (by Technology)
- 8.1. Overview
- 8.2. Polymerase Chain Reaction (PCR)
- 8.3. Flow Cytometry
- 8.4. Limulus Amebocyte Lysate (LAL)
- 8.5. Enzyme-Linked Immunosorbent Assay (ELISA)
- 8.6. Chromatography
- 8.7. Mass Spectrometry
- 8.8. Western Blotting
- 8.9. Next-Generation Sequencing (NGS)
- 8.1. Electrophoresis
- 8.11. Other Technologies
9. Global Cell and Gene Therapy Manufacturing QC Market (by Application)
- 9.1. Overview
- 9.2. Safety Testing
- 9.2.1. By Safety Testing Type
- 9.3. Potency Testing
- 9.3.1. By Potency Testing Type
- 9.4. Identity Testing
- 9.4.1. By Identity Testing Type
- 9.5. Stability and Genetic Fidelity Testing
- 9.5.1. By Stability Testing and Genetic Fidelity Testing Type
- 9.6. Others
10. Global Cell and Gene Therapy Manufacturing QC Market (by Region)
- 10.1. Overview
- 10.2. North America
- 10.2.1. North America Cell and Gene Therapy Manufacturing QC Market (by Offering)
- 10.2.2. North America Cell and Gene Therapy Manufacturing QC Market (by Therapy Type)
- 10.2.3. North America Cell and Gene Therapy Manufacturing QC Market (by Process)
- 10.2.4. North America Cell and Gene Therapy Manufacturing QC Market (by Technology)
- 10.2.5. North America Cell and Gene Therapy Manufacturing QC Market (by Application)
- 10.2.6. U.S.
- 10.2.6.1. U.S. Cell and Gene Therapy Manufacturing QC Market (by Offering)
- 10.2.6.2. U.S. Cell and Gene Therapy Manufacturing QC Market (by Therapy Type)
- 10.2.6.3. U.S. Cell and Gene Therapy Manufacturing QC Market (by Process)
- 10.2.6.4. U.S. Cell and Gene Therapy Manufacturing QC Market (by Technology)
- 10.2.6.5. U.S. Cell and Gene Therapy Manufacturing QC Market (by Application)
- 10.2.7. Canada
- 10.2.7.1. Canada Cell and Gene Therapy Manufacturing QC Market (by Offering)
- 10.2.7.2. Canada Cell and Gene Therapy Manufacturing QC Market (by Therapy Type)
- 10.2.7.3. Canada Cell and Gene Therapy Manufacturing QC Market (by Process)
- 10.2.7.4. Canada Cell and Gene Therapy Manufacturing QC Market (by Technology)
- 10.2.7.5. Canada Cell and Gene Therapy Manufacturing QC Market (by Application)
- 10.3. Europe
- 10.3.1. Europe Cell and Gene Therapy Manufacturing QC Market (by Offering)
- 10.3.2. Europe Cell and Gene Therapy Manufacturing QC Market (by Therapy Type)
- 10.3.3. Europe Cell and Gene Therapy Manufacturing QC Market (by Process)
- 10.3.4. Europe Cell and Gene Therapy Manufacturing QC Market (by Technology)
- 10.3.5. Europe Cell and Gene Therapy Manufacturing QC Market (by Application)
- 10.3.6. U.K.
- 10.3.6.1. U.K. Cell and Gene Therapy Manufacturing QC Market (by Offering)
- 10.3.6.2. U.K. Cell and Gene Therapy Manufacturing QC Market (by Therapy Type)
- 10.3.6.3. U.K. Cell and Gene Therapy Manufacturing QC Market (by Process)
- 10.3.6.4. U.K. Cell and Gene Therapy Manufacturing QC Market (by Technology)
- 10.3.6.5. U.K. Cell and Gene Therapy Manufacturing QC Market (by Application)
- 10.3.7. Germany
- 10.3.7.1. Germany Cell and Gene Therapy Manufacturing QC Market (by Offering)
- 10.3.7.2. Germany Cell and Gene Therapy Manufacturing QC Market (by Therapy Type)
- 10.3.7.3. Germany Cell and Gene Therapy Manufacturing QC Market (by Process)
- 10.3.7.4. Germany Cell and Gene Therapy Manufacturing QC Market (by Technology)
- 10.3.7.5. Germany Cell and Gene Therapy Manufacturing QC Market (by Application)
- 10.3.8. France
- 10.3.8.1. France Cell and Gene Therapy Manufacturing QC Market (by Offering)
- 10.3.8.2. France Cell and Gene Therapy Manufacturing QC Market (by Therapy Type)
- 10.3.8.3. France Cell and Gene Therapy Manufacturing QC Market (by Process)
- 10.3.8.4. France Cell and Gene Therapy Manufacturing QC Market (by Technology)
- 10.3.8.5. France Cell and Gene Therapy Manufacturing QC Market (by Application)
- 10.3.9. Italy
- 10.3.9.1. Italy Cell and Gene Therapy Manufacturing QC Market (by Offering)
- 10.3.9.2. Italy Cell and Gene Therapy Manufacturing QC Market (by Therapy Type)
- 10.3.9.3. Italy Cell and Gene Therapy Manufacturing QC Market (by Process)
- 10.3.9.4. Italy Cell and Gene Therapy Manufacturing QC Market (by Technology)
- 10.3.9.5. Italy Cell and Gene Therapy Manufacturing QC Market (by Application)
- 10.3.10. Spain
- 10.3.10.1. Spain Cell and Gene Therapy Manufacturing QC Market (by Offering)
- 10.3.10.2. Spain Cell and Gene Therapy Manufacturing QC Market (by Therapy Type)
- 10.3.10.3. Spain Cell and Gene Therapy Manufacturing QC Market (by Process)
- 10.3.10.4. Spain Cell and Gene Therapy Manufacturing QC Market (by Technology)
- 10.3.10.5. Spain Cell and Gene Therapy Manufacturing QC Market (by Application)
- 10.3.11. Rest-of-Europe
- 10.3.11.1. Rest-of-Europe Cell and Gene Therapy Manufacturing QC Market (by Offering)
- 10.3.11.2. Rest-of-Europe Cell and Gene Therapy Manufacturing QC Market (by Therapy Type)
- 10.3.11.3. Rest-of-Europe Cell and Gene Therapy Manufacturing QC Market (by Process)
- 10.3.11.4. Rest-of-Europe Cell and Gene Therapy Manufacturing QC Market (by Technology)
- 10.3.11.5. Rest-of-Europe Cell and Gene Therapy Manufacturing QC Market (by Application)
- 10.4. Asia-Pacific
- 10.4.1. Asia-Pacific Cell and Gene Therapy Manufacturing QC Market (by Offering)
- 10.4.2. Asia-Pacific Cell and Gene Therapy Manufacturing QC Market (by Therapy Type)
- 10.4.3. Asia-Pacific Cell and Gene Therapy Manufacturing QC Market (by Process)
- 10.4.4. Asia-Pacific Cell and Gene Therapy Manufacturing QC Market (by Technology)
- 10.4.5. Asia-Pacific Cell and Gene Therapy Manufacturing QC Market (by Application)
- 10.4.6. China
- 10.4.6.1. China Cell and Gene Therapy Manufacturing QC Market (by Offering)
- 10.4.6.2. China Cell and Gene Therapy Manufacturing QC Market (by Therapy Type)
- 10.4.6.3. China Cell and Gene Therapy Manufacturing QC Market (by Process)
- 10.4.6.4. China Cell and Gene Therapy Manufacturing QC Market (by Technology)
- 10.4.6.5. China Cell and Gene Therapy Manufacturing QC Market (by Application)
- 10.4.7. Japan
- 10.4.7.1. Japan Cell and Gene Therapy Manufacturing QC Market (by Offering)
- 10.4.7.2. Japan Cell and Gene Therapy Manufacturing QC Market (by Therapy Type)
- 10.4.7.3. Japan Cell and Gene Therapy Manufacturing QC Market (by Process)
- 10.4.7.4. Japan Cell and Gene Therapy Manufacturing QC Market (by Technology)
- 10.4.7.5. Japan Cell and Gene Therapy Manufacturing QC Market (by Application)
- 10.4.8. South Korea
- 10.4.8.1. South Korea Cell and Gene Therapy Manufacturing QC Market (by Offering)
- 10.4.8.2. South Korea Cell and Gene Therapy Manufacturing QC Market (by Therapy Type)
- 10.4.8.3. South Korea Cell and Gene Therapy Manufacturing QC Market (by Process)
- 10.4.8.4. South Korea Cell and Gene Therapy Manufacturing QC Market (by Technology)
- 10.4.8.5. South Korea Cell and Gene Therapy Manufacturing QC Market (by Application)
- 10.4.9. Australia
- 10.4.9.1. Australia Cell and Gene Therapy Manufacturing QC Market (by Offering)
- 10.4.9.2. Australia Cell and Gene Therapy Manufacturing QC Market (by Therapy Type)
- 10.4.9.3. Australia Cell and Gene Therapy Manufacturing QC Market (by Process)
- 10.4.9.4. Australia Cell and Gene Therapy Manufacturing QC Market (by Technology)
- 10.4.9.5. Australia Cell and Gene Therapy Manufacturing QC Market (by Application)
- 10.4.10. India
- 10.4.10.1. India Cell and Gene Therapy Manufacturing QC Market (by Offering)
- 10.4.10.2. India Cell and Gene Therapy Manufacturing QC Market (by Therapy Type)
- 10.4.10.3. India Cell and Gene Therapy Manufacturing QC Market (by Process)
- 10.4.10.4. India Cell and Gene Therapy Manufacturing QC Market (by Technology)
- 10.4.10.5. India Cell and Gene Therapy Manufacturing QC Market (by Application)
- 10.4.11. Rest-of-Asia-Pacific
- 10.4.11.1. Rest-of-Asia-Pacific Cell and Gene Therapy Manufacturing QC Market (by Offering)
- 10.4.11.2. Rest-of-Asia-Pacific Cell and Gene Therapy Manufacturing QC Market (by Therapy Type)
- 10.4.11.3. Rest-of-Asia-Pacific Cell and Gene Therapy Manufacturing QC Market (by Process)
- 10.4.11.4. Rest-of-Asia-Pacific Cell and Gene Therapy Manufacturing QC Market (by Technology)
- 10.4.11.5. Rest-of-Asia-Pacific Cell and Gene Therapy Manufacturing QC Market (by Application)
- 10.5. Middle East and Africa
- 10.5.1. Middle East and Africa Cell and Gene Therapy Manufacturing QC Market (by Offering)
- 10.5.2. Middle East and Africa Cell and Gene Therapy Manufacturing QC Market (by Therapy Type)
- 10.5.3. Middle East and Africa Cell and Gene Therapy Manufacturing QC Market (by Process)
- 10.5.4. Middle East and Africa Cell and Gene Therapy Manufacturing QC Market (by Technology)
- 10.5.5. Middle East and Africa Cell and Gene Therapy Manufacturing QC Market (by Application)
- 10.5.6. Israel
- 10.5.6.1. Israel Cell and Gene Therapy Manufacturing QC Market (by Offering)
- 10.5.6.2. Israel Cell and Gene Therapy Manufacturing QC Market (by Therapy Type)
- 10.5.6.3. Israel Cell and Gene Therapy Manufacturing QC Market (by Process)
- 10.5.6.4. Israel Cell and Gene Therapy Manufacturing QC Market (by Technology)
- 10.5.6.5. Israel Cell and Gene Therapy Manufacturing QC Market (by Application)
- 10.5.7. Turkiye
- 10.5.7.1. Turkiye Cell and Gene Therapy Manufacturing QC Market (by Offering)
- 10.5.7.2. Turkiye Cell and Gene Therapy Manufacturing QC Market (by Therapy Type)
- 10.5.7.3. Turkiye Cell and Gene Therapy Manufacturing QC Market (by Process)
- 10.5.7.4. Turkiye Cell and Gene Therapy Manufacturing QC Market (by Technology)
- 10.5.7.5. Turkiye Cell and Gene Therapy Manufacturing QC Market (by Application)
- 10.5.8. K.S.A.
- 10.5.8.1. K.S.A. Cell and Gene Therapy Manufacturing QC Market (by Offering)
- 10.5.8.2. K.S.A. Cell and Gene Therapy Manufacturing QC Market (by Therapy Type)
- 10.5.8.3. K.S.A. Cell and Gene Therapy Manufacturing QC Market (by Process)
- 10.5.8.4. K.S.A. Cell and Gene Therapy Manufacturing QC Market (by Technology)
- 10.5.8.5. K.S.A. Cell and Gene Therapy Manufacturing QC Market (by Application)
- 10.5.9. U.A.E.
- 10.5.9.1. U.A.E. Cell and Gene Therapy Manufacturing QC Market (by Offering)
- 10.5.9.2. U.A.E. Cell and Gene Therapy Manufacturing QC Market (by Therapy Type)
- 10.5.9.3. U.A.E. Cell and Gene Therapy Manufacturing QC Market (by Process)
- 10.5.9.4. U.A.E. Cell and Gene Therapy Manufacturing QC Market (by Technology)
- 10.5.9.5. U.A.E. Cell and Gene Therapy Manufacturing QC Market (by Application)
- 10.5.10. South Africa
- 10.5.10.1. South Africa Cell and Gene Therapy Manufacturing QC Market (by Offering)
- 10.5.10.2. South Africa Cell and Gene Therapy Manufacturing QC Market (by Therapy Type)
- 10.5.10.3. South Africa Cell and Gene Therapy Manufacturing QC Market (by Process)
- 10.5.10.4. South Africa Cell and Gene Therapy Manufacturing QC Market (by Technology)
- 10.5.10.5. South Africa Cell and Gene Therapy Manufacturing QC Market (by Application)
- 10.5.11. Rest-of-Middle East and Africa
- 10.5.11.1. Rest-of-Middle East and Africa Cell and Gene Therapy Manufacturing QC Market (by Offering)
- 10.5.11.2. Rest-of-Middle East and Africa Cell and Gene Therapy Manufacturing QC Market (by Therapy Type)
- 10.5.11.3. Rest-of-Middle East and Africa Cell and Gene Therapy Manufacturing QC Market (by Process)
- 10.5.11.4. Rest-of-Middle East and Africa Cell and Gene Therapy Manufacturing QC Market (by Technology)
- 10.5.11.5. Rest-of-Middle East and Africa Cell and Gene Therapy Manufacturing QC Market (by Application)
- 10.6. Latin America
- 10.6.1. Latin America Cell and Gene Therapy Manufacturing QC Market (by Offering)
- 10.6.2. Latin America Cell and Gene Therapy Manufacturing QC Market (by Therapy Type)
- 10.6.3. Latin America Cell and Gene Therapy Manufacturing QC Market (by Process)
- 10.6.4. Latin America Cell and Gene Therapy Manufacturing QC Market (by Technology)
- 10.6.5. Latin America Cell and Gene Therapy Manufacturing QC Market (by Application)
- 10.6.6. Brazil
- 10.6.6.1. Brazil Cell and Gene Therapy Manufacturing QC Market (by Offering)
- 10.6.6.2. Brazil Cell and Gene Therapy Manufacturing QC Market (by Therapy Type)
- 10.6.6.3. Brazil Cell and Gene Therapy Manufacturing QC Market (by Process)
- 10.6.6.4. Brazil Cell and Gene Therapy Manufacturing QC Market (by Technology)
- 10.6.6.5. Brazil Cell and Gene Therapy Manufacturing QC Market (by Application)
- 10.6.7. Mexico
- 10.6.7.1. Mexico Cell and Gene Therapy Manufacturing QC Market (by Offering)
- 10.6.7.2. Mexico Cell and Gene Therapy Manufacturing QC Market (by Therapy Type)
- 10.6.7.3. Mexico Cell and Gene Therapy Manufacturing QC Market (by Process)
- 10.6.7.4. Mexico Cell and Gene Therapy Manufacturing QC Market (by Technology)
- 10.6.7.5. Mexico Cell and Gene Therapy Manufacturing QC Market (by Application)
- 10.6.8. Rest-of-Latin America
- 10.6.8.1. Rest-of-Latin America Cell and Gene Therapy Manufacturing QC Market (by Offering)
- 10.6.8.2. Rest-of-Latin America Cell and Gene Therapy Manufacturing QC Market (by Therapy Type)
- 10.6.8.3. Rest-of-Latin America Cell and Gene Therapy Manufacturing QC Market (by Process)
- 10.6.8.4. Rest-of-Latin America Cell and Gene Therapy Manufacturing QC Market (by Technology)
- 10.6.8.5. Rest-of-Latin America Cell and Gene Therapy Manufacturing QC Market (by Application)
11. Company Profiles
- 11.1. Overview
- 11.2. Manufacturers
- 11.2.1. Bio-Techne Corporation
- 11.2.1.1. Company Overview
- 11.2.1.2. Role of Bio-Techne Corporation in the Global Cell and Gene Therapy Manufacturing QC Market
- 11.2.1.3. Key Competitors
- 11.2.1.4. Financials
- 11.2.1.5. Analyst Perspective
- 11.2.2. bioMerieux SA
- 11.2.2.1. Company Overview
- 11.2.2.2. Role of bioMerieux SA in the Global Cell and Gene Therapy Manufacturing QC Market
- 11.2.2.3. Key Competitors
- 11.2.2.4. Financials
- 11.2.2.5. Analyst Perspective
- 11.2.3. Danaher. (Cytiva)
- 11.2.3.1. Company Overview
- 11.2.3.2. Role of Danaher. (Cytiva) in the Global Cell and Gene Therapy Manufacturing QC Market
- 11.2.3.3. Key Competitors
- 11.2.3.4. Financials
- 11.2.3.5. Key Insights about the Financial Health of the Company
- 11.2.3.6. Analyst Perspective
- 11.2.4. F. Hoffmann-La Roche Ltd
- 11.2.4.1. Company Overview
- 11.2.4.2. Role of F. Hoffmann-La Roche Ltd in the Global Cell and Gene Therapy Manufacturing QC Market
- 11.2.4.3. Key Competitors
- 11.2.4.4. Financials
- 11.2.4.5. Key Insights about the Financial Health of the Company
- 11.2.4.6. Analyst Perspective
- 11.2.5. Lonza.
- 11.2.5.1. Company Overview
- 11.2.5.2. Role of Lonza. in the Global Cell and Gene Therapy Manufacturing QC Market
- 11.2.5.3. Key Competitors
- 11.2.5.4. Financials
- 11.2.5.5. Analyst Perspective
- 11.2.6. Miltenyi Biotec B.V. & Co. KG
- 11.2.6.1. Company Overview
- 11.2.6.2. Role of Miltenyi Biotec B.V. & Co. KG in the Global Cell and Gene Therapy Manufacturing QC Market
- 11.2.6.3. Key Competitors
- 11.2.6.4. Analyst Perspective
- 11.2.7. Sartorius AG
- 11.2.7.1. Company Overview
- 11.2.7.2. Role of Sartorius AG in the Global Cell and Gene Therapy Manufacturing QC Market
- 11.2.7.3. Key Competitors
- 11.2.7.4. Financials
- 11.2.7.5. Analyst Perspective
- 11.2.8. Thermo Fisher Scientific Inc.
- 11.2.8.1. Company Overview
- 11.2.8.2. Role of Thermo Fisher Scientific Inc. in the Global Cell and Gene Therapy Manufacturing QC Market
- 11.2.8.3. Key Competitors
- 11.2.8.4. Financials
- 11.2.8.5. Key Insights about the Financial Health of the Company
- 11.2.8.6. Analyst Perspective
- 11.2.9. WuXi AppTec
- 11.2.9.1. Company Overview
- 11.2.9.2. Role of WuXi AppTec in the Global Cell and Gene Therapy Manufacturing QC Market
- 11.2.9.3. Key Competitors
- 11.2.9.4. Financials
- 11.2.9.5. Analyst Perspective
- 11.2.1. Bio-Techne Corporation
- 11.3. Service
- 11.3.1. AGC Biologics.
- 11.3.1.1. Company Overview
- 11.3.1.2. Role of AGC Biologics. in the Global Cell and Gene Therapy Manufacturing QC Market
- 11.3.1.3. Key Competitors
- 11.3.1.4. Analyst Perspective
- 11.3.2. Charles River Laboratories International, Inc.
- 11.3.2.1. Company Overview
- 11.3.2.2. Role of Charles River Laboratories International, Inc. in the Global Cell and Gene Therapy Manufacturing QC Market
- 11.3.2.3. Key Competitors
- 11.3.2.4. Financials
- 11.3.2.5. Analyst Perspective
- 11.3.3. Catalent, Inc
- 11.3.3.1. Company Overview
- 11.3.3.2. Role of Catalent, Inc in the Global Cell and Gene Therapy Manufacturing QC Market
- 11.3.3.3. Key Competitors
- 11.3.3.4. Financials
- 11.3.3.5. Analyst Perspective
- 11.3.4. Eurofins Scientific
- 11.3.4.1. Company Overview
- 11.3.4.2. Role of Eurofins Scientific in the Global Cell and Gene Therapy Manufacturing QC Market
- 11.3.4.3. Key Competitors
- 11.3.4.4. Financials
- 11.3.4.5. Analyst Perspective
- 11.3.5. Merck KGaA
- 11.3.5.1. Company Overview
- 11.3.5.2. Role of Merck KGaA in the Global Cell and Gene Therapy Manufacturing QC Market
- 11.3.5.3. Key Competitors
- 11.3.5.4. Financials
- 11.3.5.5. Analyst Perspective
- 11.3.1. AGC Biologics.
- 11.4. Emerging Companies
- 11.4.1. Applied StemCell, Inc.
- 11.4.2. Vineti